Abstract
Energy needs and environmental concerns are leading to the search for alternative renewable fuels such as biodiesel. Biodiesel from microalgae has recently gained attention due to the drawbacks of other feedstocks such as edible oils. Recent research is focussing on techniques to convert feedstock into quality biodiesel in a cost-effective way. Here, we review conventional and in situ biodiesel synthesis from microalgae. We present the various catalysts and ultrasonic reactors. We found that biodiesel production through ultrasonication assisted in situ processing of wet microalgae is at least three times more expensive than biodiesel production through conventional mechanisms from feedstocks such as waste cooking oil. Finally, we discuss the feasibility of ultrasound-intensified biodiesel production from microalgae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing population and modernization, the total primary energy consumption is increasing continuously and is expected to increase by 57% from 2010–2040 (Lee et al. 2010). Increased energy consumption directly has an impact on the use of the total available fossil fuels since fossil fuels provide more than 80% of the total energy consumed (Kumar and Sharma 2016). Energy utilization from natural resources has long been an area of active research. In the dual crisis of fossil fuel depletion and environmental degradation, biofuels, e.g., biodiesel are considered as one of the most potential source of alternative fuels (Schmidt 2007). Biodiesel is considered as an possible replacement of petro-diesel as biodiesel is non-toxic, biodegradable and renewable (Lei et al. 2011). However, more than 75% of the biodiesel production cost is toward the cost of feedstock (Atabani et al. 2012).
In the present scenario, the plant seed oil is a major source of biodiesel production (Naik et al. 2010; Khiratkar et al. 2018). Generally, biodiesel can be classified into three generations. The extraction of oil from edible plant seeds, which refers to the first generation, puts pressure on the supply of food and hence problems with the food chain (Rodionova et al. 2017). Other drawbacks are-i) negative impact on the arable land and ii) limited product range (Zhang et al. 2014). In the second generation, other sources of biodiesel were explored which ranged from lignocellulosic feedstocks to municipal solid wastes (Meher et al. 2006). Biodiesel from micro- or macro-algal biomass is referred to as the third generation, which is considered as the best alternative mainly because it does not alter the food chain and leads to lesser pressure on the arable lands as well as the environment (Sara et al. 2016; Srivastava et al. 2020; Pavithra et al. 2020).
Algae, especially microalgae, are considered as an excellent source for biodiesel production as microalgae have better growth rates as compared to the terrestrial crops. The oil yield from them is approximated to be from 20,000 to 80,000 L per acre per year, which is about 7–31 times higher than the most widely used source, i.e., Palm oil (Demirbas 2009; Demirbas and Demirbas 2011). Additionally, algal biomass can be converted into bioethanol and biohydrogen via various chemical as well as biological methods in addition to the biodiesel production from algal lipids (Demirbas 2010). In recent years, various algae have been cultured and tested for the lipid contents. Moreover, the past decade has witnessed great advancements in the lipid extraction techniques. Since oil, i.e., lipid from algae is considered to be a great source for biodiesel synthesis, the paradigm has shifted toward increasing the concentration of lipids in algae and optimization of the biodiesel synthesis process. Different algae culture devices and purification methods have been reported by Kelliher et al. (2016). The characterization of algae into macro and micro has helped in various specific methods for the extraction of lipids from them (Griffiths et al. 2016). Macroalgae by virtue of the ease of handling and visible features were the first to be studied for optimization of various extraction processes. Subsequently, the research trend shifted toward the use of microalgae because microalgae have high potential for oil production (Scragg et al. 2003; Mondal et al. 2017).
The cost of biodiesel production from microalgae varies widely because of the diverse variety of algal species and wide range of the lipid contents (Slade and Bauen 2013). Sun et al. (2011) reported microalgal biodiesel cost as $4.92 per gallon, whereas it was reported to be $13.32 per gallon in another report (Sun et al. 2011; Solecki et al. 2013). Lipid extraction is often dependent on the type of algae, which causes an irregularity in the process development. Also, choice of the extraction method can cause a difference in the amount of lipid extracted. Lee et al. (2010) carried out the extraction of Botryococcus sp, Chlorella vulgaris, and Scenedesmus sp using several methods like autoclaving, bead-beating, microwaves, sonication, and obtained a varied amount of lipid. Such irregularity in the sample and in the extraction process makes it difficult to find the most optimum method.
Apart from the above challenges, the presence of water plays a negative role in the synthesis of biodiesel from wet algal biomass (Atadashi et al. 2012). Significantly higher content of water in wet microalgae, i.e., up to about 98%, poses difficulty in the extraction of lipids as the water around algal cells generates a hydrated shell. A hydrated shell acts as an obstacle for both energy as well as mass transfer (Martinez-Guerra et al. 2018). Various sources of lipids with their water content and respective percentage biodiesel yield are presented in Table 1. Table 1 depicts that the ester yield for Chlorella Sp. is least (i.e., 60 wt%) which has a high water content of about 98%.
Conventionally, oil is extracted from the algae in the first step and then the oil is transesterified into the biodiesel in the subsequent steps. Recently, researchers are working on the direct, i.e., in situ, transesterification of the algal biomass, where biodiesel synthesis occurs simultaneously with the extraction of oil (Velasquez-Orta et al. 2013; Zhang et al. 2014; Sara et al. 2016; Martinez-Guerra et al. 2018; Al-Ameri and Al-Zuhair 2019). As in situ biodiesel synthesis combines three processes, i.e., extraction of oil, esterification of free fatty acids, and transesterification of triglycerides in a single stage, it affects the process economics as discussed in Sect. 8. In situ processing simplifies the production process and can give improved biodiesel yield with fewer processing steps. It offers a minimal loss of oil as a result of simultaneous oil extraction and reaction.
Park et al. (2015a) reviewed the advances in direct transesterification of algal oils from wet biomass and suggested the need for purification of microalgal oils and upgrading of biodiesel properties. Faried et al. (2017) reviewed the processes, technologies, and recent advancements for the biodiesel production from microalgae. Recently, Mofijur et al. (2019) reviewed recent developments in microalgal biodiesel in terms of the oil extraction techniques, challenges in oil extraction, production of biodiesel from microalgal oil and fuel properties. Kim et al. (2019) recently reviewed current research on in situ transesterification targeting biodiesel production from wet microalgae. Later, authors also suggested the future prospects of in situ transesterification based on the techno-economic analysis of existing studies. Sati et al. (2019) reviewed microalgal lipid extraction strategies for biodiesel production and presented the comparative analysis of different extraction methods. Peng et al. (2020) reviewed biofuel production from microalgae, including cultivation, harvesting, drying, extraction, and conversion of microalgal lipids. Authors stated that the cost-effectiveness can be obtained by enhancement in (i) upstream method, in which more productive strains are obtained by proper strain selection, genetic engineering and metabolic engineering, and (ii) downstream method, in which high biofuel yields are obtained by improving the lipid content and by novel conversion of microalgae to biofuels. Gude and Martinez-Guerra (2018) discussed the concept of green chemistry and intensification strategies for biodiesel synthesis followed by specific examples on green metrics of microwave- and ultrasound-intensified biodiesel production. Authors also discussed the effect of catalysts and solvents including discussion about transesterification reaction kinetics. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production targeting environmental pollution control is presented by Yin et al. (2020). All the above articles systematically discussed the production of microalgal biodiesel through the direct transformation of biomass. Some articles also reviewed the cultivation conditions for biomass growth and lipid enhancement as well as the harvesting and lipid extraction technologies. However, above-mentioned articles do not cover the in situ biodiesel synthesis from microalgae using ultrasonic intensification addressing the technology as well as economic feasibility, which is one of the major contributions of this article. Recently, Kumar et al. (2020) discussed the potential of algae as a feedstock for the production of biofuels and value-added chemicals with major emphasis on the opportunities and involved challenges.
Although biodiesel production from algal biomass has potential to be used as a renewable fuel, there are many aspects which require further investigations. Ultrasonication intensified in situ synthesis of biodiesel could be one such way to produce good quality renewable fuel with high yield. Keeping the above challenges in mind, we present in this article up-to-date review of the studies reported in the literature on in situ algal biodiesel production from the year 2000 till 2019 to help readers update themselves with the current status. This article discusses (i) the cultivation of microalgae; (ii) suitability of microalgae for biodiesel synthesis; (iii) ultrasonication aided in situ biodiesel synthesis; (iv) the application of various catalysts in biodiesel synthesis; (v) the quality of biodiesel produced from algal biomass; (vi) the novel ultrasonic reactor for biodiesel synthesis; and (vii) presents the contextual discussion on the feasibility and future scopes. This article also discusses the challenges and futuristic vision on ultrasound-intensified biodiesel production using algal biomass. From this article, the researchers will get up-to-date information on the recent advancements in the field which will direct the researchers to search for the solutions to the present obstacles. The present article presents recent ultrasonication techniques and challenges in biodiesel synthesis for the researchers to investigate, leading to the commercial algal biodiesel production.
Cultivation of microalgae
Microalgae have been studied extensively and considered as one of the most promising species for biodiesel production due to their higher photosynthesis efficiency, growth rate, lipid accumulation, and CO2 sequestration (Mondal et al. 2017; Al-Ameri and Al-Zuhair 2019). However, cultivating microalgae on a large scale is a tedious task (Salama et al. 2017). Mainly, there are four different modes of microalgae cultivation: autotrophic, heterotrophic, mixotrophic, and photo-heterotrophic, as shown in Table 2 (Patel et al. 2018; Pandey et al. 2019). Artificial or natural light source plus the nutrient availability, such as, macro- and micronutrients, play an important role in the cultivation of microalgae at pilot scale. However, in addition to the nutrient requirement, the environmental conditions such as temperature, pH, light intensity, and photoperiod also play a vital role (Khan et al. 2017).
In the autotrophic mode of cultivation, the microalgae use inorganic carbon in the form of CO2 and sunlight energy to produce organic matter (Jerney and Spilling 2018). Till now, only phototrophic method is found to be technically and economically feasible to culture microalgae on a large scale, especially at outdoor environment having abundant sunlight (Duan and Shi 2014). A heterotrophic mode is independent of light and utilizes exogenous organic substrate (like glucose, acetate and glycerol) as energy as well as carbon source. In a mixotrophic mode of cultivation, microalgae assimilate both CO2 and exogenous organic carbons for energy, and both respiratory and photosynthetic metabolism operate concurrently (Perez-Garcia and Bashan 2015; Huang et al. 2015). In contrast, exogenous organic material is the sole source of carbon for the microalgae for photoheterotrophic cultivation, but a light supply is still needed to serve as the energy source (Patel et al. 2018). The type of organic carbon source is generally the most significant factor influencing the production of microalgae in heterotrophic as well as a photoheterotrophic mode of cultivation (Perez-Garcia and Bashan 2015). A study by Hsia and Yang (2015) reported that ultrasound treatment can improve the growth rate of algae. Authors first found the natural ultrasound frequency of freshwater Chlorella in order to set the transducer frequency so that the freshwater Chlorella would resonate optimally for biological effect. Authors reported an increase of 8.23% in the growth rate of algae.
The two approaches that can fit to pilot scale cultivation of microalgae are open (raceway pond) and closed (photobioreactors) cultivation system (Jerney and Spilling 2018). Table 3 depicts the merits and demerits of open and closed algal cultivation system. Open and closed algal cultivation system differ by (i) operation—gas exchange and cooling, (ii) outside involvement—the introduction of unwanted materials and organisms, and (iii) the capital input—operation and the setup. Open systems such as natural ponds are one of the most used systems for several decades. However, maintaining the culture conditions such as pH, temperature, and gas exchange is difficult in open ponds (Slade and Bauen 2013; Khan et al. 2017). Most importantly, there is a high risk of contamination in open systems. Consequently, only highly resistant microalgae can be cultivated for a longer batch length (Lammers et al. 2017). Closed systems, such as tubular, flat plate, column (airlift and bubble), and hybrid photobioreactors, provide the solution to the problems faced in open cultivation system (Singh and Sharma 2012; Duan and Shi 2014; Huang et al. 2015). Closed systems also reduce the risk of contamination by different species (Vo et al. 2019). Besides the advantages, the closed system has some limitations. The culture sticks to the reactor wall in closed system, which in turn leads to the increase in oxygen accumulation and leads to the negative impact on microalgal growth (Zhang et al. 2014). The heat accumulation is one of the major problems associated with photobioreactors. However, above-mentioned problems can be tackled by applying various engineering principles (Perez-Garcia and Bashan 2015; Pruvost et al. 2016; Jerney and Spilling 2018).
Biodiesel synthesis from microalgae
Third generation biodiesel is referred to the biodiesel obtained from the sources such as algae (Dragone et al. 2010, 2011). Different algae have a different amount of lipid content. Lipid content of various species is presented in Fig. 1a (Demirbas and Demirbas 2011; Sun et al. 2018). The lipid content in the algae is between 2 and 40%, approximately (Fig. 1a). Therefore, selection of an appropriate algal species is an important step in biodiesel production. Typical feedstock with their gallons per year capacity for biodiesel production is given in Fig. 1b (Brown et al. 1994; Chisti 2007; Khan et al. 2009; Patle et al. 2020). Clearly, yield per acre per year in case of microalgae is more than 100 times of that of first-generation sources. The properties of algae can be altered so that the energy required for harvesting the algae is minimum. At concentrations more than 60 kg/m3, the green algae species start behaving as shear thinning fluids (non-Newtonian fluids), whereas diatomic species behave as Newtonian fluids for all concentrations up to 80 kg/m3. Above behaviors at various concentrations depict the properties of algae in the reactor (Wileman et al. 2012).
a Lipid content of various species. The lipid content of microalgae is generally 20–50% of the on dry basis and can be as high as 80% under controlled conditions; b Typical feedstock with their gallons per year biodiesel capacity. Microalgae have an oil yield of about 20,000 to 80,000 L per acre per year
As mentioned in Sect. 2, the algae can be grown via a photobioreactor or in raceway ponds. The culture medium is prepared by adding nutrients and followed by filter sterilization (Weissman et al. 1988; Salim 2013). In comparison with raceway ponds where filtration is not required, a photobioreactor is more efficient (Lardon et al. 2009). The flowsheet explaining the production of algal biomass in photobioreactors and raceway ponds is given in Fig. 2. The end product is the wet biomass that has around 15% dry biomass. Figure 3 presents a detailed insight into the raceway pond, where microalgae are further dried and concentrated. Biodiesel is produced from the microalgae through a conventional two-step method or an in situ method as discussed below.
Algae cultivation in photobioreactors and raceway ponds (Slade and Bauen 2013). Both photobioreactor and raceway pond lead to carbon fixation
Cultivation and harvesting of microalgae (Ramos-Tercero et al. 2014). Typical steps include a reactor system, a sedimentation unit and a centrifugation unit
Conventional two-step method
After the algae are cultivated, the next important step is to extract the oil and use it for biodiesel production (Khan et al. 2017). Figure 4 presents a two-step process of biodiesel production where oil extraction is followed by the transesterification.
Typically, there are two methods for lipid extraction, both employing solvent extraction. One is a short protocol using Soxhlet extraction apparatus, and the other one is long protocol using modified Bligh and Dyer (Bligh and Dyer 1959). Two-step process invariably requires a greater number of processing steps as oil extraction, and the transesterification takes place in separate equipment’s. For example, Trichosporon oleaginosus was transesterified through the conventional two-step method that yielded a fatty acid methyl ester (FAME), i.e., biodiesel yield of 95% using 1 wt% H2SO4 catalyst in 24 h (Zhang et al. 2016). Although a higher yield is obtained, the reaction time of 24 h indicates the need of a very large reactor if is to be scaled up to industrial scale. The large reactor, in turn, will require a higher investment. Therefore, intensification of the process to reduce a reaction time is a lucrative alternative, which is discussed in Sect. 4. Various sources of lipids and lipid conversion to the biodiesel are presented in Table 4. Table 4 clearly depicts that high biodiesel conversion can be obtained from microalgae at optimum conditions. Up to 99% biodiesel yield is obtained from soybean oil using sulfuric acid catalyst. However, the yield varies from 48 to 99% depending on the source of lipids, reaction conditions, and catalyst used. Conversion of wet microalgae to biodiesel showed the least yield, i.e., 48%, at optimal conditions. Lesser yield implies the need to recycle a large amount of unreacted feed.
In situ method
The development of an efficient method to convert lipids to biodiesel has been given more focus recently. Combining the extraction of lipids and lipids conversion to biodiesel into a single step, called as in situ transesterification, can minimize the requirement of solvents (Sara et al. 2016). However, conventional techniques (i.e., without the aid-in of techniques such as ultrasound) require large reaction time, resulting in a larger reactor as mentioned above (Zhang et al. 2014, 2016).
Ultrasonication-aided in situ method
As in situ processing denotes simultaneous lipid extraction and the transesterification, the process can be feasible when lipid extraction from the biomass is maximized and the rate of transesterification is enhanced. Such enhancements are possible with the help of suitable process intensification, such as ultrasonication aided process intensification. The ultrasound intensification is attributed to the traveling of acoustic, i.e., sound waves through the solvent, resulting in the phenomenon called cavitation. The constant formation of the cavitation bubbles generates micro-turbulence, high-velocity inter-particle collisions and perturbation in micro-porous particles of the biomass which accelerates the internal diffusion and eddy diffusion (Toma et al. 2001; Valachovic et al. 2001; Vilkhu et al. 2008; Paniwnyk et al. 2009). Since the vessel volume is constant, the bubbles collapse irregularly instead of expanding. Such collapses result in the significant liquid circulation currents coupled with severe turbulence. Also, if cavitation occurs on the surface then surface peeling and particle breakdown occurs (Li et al. 2004). Figure 5 presents the ultrasound-assisted mechanism for biodiesel production from microalgae. Ultrasound waves generated at suitable conditions break the cell walls. Subsequently, the solvent extracts the lipids from the microalgal cells. Lipids are then converted to biodiesel and glycerol in the presence of alcohol and suitable catalyst.
Park et al. (2015b) studied the sonication-assisted homogenization system for increased lipid extraction from Chlorella vulgaris microalgae. Authors concluded that the sonication-assisted homogenization system breaks up microalgal cell walls effectively. Patil et al. (2011) reported the dominant mechanical effects of ultrasonication using scanning electron microscopy of the samples of soybean flakes and almond powder. Authors reported that the microfractures appeared in the soybean flakes after application of ultrasonication. Many other researchers have successfully applied ultrasonication to extract lipid from microbes for biodiesel synthesis and reported similar findings (Zhang et al. 2014, 2016; Martinez-Guerra et al. 2018). In addition to the enhancement of lipid extraction, ultrasonication improves the rate of transesterification due to the cavitation phenomenon. However, the success of the ultrasonication depends on various operating parameters that have a great influence on the product yield (Sancheti and Gogate 2017):
Power The enhanced generation of active cavitation bubbles occurs as the ultrasonication power increases. At higher power dissipation levels, cushioning effects may be observed. As reported by Safari and Javadian, an increase in ultrasonication power till an optimum of 160 W gave higher yield and shorter reaction time, whereas an increase beyond 160 W yielded marginal reduction in product yield (Safari and Javadian 2015). For the microalgae, Spirulina sp., the effect of ultrasound power on in situ transesterification was investigated by Martinez-Guerra et al. (2014). The study was conducted at 80 W, 108 W, 144 W, and 180 W. The results conclusively showed that the maximum ester content was obtained at 180 W (Martinez-Guerra et al. 2014).
Frequency According to the bubble dynamics studies, the bubble size reduces as the frequency increases (Thompson and Doraiswamy 1999; Gogate 2008; Son et al. 2009; Li et al. 2014). In general, low frequencies are better as lower frequencies generate dominant physical effects (Sawarkar 2019). It has also been shown that ultrasound with low frequency (in the order of kHz) and a high amplitude induces cell rupture and ultrasound with high frequency (in the order of MHz) and low amplitude makes the cells to aggregate (Kim et al. 2013).
Duty Cycle, i.e., Cycle Time Duty cycle is the factor which decides the exposure time of irradiation in one cycle. The duty cycle applied to any chemical reaction can be altered using ON–OFF time. The pulse mode of operation is recommended as it increases the lifespan of transducers and also decreases the local temperature rise (Avhad et al. 2014; Raskar et al. 2014).
Temperature The temperature has a critical effect on the biodiesel yield. Although the increase in temperature until some limit will lead to improved chemical kinetics, the cavitational intensity may suffer (Ammar et al. 2015). Therefore, determination of the optimum temperature for the cavitation-induced biodiesel production is important.
Due to the generation of sound waves, which propagate through the fluid leading to the alternate cycles of high and low pressure, the yield of biodiesel is enhanced. In the low-pressure cycle, the small bubbles are formed which violently collapse in the high-pressure cycle resulting in a phenomenon called cavitation. During cavitation, shear force is created due to the high pressure and liquid velocities which mechanically breaks the cellular structures of the microalgae and enhances the extraction of lipids. Lipid yield improves between 50 to 500%, and the extraction time is reduced by tenfold (Mubarak et al. 2015). Keris-Sen et al. (2014), on the other hand, tested different ultrasound intensities (0.1–0.5 W/mL) at 30 kHz frequency for 5 to 60 min cycles. Authors studied the effect of ultrasonication on the lipid extraction efficiency using hexane or a chloroform–methanol mixture as co-solvents. Martínez et al. (2017) showed that ultrasound is an effective method to enhance lipid extraction from biomass as it results in sufficient cellular disruption. Various studies on ultrasonication assisted cell disruption of algal biomass for biodiesel production are presented in Table 5. Biomass concentration (g/L), volume (mL), frequency (kHz), power (W), and treatment time (min) are summarized in Table 5. Each of the studies concluded that the reaction time, amount of alcohol (used as a reactant), and the amount of catalyst can be reduced as a result of physio-chemical effects of ultrasonication. Table 5 shows that the process completed within a few minutes (i.e., 2–30 min), which is significantly lesser than the conventional counterpart where several hours are required.
The add-in of ultrasonication also increases the chemical reaction rate by virtue of enhancement of chemical, physical, or both effects. Additional benefits of ultrasound include the low requirement of alcohol and catalyst. In comparison with the conventional mechanical stirring method, ultrasound-assisted method is more efficient as it enhances lipid extraction as well as transesterification. The cavitation, which depends on the optimal irradiation frequency, is one of the main factors for obtaining a higher yield of biodiesel under the influence of ultrasonic irradiation by improving the mass transfer (Patle et al. 2018). For instance, the production of fatty acid methyl esters, i.e., biodiesel from the transesterification of Brassica campestris with methanol using ultrasonic irradiation was found to be greater than 99% within 50 min, and ultrasound-assisted biodiesel synthesis in an in situ conversion of Trichosporon oleaginosus was found to be 95% in just 50 min instead of several hours by a conventional method (Thanh et al. 2010; Zhang et al. 2016).
Several researchers have successfully applied ultrasonication to extract lipid from microbes (Zhang et al. 2014, 2016; Martinez-Guerra et al. 2018). Although the extraction time reduces, non-degradable materials could also be broken down during ultrasonication-aided lipid extraction and some compounds may either react or mix with lipid, which may affect the lipid quality (Cho et al. 2012; Gadhe et al. 2014). Consequently, the biodiesel in ultrasonication aided in situ transesterification may also be affected. Similar findings were reported by Zhang et al. (2014). Other researchers (Gogate 2004, 2008; Patil et al. 2011, 2012) also discussed the application of ultrasonication on the product yield.
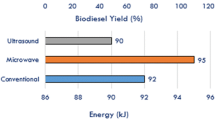
Ultrasonication also improves the rate of transesterification reaction under suitable reaction conditions (Martinez-Guerra et al. 2018). The mass transfer in an ultrasound aided process is about 10 times faster than the mass transfer in a conventional mode of stirring (Gole and Gogate 2012). Gole and Gogate (2012) reported an intensification of biodiesel synthesis from non-edible oil using the sequential effect of microwave and ultrasonication. Authors showed that the reaction time, alcohol requirement, and the reaction temperature can be reduced using ultrasonication technique as compared to the conventional process. Authors also reported that a low methanol to oil molar ratio is generally needed for ultrasound assisted transesterification. Guldhe et al. (2014) compared the microwave and ultrasonication technique and reported that sonication yielded a higher conversion of Scenedesmus sp. to biodiesel (i.e., ∼71% using ultrasonication as compared to ∼52% using the microwave). Apart from high yield, a low reaction temperature (about 50 °C) was warranted for the transesterification. Zhang et al. (2016) reported an ultrasonication-assisted in situ transesterification using an ultrasonic processor CPX 750 (Cole-Parmer Instrument, IL) at 20 kHz. Methanol was added to 0.2 g of dry biomass, and then, the ultrasound horn was directly immersed in the solution that was maintained at 25 °C (Zhang et al. 2016). A cycle time of 5 min was used with a pause of 2 min. Various concentrations of methanol to biomass ratio were taken, and the biodiesel yield was observed. Authors concluded that enhanced biodiesel yield, i.e., 95% in lesser time, i.e., 60 min was achieved in the ultrasonication assisted mechanism.
Ehimen et al. (2012) studied biodiesel synthesis from Scenedesmus sp. using Tungstated zirconia catalyst. Authors reported 71% biodiesel yield in 20 min with 4 wt% catalyst loading. Martinez-Guerra et al. (2014) studied the in situ transesterification of lipids from Chlorella sp. under the influence of ultrasonication using ethanol as a co-solvent for lipid extraction and as a reactant in the transesterification reaction. Authors reported the optimal conditions as microalgae-to-ethanol ratio (w/v) of 1:6 to 1:9, NaOH catalyst amount of 2 wt%, ultrasonication power of 490 W, and reaction time of 6 min. Zhang et al. (2014) reported that high biodiesel yield, i.e., up to 92.1 wt% was obtained in 20 min reaction time using dry (i.e., lyophilized) oleaginous yeast biomass using ultrasonication assisted in situ transesterification. The biodiesel yield of 18.5% with 95% conversion was obtained.
Sara et al. (2016) investigated the transesterification of Trichosporon oleaginous using NaOH catalyst. Authors obtained about 95% conversion in just 20 min with 1 wt% catalyst at moderate reaction temperature of 25 °C. The elimination of the use of toxic solvents for lipid extraction and lyophilization or drying the wet biomass was accomplished by Yellapu et al. (2017) with the help of sonication. Authors showed that the conversion of wet biomass with 83.8% moisture to biodiesel using N-lauroyl sarcosine treatment followed by ultrasonication assisted in situ transesterification could be a potential approach. Martinez-Guerra et al (2018) reported 48.2% biodiesel yield from Nannochloropsis sp using NaOH catalyst in 7 min. Wahidin et al. (2018) reported ionic liquid-catalyzed and microwave-assisted single-step biodiesel production from wet microalgae, i.e., Nannochloropsis sp. Authors obtained the maximum biodiesel yield of 40.9% using wet algae-to-methanol (wt/vol) ratio of 1:4 and methanol-to-catalyst ratio of 1:0.5 in 25 min.
Reaction kinetics for ultrasound assisted esterification and/or transesterification for biodiesel production from various feedstocks, such as vegetable oils or waste cooking oil, has been widely reported in the literature (for example, Sarve et al. 2016), while the same for the microalgal biodiesel is not as common. Recently, Martinez-Guerra et al. (2018) reported the kinetics for ultrasound-microwave assisted biodiesel production from Nannochloropsis sp microalgae. The study suggested a first-order reaction. Pre-exponential factor and activation energy were found to be 1.18 s−1 and 17,298 J/mol, respectively. These values, i.e., larger pre-exponential factor and smaller activation energy, indicate the fast reaction at lower temperature which is a direct consequence of the ultrasonication-microwave intensification. Ghosh et al. (2017) reported pre-exponential factor and an activation energy for the conversion of Chlorella MJ11/11 without ultrasonication as 0.054 s−1 and 22,828 J/mol, respectively. A pre-exponential factor indicates the molecular mobility that depends on the frequency of vibrations of the molecules at the reaction interface, while the activation energy is the energy required to initiate the reaction (Martinez-Guerra et al. 2014). A detailed information on the kinetic analyses of ultrasonic intensification processes along with ultrasound assisted reactor design is presented by Naveena et al. (2015).
Table 6 summarizes some of the recent studies on biodiesel synthesis considering various microalgal and sludge-derived lipids with the respective biodiesel yield at given reaction conditions under the influence of ultrasonication. Conversion of Nannochloropsis sp. (entries 7 and 8 in the table) to biodiesel showed lesser yield, which is due to the fact that the source of lipid is wet microalgae. Water acts as an obstacle in effective lipid extraction as well as in the transesterification (Martinez-Guerra et al. 2018). For ultrasound-assisted processes, an optimum temperature needs to be determined as (1) lower temperature results in reduced cavitation and (2) higher temperature may reduce the ultrasonication efficiency due to the excessive evaporation of solvent and solvent entrapment in the bubbles. Choice of solvent and bulk working temperature are important factors that must be considered while selecting the reaction conditions in ultrasound-assisted reactions. Increase in solvent vapor pressure would decrease the temperature and pressure of maximum bubble collapse. Consequently, the reactions having cavitational collapse as a primary cause of sonochemical activation would require a low bulk temperature. On the contrary, high boiling solvent would be suitable for reactions requiring higher temperatures (Mason and Lorimer 2002). Studies reported in Table 6 did not discuss the effect of pH on ultrasound-assisted synthesis of biodiesel from algal biomass. However, Ren et al. (2013) reported that pH has a considerable effect on algal growth and lipid accumulation. Authors found that the algal growth and lipid accumulation were slightly affected by the pH of the medium between 6.0 and 11.0. On the other hand, the algal cells showed poor growth and lipid productivity at two extremes of pH, i.e., < 3.0 and > 12.0. Although pH of the medium is unlikely to affect the ultrasonication efficiency, it may affect the transesterification depending on whether the transesterification reaction is acid- or alkali-catalyzed.
Table 6 clearly shows that the reaction time for ultrasonication intensified in situ process varies from a few minutes to about an hour, which is manifold (10–15 times) lesser than the time requirement in the conventional counterparts. Another important observation from Table 6 is that the biodiesel is obtained in shorter time, i.e., a few minutes, in processes with basis catalyst such as NaOH and KOH. Although there has been increasing interest toward the use of ultrasonication aided in situ transesterification in recent years and the fact that intensified in situ process may be a way for efficient and cost-effective biodiesel synthesis, significant research efforts are required to cope with the challenges. The challenges include: handling of high volume of water when processing the wet microalgae, controlled sonication at larger volumes, process scale-up, and removal of impurities such as carbohydrates, proteins, caratenoids, and chlorophyll. Various catalysts used for biodiesel synthesis are discussed in the following section.
Catalysts for biodiesel synthesis
Transesterification reaction can be catalyzed by an acid or an alkaline catalyst, and the catalysts can be homogeneous or heterogeneous (Tiwari et al. 2007; Aransiola et al. 2010; Jain and Sharma 2010; Juan et al. 2011). Enzymes have also been used for transesterification reactions (Wang et al. 2011; He et al. 2020). Above-mentioned catalysts can be used irrespective of the absence or presence of ultrasonication. Depending on the nature of the catalyst, the transesterification mechanism also varies.
Homogenous catalysts Homogenous catalysts are usually applied in liquid form. Catalysts may be acidic or basic in nature. The separation of the homogeneous catalysts is tough and requires additional equipments for the neutralization and the subsequent separation of homogeneous catalysts.
Acid catalysts are generally used for feed that has a high content of free fatty acids and water as acid catalysts do not lead to the saponification, unlike base catalysts (Aransiola et al. 2010; Srivastava and Prasad 2000). However, homogenous acid-catalyzed reaction is about 400 times slower than the base-catalyzed reaction. Some of the popular acid catalysts for biodiesel synthesis include sulfuric acid, hydrochloric acid, sulfonic acid, and phosphoric acid. Ehimen et al. (2012) reported the reaction time of 2 h for ultrasound-assisted in situ transesterification of Chlorella sp. using sulfuric acid catalyst; Authors obtained a biodiesel yield of 99.9%. Suganya et al. (2014) reported that ultrasound intensified transesterification of Enteromorpha compressa using sulfuric acid yielded around 99% biodiesel in 90 min. There are many notable studies on biodiesel synthesis using many acid catalyst (Fukuda et al. 2001; Helwani et al. 2009; Ehimen et al. 2012; Suganya et al. 2014). In addition to slow kinetics for acid-catalyzed reaction, the reaction is required to be carried out at high alcohol-to-oil ratio with high acid catalyst concentration (Fukuda et al. 2001; Demirbas 2009; Helwani et al. 2009). In acid-catalyzed transesterification, direct protonation of triglycerides by acid catalyst takes place that initiates the reaction.
Base catalysts are more popular both at the laboratory as well as the industrial level (Noureddini et al. 2005; Frascari et al. 2008). Various examples include alkaline metal hydroxides, alkoxides, sodium or potassium carbonates (Ma and Hanna 1999; Çetinkaya and Karaosmanoǧlu 2004). As presented above, the reaction times are significantly lesser using such catalysts as compared to the acid catalysts (Fukuda et al. 2001). However, base, i.e., alkaline catalysts are very sensitive to the free fatty acids and water content. As reported, Trichosporon oleaginosus yielded 95.1 ± 0.2% methyl ester from 3 wt% sodium hydroxide-catalyzed transesterification at 25 °C in just 20 min under the influence of ultrasonication (Sara et al. 2016). The base-catalyzed mechanism is different than the mechanism using any acid catalyst where alkoxide anions are generated instead of the direct protonation of glycerides by the catalyst (Moholkar et al. 2015).
Heterogeneous catalyst The major advantage of using a heterogeneous catalyst is the easy catalyst separation, thereby reducing the cost of catalyst recovery (Tran et al. 2017). Nafion-NR50, sulfated zirconia, and tungstate zirconia are some of the common examples of heterogeneous catalysts (Helwani et al. 2009). Moreover, a basic heterogeneous catalyst such as calcium oxide, calcium carbonate, calcium hydroxide, and magnesium oxide also reduce the environmental impact as well as the process cost (Zhang et al. 2010). Despite the obvious advantages of the heterogeneous catalysts, catalyst recyclability remains to be a challenge as catalysts should be able to perform well even after repeated for economical biodiesel production.
Despite the fact that the homogeneous acid and base catalysts are efficient and have been used extensively, demerits of the acid and base catalysts are (i) non-renewability, (ii) corrosiveness, (iii) environmentally harmfulness, (iv) vulnerability toward saponification, and (v) difficulty in separation (Patle et al. 2018). Given that the catalysts mentioned above are not environmentally benign, designing the new environment-friendly catalysts for the biodiesel production is crucial. In recent years, researchers have started to investigate the use of ionic liquid catalyst for transesterification reaction. Ionic liquid is known as green solvents and is an organic salt that entirely consists of organic cations and organic or inorganic anions. Major highlights of ionic liquids are (i) negligible vapor pressure, (ii) applicability for a complete range of (in)organic materials, (iii) immiscibility with organic solvents, (iv) good thermal and chemical stability, and (v) non-flammability (Khiratkar et al. 2018). Therefore, an application of ionic liquid catalysts in biodiesel production may provide an answer to the non-benignness of the existing conventional catalysts. Khiratkar et al. (2018) reported some of the applications of ionic liquid catalysts in biodiesel synthesis from non-edible oil.
Recently, Wahidin et al. (2018) reported the successful application of ionic liquid catalyst (1-ethyl-3-methylimmidazolium methyl sulfate [EMIM][MeSO4]) in single-step biodiesel production from wet microalgae, i.e., Nannochloropsis sp. Transesterification reaction mechanism for conventional catalyst such as NaOH or H2SO4 is widely studied and is available in the literature. Some of the studies are mentioned above. However, reaction mechanism for ionic liquid catalyzed transesterification of microalgal lipids is scarce to find. Based on our previous studies (Khiratkar et al. 2018; Patle et al. 2018), a plausible mechanism of the transesterification of microalgal lipids with methanol using benzimidazolium-based Brønsted acid ionic liquid catalyst (BBAIL) catalyst is shown in Fig. 6. Preparation of BBAIL catalyst is discussed in detail by Khiratkar et al. (2018). At first, activation of microalgal lipid (i.e., triglycerides) takes place by the protonation of carbonyl group by BBAIL. The activation is followed by nucleophilic attack of an alcohol (usually methanol or ethanol) on electrophilic carbon, and then, the attack of a lone pair of oxygen to abstract the proton leading to the electrophilic oxygen. Finally, biodiesel is synthesized along with glycerol as a side product. BBAIL is then regenerated with the elimination of H+, as depicted in Fig. 6. If the catalyst is basic in nature, the reaction mechanism is different as the alkoxide anions are generated while using base catalyst (Patle et al. 2018). It should be noted that a microalgae have several glycerides, generally ranging from C12 to C25. Overall simplified biodiesel synthesis from one such triglyceride, i.e., (triolein) and methanol using ionic liquid catalyst is presented in Fig. 7.
Plausible mechanism of the transesterification of microalgal lipids with alcohol using BBAIL catalyst [Here, R denotes the alkyl chain of the triglycerides and R' denotes the alkyl group of alcohol; BBAIL: benzimidazolium-based Brønsted acid ionic liquid catalyst]. Direct protonation of the glycerides takes place in this mechanism
Poly-ionic liquids catalyst have also been used for biodiesel synthesis (Bian et al. 2020). Poly-ionic liquids offer large surface area and rich mesopores in addition to specific characteristics of general ionic liquids such as high catalytic activity and good thermal stability. Next section discusses the quality of algal biodiesel as biodiesel quality is of primary focus for the practical applications.
Properties of biodiesel obtained from algal biomass
Irrespective of the source of glycerides, biodiesel obtained from any algal biomass needs to follow the quality standards, namely European standards (EN), American Society for Testing and Materials (ASTM) or Bureau of Indian Standards (BIS). Various properties, such as viscosity, density, solidifying point, cold filter plugging point, heating value, H/C ratio, flash point, sulfur content, cetane number, sulfated ash content, water and sediment, acid value, free glycerol, total glycerine, phosphorous content and carbon residue, need to be determined for the obtained algal biodiesel and compared against the standards. Table 7 presents the quality of the biodiesel obtained from the algal biomass, i.e., Chlorella MJ 11/11 and Nannochloropsis sp. Table 7 depicts that the algal biodiesel follows many of the prescribed ASTM, EN14214 and IS 15,607 standards.
Table 7 suggests that the properties such as density, calorific value, acid value, and flash point improve when biodiesel is blended with the petro-diesel. Note that different fatty acid compositions present in the microalgae can affect the quality of algal biodiesel. For example, the content for linolenic acid (C18:3) should not be greater than 12%, which is the maximum limit allowed in the EN regulation (EN 14,214). Also, the sum of the percentages of polyunsaturated fatty acids (with 4 or more double bonds) should not be greater than 1% as per EN 14,214.
New ultrasonic reactor designs
Various reactor designs have been proposed and studied for an ultrasonic reactor. The ultimate objective is to achieve uniform cavitation activity with. Ultrasound-assisted reactor has a transducer that converts alternating current into ultrasonic vibrations using piezoelectric materials. The transducer is actuated by an amplifier driven with a sine wave from a signal generator. Depending on the transducer-type, ultrasound-assisted reactors can be classified as piezoelectric plate-based reactors or Langevin-based transducer-based reactors. Some of the attractive designs are discussed in this section. Note that the designs discussed below have not been necessarily applied for biodiesel synthesis, but the discussed designs can be potential options. Mason (2000) used a flexible sheet of embedded piezoelectric pillars in the reactor design. Suri et al. (2002) did some design modifications by altering the locations of the two transducers with irradiating frequency as 1 MHz and 750 kHz. Cravotto et al. (2003) suggested a modified horn type reactor which irradiated a frequency of 20 kHz with a power rating of 1000 W.
Bhirud et al. (2004) tested the energy efficiency of an ultrasonic reactor equipped with longitudinally vibrating horn operating at a frequency of 36 kHz. The energy efficiency of such a reactor is reported to be higher than traditional reactors. A triple frequency hexagonal flow cell type of reactor is reported by Gogate (2008). Some of the designs of transducer horns are shown in Fig. 8; each design has different cavitation behavior as reported by Berlan and Mason (1992).
A configuration for a large-scale rectangular sonochemical reactor (dimensions: 0.508 m × 0.508 m × 0.672 m and operating capacity: 112 L) was reported by Asakura et al. (2008). The design used 12 transducers with a frequency of 500 kHz and maximum power rating of 620 W. Similarly, Son et al. (2009) reported a reactor of dimensions 1.2 m × 0.6 m × 0.4 m with a working volume of 250 L. A continuous stage ultrasonic reactor is shown in Fig. 9, where three ultrasound generators are placed at different locations in the reactor (Gondrexon et al. 1998). Aljbour et al. (2009) reported an ultrasonication-assisted capillary microreactor setup as shown in Fig. 10. The capillary microreactor is immersed in the ultrasound bath of dimension 150 mm × 135 mm × 65 mm.
Ultrasonication-assisted capillary microreactor setup (reused with permission from Aljbour et al. (2009). This arrangement combines a microreactor and an ultrasonicator, both of which could result in improved reactor performance
Many such laboratory scale reactor designs have been tested till date. However, the industrial prospects of such reactors are currently limited, and their application still demands a lot of research in terms of the scalability to a large scale production. Challenges with respect to the industrial operation are discussed in Sect. 8. It might be interesting to study the application of the above instruments in microalgal biodiesel synthesis. The following section presents a contextual discussion on the feasibility and future scopes.
Challenges in microalgal biodiesel production
The central issue in microalgal biodiesel production is a small amount of lipid content. Hence, the microalgae should be grown in such a way that it has higher lipid content. Growing microalgae in nitrogen starved condition is reported to yield higher oil content (Jorquera et al. 2010). However, it may reduce the overall biomass concentration. Also, the farming of microalgae is tough and complex in comparison with other conventional oil crops. The natural biological characteristics of microalgae, such as size, density, shape, the surface charge of cells, hydrophobicity, medium salinity, adhesion with cohesion features, and settling velocity, affect the efficiency of harvesting (Zhang et al. 2016). Different algae have different compositions, which causes an irregularity in the development of a process. Also, various properties of the biodiesel, such as cetane number, oxidative stability, and degree of saturation, depend on the fatty acid composition of the raw material (Martinez-Guerra et al. 2018). The presence of water in the wet algal biomass also impacts the synthesis of biodiesel negatively (Atadashi et al. 2012).
Another main problem with the biodiesel production from wet microalgae is associated with the downstream processing. The nature and amount of catalyst used in the processing can have a considerable environmental impact. Large amounts of the catalyst may result in soap formation in case of basic catalyst and some portion of the catalyst may remain unutilized in the biodiesel. After the completion of transesterification reaction, biodiesel is separated out and washed with water to remove the remaining catalyst, soap, and glycerine. Washing generates a lot of wastewater that needs to be treated, demanding further investment (Santos et al. 2009). Therefore, application of green catalysts should be explored. Also, the process should be designed in such a way that it operates at moderate operating conditions so as to ensure better process controllability and safety.
Although various ultrasonic reactor designs have been proposed for biodiesel production, industrial implementation of the discussed designs is difficult. The major challenge lies in the scale-up of sonochemical reactors and the optimization of diverse parameters. The challenge involves the integration of material prospects with the cavitation intensity as well as the engineering aspects of the design (Gogate 2004, 2008). In spite of the several merits of ultrasonication such as small footprint and better yield, ultrasound-induced algae harvesting may be challenging on an industrial scale. Zhang et al. (2016) successfully used ultrasound as an assisting method for microalgal harvesting combined with polyaluminum chloride in order to harvest freshwater microalgae at the laboratory scale. The industrial scale processing of ultrasound aided reactors is very difficult than the laboratory scale operation (Patle et al. 2018). At the industrial level, high-capacity industrial ultrasonicators are desired as the requirement of power is more. Therefore, the ultrasonic instruments should operate with no or minimal loss of energy. Also, ultrasonic instruments should be able to operate continuously at an industrial environment for a long time. Design of ultrasound-assisted reactors is crucial for obtaining maximum benefits. Quantitative prediction and analysis of acoustic streaming, power dissipation, mass transfer, and cavitational activity in the reactor can assist in designing a scaled-up sonication-assisted reactor. An efficient scale-up of the ultrasound assisted reactor can be achieved if the energy dissipation mechanism in the reactor is understood (Naveena et al. 2015).
Generally, biodiesel is synthesized from microalgae in two ways: i) using dry microalgae and ii) using wet microalgae. A high amount of energy required for drying of the microalgae makes the first approach unattractive. Using wet microalgae directly (thereby avoiding the need for drying) also has some disadvantages. A major disadvantage is a need for a larger volume of process equipments arising from the need to process a large number of wet microalgae due to high water content (up to 98%), resulting in higher capital and operating cost. For example, Martinez-Guerra et al. (2018) reported that the microalgae paste contained 18.4% of dry biomass having 52% protein, 0.89% chlorophyll, and 16% carbohydrates with about 27% lipids. Water content in the microalgae paste was 81.6%. In this case, 100 g of a perfectly dried microalgae will have approximately 27 g lipids, whereas a wet microalgae having 81.6% water will have just about 5 g of lipids. Overall, the main encumbrance to the cost-effective production of algal biodiesel is the higher production costs of lipids in addition to the other costs such as processing and capital investments, which lead to a negative energy balance (Kumar et al. 2020). Our recent study (Patle et al. 2020) on in situ biodiesel production using ultrasonication and microwave intensification suggested that the total module cost of a plant processing 20 kt per annum of wet microalgae is $ 11.3 million excluding the cost of ultrasonication and microwave. In contrast, the total module cost is $ 2.88 million for the plant of same capacity processing waste cooking oil without ultrasonication and microwave (Sharma and Rangaiah 2013). In other words, the plant processing wet microalgae is about four times expensive than the process using a waste cooking oil despite the former's benefits such as smaller number of processing steps and lesser loss of oil.
Similarly, West et al. (2008) reported a total module cost of $ 1.1 million using chemical engineering plant cost index (CEPCI) of 394 for a biodiesel plant having a capacity of 8 kt/yr processing waste cooking oil. Our other study reported a total module cost of $ 12.95 million using CPECI of 600 for a biodiesel plant of capacity 120 kt/yr processing waste cooking oil (Patle et al. 2018). Projected total module cost for a plant capacity of 20 kt/yr, using the six-tenths rule and CEPCI of 602 for year the 2018, is $ 2.9 million (based on West et al. 2008) and $ 4.43 million (based on Patle et al. 2014). Total module cost of ultrasound intensified in situ biodiesel production from wet microalgae obtained by Patle et al. (2020) is about 4 and 2.5 times the total module cost of the process reported by West et al. (2008) and Patle et al. (2014), respectively. Cost of manufacturing of the process reported by Patle et al. (2020) in processing 20 kt per annum of wet microalgae is about $ 65 million, which is more than five times than that of the process of same capacity processing waste cooking oil, i.e., $ 13.86 million (Sharma and Rangaiah 2013). Patle et al. (2014) reported the cost of manufacturing of $ 73.5 million for a process of capacity 120 kt per annum processing waste cooking oil, which translates to about $ 12.25 million for a 20 kt/yr plant. Hence, a cost of manufacturing of the ultrasound-intensified in situ biodiesel production from wet microalgae has to be reduced significantly for it to be economically viable. Cost of algal biodiesel obtained by Patle et al. (2020) is $ 3.13 per kg excluding the cost of ultrasound and microwave, whereas the cost is about $ 1 per kg of biodiesel produced from waste cooking oil. Estimated carbon emission of the process proposed by Patle et al. (2020) is 186 kt/yr, which was calculated based on the carbon dioxide emission index of steam and electrical energy given by Oni et al. (2011).
Multiple units and multiple recycles in a large-scale biodiesel process pose a challenge in effective process control. On the one hand, units such as reactors and distillation columns may show intricate dynamics, whereas presence of recycles may exhibit snowballing effect on the other hand. Therefore, efficient plantwide control structure having many controllers such as several temperature controllers, pressure controllers, level controllers, flow controllers, pH controllers, and composition controllers is inevitable. Control design requires a systematic analysis of control degree of freedom to understand the available manipulated variables for controlling the required controlled variables. Ultrasound is known to produce favorable outcomes in terms of lipids extraction and efficient reaction at optimal parameters such as optimal frequency, optimal power, and optional duty cycle.
Understandably, the application of ultrasonication requires extensive capital as well as operational investment. Biodiesel production at large scale invariably requires effective solutions to above challenges. Concept of microalgal photobiorefinery that integrates the production of biofuels and bioproducts with the use of alternative sources of nutrients, making the process of obtaining energy economically viable, is a potential answer to the cost intensive process. Concerns mentioned above are some of the biggest hurdles in the commercial full-scale production of algal biodiesel. Therefore, although microalgae have potentials to serve as a great source of lipids, a lot of research efforts need to be devoted targeting technological advancements to obtain higher lipid content in microalgae and reduced cost of transesterification, for economically viable biodiesel production.
Conclusion
This article thoroughly discussed conventional and in situ method of biodiesel synthesis, effects of various catalysts on biodiesel production, multiple facets of ultrasonication, and novel ultrasonic reactors for biodiesel production. Algal biomass can serve as potential feedstock considering limitations associated with vegetable oils and animal fats. Algal biodiesel production can assist to cover the growing demand for fuel through the large-scale algal production on non-arable lands to produce a large amount of algal biomass and further transesterifying it via in situ methods to the biodiesel. However, technological advancements, especially suitable process intensifications, to reduce the cost of biodiesel production remain a key for the increased biodiesel usage. Recent advancements, namely use of ultrasonication and in situ processing using an efficient catalyst, are cost intensive despite the attractiveness in terms of biodiesel production in reduced time with lesser processing steps. This article can serve as a resource for researchers where researchers can find a motivation to look beyond the conventional techniques for efficient biodiesel production.
Abbreviations
- ASTM:
-
American society for testing and materials
- BBAIL:
-
Benzimidazolium-based Brønsted acid ionic liquid
- BIS:
-
Bureau of Indian standards
- CPECI:
-
Chemical engineering plant cost index
- EN:
-
European standards
- FAME:
-
Fatty acid methyl ester
References
Adam F, Abert-Vian M, Peltier G, Chemat F (2012) “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: a green, clean and scalable process. Bioresour Technol. 114:457–65. https://doi.org/10.1016/j.biortech.2012.02.096
Al-Ameri M, Al-Zuhair S (2019) Using switchable solvents for enhanced, simultaneous microalgae oil extraction-reaction for biodiesel production. Biochem Eng J 141:217–224. https://doi.org/10.1016/j.bej.2018.10.017
Alcantara A, Amores J, Canoira L, Fidalgo E, Franco MJ, Navarro A (2000) Catalytic production of biodiesel from soy-bean oil, used frying oil and tallow. Biomass Bioenergy 18:515–527. https://doi.org/10.1016/S0961-9534(00)00014-3
Aljbour S, Yamada H, Tagawa T (2009) Ultrasound-assisted phase transfer catalysis in a capillary microreactor. Chem Eng Process 48:1167–1172. https://doi.org/10.1016/j.cep.2009.04.004
Ammar HB, Chtourou M, Frikha MH, Trabelsi M (2015) Green condensation reaction of aromatic aldehydes with active methylene compounds catalyzed by anion-exchange resin under ultrasound irradiation. Ultrason Sonochem 22:559–564. https://doi.org/10.1016/j.ultsonch.2014.07.018
Antolín G, Tinaut FV, Briceño Y, Castanñ V (2002) Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 83(2):111–114. https://doi.org/10.1016/S0960-8524(01)00200-0
Aransiola E, Betiku E, Layokun S, Solomon B (2010) Production of biodiesel by transesterification of refined soybean oil. Int J Biol Chem Sci. https://doi.org/10.4314/ijbcs.v4i2.58132
Asakura Y, Yasuda K, Kato D et al (2008) Development of a large sonochemical reactor at a high frequency. Chem Eng J 139:339–343. https://doi.org/10.1016/j.cej.2007.08.007
Atabani AE, Silitonga AS, Badruddin IA et al (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev 16:2070–2093. https://doi.org/10.1016/j.rser.2012.01.003
Atadashi IM, Aroua MK, Abdul Aziz AR, Sulaiman NMN (2012) The effects of water on biodiesel production and refining technologies: a review. Renew Sustain Energy Rev 16:3456–3470. https://doi.org/10.1016/j.rser.2012.03.004
Avhad DN, Niphadkar SS, Rathod VK (2014) Ultrasound assisted three phase partitioning of a fibrinolytic enzyme. Ultrason Sonochem 21:628–633. https://doi.org/10.1016/j.ultsonch.2013.10.002
Berlan J, Mason TJ (1992) Sonochemistry: from research laboratories to industrial plants. Ultrasonics 30:203–212. https://doi.org/10.1016/0041-624X(92)90078-Z
Bhirud US, Gogate PR, Wilhelm AM, Pandit AB (2004) Ultrasonic bath with longitudinal vibrations: a novel configuration for efficient wastewater treatment. Ultrason Sonochem 11:143–147. https://doi.org/10.1016/j.ultsonch.2004.01.010
Bian Y, Zhang J, Liu C, Zhao D (2020) Synthesis of cross-linked poly acidic ionic liquids and its application in biodiesel production. Catal Lett 150:969–978. https://doi.org/10.1007/s10562-019-02988-0
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Brown LM, Sprague S, Jarvis EE et al (1994) Biodiesel from aquatic species. Project report: FY 1993. National Renewable Energy Laboratory, Golden, Colorado
Cetinkaya M, Karaosmanoǧlu F (2004) Optimization of base-catalyzed transesterification reaction of used cooking oil. https://doi.org/10.1021/ef049891c Accessed 3 Dec 2018
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Cho S-K, Shin H-S, Kim D-H (2012) Waste activated sludge hydrolysis during ultrasonication: two-step disintegration. Biores Technol 121:480–483. https://doi.org/10.1016/j.biortech.2012.07.024
Choi JA, Hwang JH, Dempsey BA, Abou-Shanab RAI, Min B, Song H, Lee DS, Kim JR, Cho Y, Hong S, Jeon BH (2011) Enhancement of fermentative bioenergy (ethanol/hydrogen) production using ultrasonication of Scenedesmus obliquus YSW15 cultivated in swine wastewater effluent. Energy & Environmental Science 4(9):3513
Cravotto G, Demetri A, Nano GM et al (2003) The aldol reaction under high-intensity ultrasound: a novel approach to an old reaction. Eur J Org Chem 2003:4438–4444. https://doi.org/10.1002/ejoc.200300369
Dalvi SN, Funde PE, Pokharkar RD, Mohite KC (2009) Effect of concentration of KOH, H2O, temp in in-situ transestrification reaction of Sesbania sesban, capparis deciduas seed. Renew Energy Power Qual 1(07):28–31
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energy Convers Manage 50:14–34. https://doi.org/10.1016/j.enconman.2008.09.001
Demirbas A (2010) Use of algae as biofuel sources. Energy Convers Manage 51:2738–2749. https://doi.org/10.1016/j.enconman.2010.06.010
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manage 52:163–170. https://doi.org/10.1016/j.enconman.2010.06.055
Dragone G, Fernandes B, Vicente AA, Teixeira JA (2010) Third generation biofuels from microalgae. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex, Spain, pp 1355–1366
Dragone G, Fernandes BD, Abreu AP et al (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335. https://doi.org/10.1016/j.apenergy.2011.03.012
Duan Y, Shi F (2014) Bioreactor design for algal growth as a sustainable energy source. In: Shi F (ed) Reactor and process design in sustainable energy technology. Elsevier, New York, pp 27–60
Ehimen EA, Sun Z, Carrington GC (2012) Use of ultrasound and co-solvents to improve the in-situ transesterification of microalgae biomass. Procedia Env Sci 15:47–55. https://doi.org/10.1016/j.proenv.2012.05.009
Encinar JM, González JF, Rodríguez-Reinares A (2007) Ethanolysis of used frying oil. Biodiesel preparation and characterization. Fuel Process Technol 88(5):513–522
Faried M, Samer M, Abdelsalam E et al (2017) Biodiesel production from microalgae: processes, technologies and recent advancements. Renew Sustain Energy Rev 79:893–913. https://doi.org/10.1016/j.rser.2017.05.199
Frascari D, Zuccaro M, Pinelli D, Paglianti A (2008) A pilot-scale study of alkali-catalyzed sunflower oil transesterification with static mixing and with mechanical agitation. Energy Fuels 22:1493–1501. https://doi.org/10.1021/ef700584h
Fukuda H, Kondo A, Noda H (2001) Biodiesel fuel production by transesterification of oils. J Biosci Bioeng 92:405–416. https://doi.org/10.1016/S1389-1723(01)80288-7
Gadhe A, Sonawane SS, Varma MN (2014) Evaluation of ultrasonication as a treatment strategy for enhancement of biohydrogen production from complex distillery wastewater and process optimization. Int J Hydrogen Energy 39:10041–10050. https://doi.org/10.1016/j.ijhydene.2014.04.153
Ghosh S, Banerjee S, Das D (2017) Process intensification of biodiesel production from Chlorella sp. MJ 11/11 by single step transesterification. Algal Res 27:12–20. https://doi.org/10.1016/j.algal.2017.08.021
Gogate P (2004) Sonochemical reactors: scale up aspects. Ultrason Sonochem 11:105–117. https://doi.org/10.1016/j.ultsonch.2004.01.005
Gogate PR (2008) Cavitational reactors for process intensification of chemical processing applications: a critical review. Chem Eng Process 47:515–527. https://doi.org/10.1016/j.cep.2007.09.014
Gole VL, Gogate PR (2012) Intensification of synthesis of biodiesel from nonedible oils using sonochemical reactors. Ind Eng Chem Res 51:11866–11874. https://doi.org/10.1021/ie2029442
Gondrexon N, Renaudin V, Petrier C et al (1998) Experimental study of the hydrodynamic behaviour of a high frequency ultrasonic reactor. Ultrason Sonochem 5:1–6. https://doi.org/10.1016/S1350-4177(97)00043-6
Griffiths M, Harrison STL, Smit M, Maharajh D (2016) Major commercial products from micro- and macroalgae. In: Bux F, Chisti Y (eds) Algae biotechnology: products and processes. Springer International Publishing, Cham, pp 269–300
Gude VG, Martinez-Guerra E (2018) Green chemistry with process intensification for sustainable biodiesel production. Environ Chem Lett 16:327–341. https://doi.org/10.1007/s10311-017-0680-9
Guldhe A, Singh B, Rawat I, Bux F (2014) Synthesis of biodiesel from Scenedesmus sp. by microwave and ultrasound assisted in situ transesterification using tungstated zirconia as a solid acid catalyst. Chem Eng Res Des 92:1503–1511. https://doi.org/10.1016/j.cherd.2014.05.012
He Y, Zhang B, Guo S et al (2020) Sustainable biodiesel production from the green microalgae Nannochloropsis: novel integrated processes from cultivation to enzyme-assisted extraction and ethanolysis of lipids. Energy Convers Manage 209:112618. https://doi.org/10.1016/j.enconman.2020.112618
Helwani Z, Othman MR, Aziz N et al (2009) Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process Technol 90:1502–1514. https://doi.org/10.1016/j.fuproc.2009.07.016
Hsia S-Y, Yang S-K (2015) Enhancing algal growth by stimulation with LED lighting and ultrasound. J Nanomater 2015:1–11. https://doi.org/10.1155/2015/531352
Huang J, Feng F, Wan M et al (2015) Improving performance of flat-plate photobioreactors by installation of novel internal mixers optimized with computational fluid dynamics. Biores Technol 182:151–159. https://doi.org/10.1016/j.biortech.2015.01.067
Jain S, Sharma MP (2010) Kinetics of acid base catalyzed transesterification of Jatropha curcas oil. Biores Technol 101:7701–7706. https://doi.org/10.1016/j.biortech.2010.05.034
Jerney J, Spilling K (2018) Large scale cultivation of microalgae: open and closed systems. Humana Press, Totowa
Jorquera O, Kiperstok A, Sales EA et al (2010) Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Biores Technol 101:1406–1413. https://doi.org/10.1016/j.biortech.2009.09.038
Juan JC, Kartika DA, Wu TY, Hin T-YY (2011) Biodiesel production from jatropha oil by catalytic and non-catalytic approaches: an overview. Biores Technol 102:452–460. https://doi.org/10.1016/j.biortech.2010.09.093
Kelliher A, Morrison A, Oroskar A, et al (2016) Simulated moving bed chromatographic separation process, US9321715B2, United States
Keris-Sen UD, Sen U, Soydemir G, Gurol MD (2014) An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Biores Technol 152:407–413. https://doi.org/10.1016/j.biortech.2013.11.018
Khan S, Siddique R, Sajjad W et al (2017) Biodiesel production from algae to overcome the energy crisis. HAYATI J Biosci 24:163–167. https://doi.org/10.1016/j.hjb.2017.10.003
Khan SA, Rashmi HMZ, Prasad S, Banerjee UC (2009) Prospective of biodiesel production from microalgae in India. Renew Sustain Energy Rev 13:2361–2372
Khiratkar AG, Balinge KR, Patle DS et al (2018) Transesterification of castor oil using benzimidazolium based Brønsted acid ionic liquid catalyst. Fuel 231:458–467. https://doi.org/10.1016/j.fuel.2018.05.127
Kim B, Heo HY, Son J et al (2019) Simplifying biodiesel production from microalgae via wet in situ transesterification: a review in current research and future prospects. Algal Res 41:101557. https://doi.org/10.1016/j.algal.2019.101557
Kim J, Yoo G, Lee H et al (2013) Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol Adv 31:862–876. https://doi.org/10.1016/j.biotechadv.2013.04.006
Kumar M, Sharma MP (2016) Selection of potential oils for biodiesel production. Renew Sustain Energy Rev 56:1129–1138
Kumar M, Sun Y, Rathour R, Pandey TIS, Tsang DCW (2020) Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci Total Environ 716:137116
Kumar Tiwari A, Kumar A, Raheman H (2007) Biodiesel production from Jatropha oil (Jatropha curcas) with high free fatty acids: an optimized process. Biomass Bioenerg 31:569–575. https://doi.org/10.1016/j.biombioe.2007.03.003
Lammers PJ, Huesemann M, Boeing W et al (2017) Review of the cultivation program within the national alliance for advanced biofuels and bioproducts. Algal Res 22:166–186. https://doi.org/10.1016/j.algal.2016.11.021
Lardon L, Hélias A, Sialve B et al (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43:6475–6481. https://doi.org/10.1021/es900705j
Lee J-Y, Yoo C, Jun S-Y et al (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101(Suppl 1):S75–77. https://doi.org/10.1016/j.biortech.2009.03.058
Lei H, Wang Z, Zhao X, Ding X, Chen X, Zhang H (2011) A simple and promising route for biodiesel production from low-quality lipids. Environ Chem Lett 9:279–283. https://doi.org/10.1007/s10311-010-0280-4
Li D-J, Song J-F, Xu A-Q, Liu C-Q (2014) Optimization of the ultrasound-assisted synthesis of lutein disuccinate using uniform design. Ultrason Sonochem 21:98–103. https://doi.org/10.1016/j.ultsonch.2013.06.004
Li H, Pordesimo L, Weiss J (2004) High intensity ultrasound-assisted extraction of oil from soybeans. Food Res Int 37:731–738. https://doi.org/10.1016/j.foodres.2004.02.016
Liu X, He H, Wang Y, Zhu S, Piao X (2008) Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87(2):216–221
Ma F, Hanna MA (1999) Biodiesel production: a review. Biores Technol 70:1–15. https://doi.org/10.1016/S0960-8524(99)00025-5
Martínez N, Callejas N, Morais EG et al (2017) Obtaining biodiesel from microalgae oil using ultrasound-assisted in-situ alkaline transesterification. Fuel 202:512–519. https://doi.org/10.1016/j.fuel.2017.04.040
Martinez-Guerra E, Gude VG, Mondala A et al (2014) Microwave and ultrasound enhanced extractive-transesterification of algal lipids. Appl Energy 129:354–363. https://doi.org/10.1016/j.apenergy.2014.04.112
Martinez-Guerra E, Howlader MS, Shields-Menard S et al (2018) Optimization of wet microalgal FAME production from Nannochloropsis sp. under the synergistic microwave and ultrasound effect. Int J Energy Res 42:1934–1949. https://doi.org/10.1002/er.3989
Mason TJ (2000) Large scale sonochemical processing: aspiration and actuality. Ultrason Sonochem 7:145–149. https://doi.org/10.1016/S1350-4177(99)00041-3
Mason TJ, Lorimer JP (2002) Applied sonochemistry and processing. Wiley, New York. https://doi.org/10.1002/352760054X
Meher LC, Vidya Sagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification—a review. Renew Sustain Energy Rev 10:248–268. https://doi.org/10.1016/j.rser.2004.09.002
Miranda JR, Passarinho PC, Gouveia L (2012) Bioethanol production from Scenedesmus obliquus sugars: the influence of photobioreactors and culture conditions on biomass production. Appl Microbiol Biotechnol 96(2):555–564
Mofijur M, Rasul MG, Hassan NMS, Nabi MN (2019) Recent development in the production of third generation biodiesel from microalgae. Energy Procedia 156:53–58. https://doi.org/10.1016/j.egypro.2018.11.088
Moholkar VS, Choudhury HA, Singh S et al (2015) Physical and chemical mechanisms of ultrasound in biofuel synthesis. In: Fang Z, Smith RL, Qi X (eds) Production of biofuels and chemicals with ultrasound. Springer, Dordrecht, pp 35–86
Mondal M, Goswami S, Ghosh A et al (2017) Production of biodiesel from microalgae through biological carbon capture: a review. Biotech. https://doi.org/10.1007/s13205-017-0727-4
Mubarak M, Shaija A, Suchithra TV (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7:117–123. https://doi.org/10.1016/j.algal.2014.10.008
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597. https://doi.org/10.1016/j.rser.2009.10.003
Naveena B, Armshaw P, Tony Pembroke J (2015) Ultrasonic intensification as a tool for enhanced microbial biofuel yields. Biotechnol Biofuels 8:140. https://doi.org/10.1186/s13068-015-0321-0
Ngamcharussrivichai C, Totarat P, Bunyakiat K (2008) Ca and Zn mixed oxide as a heterogeneous base catalyst for transesterification of palm kernel oil. Appl Catal A: Gen 341(1–2):77–85
Noureddini H, Gao X, Philkana RS (2005) Immobilized Pseudornonas cepacia lipase for biodiesel fuel production from soybean oil. Biores Technol 96:769–777
Oni A, Fadare D, Waheed M, Adewumi A, Adejobi O, Sulaiman M (2011) Exergetic assessment of a crude oil distillation plant. In: Proceeding of the science, engineering and technology conference, Osun State University Nigeria, pp 151–158
Pandey A, Singh MP, Kumar S, Srivastava S (2019) Phycoremediation of persistent organic pollutants from wastewater: retrospect and prospects. In: Gupta SK, Bux F (eds) Application of microalgae in wastewater treatment. Springer International Publishing, Cham, pp 207–235
Paniwnyk L, Cai H, Albu S et al (2009) The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason Sonochem 16:287–292. https://doi.org/10.1016/j.ultsonch.2008.06.007
Park J-Y, Park MS, Lee Y-C, Yang J-W (2015a) Advances in direct transesterification of algal oils from wet biomass. Biores Technol 184:267–275. https://doi.org/10.1016/j.biortech.2014.10.089
Park J-Y, Lee K, Choi S-A, Jeong M-J, Kim B, Lee J-S, Oh Y-K (2015b) Sonication-assisted homogenization system for improved lipid extraction from Chlorella vulgaris. Renew Energy 79:3–8. https://doi.org/10.1016/j.renene.2014.10.001
Patel A, Matsakas L, Rova U, Christakopoulos P (2018) Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol Biofuels 11:169. https://doi.org/10.1186/s13068-018-1173-1
Patil PD, Gude VG, Mannarswamy A et al (2011) Optimization of microwave-assisted transesterification of dry algal biomass using response surface methodology. Biores Technol 102:1399–1405. https://doi.org/10.1016/j.biortech.2010.09.046
Patil PD, Gude VG, Mannarswamy A et al (2012) Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel 97:822–831. https://doi.org/10.1016/j.fuel.2012.02.037
Patle DS, Sharma S, Ahmad Z, Rangaiah GP (2014) Multi-objective optimization of two alkali catalyzed processes for biodiesel from waste cooking oil. Energy Convers Manage 85:361–372. https://doi.org/10.1016/j.enconman.2014.05.034
Patle DS, Sharma S, Gadhamsetti AP et al (2018) Ultrasonication-assisted and benzimidazolium-based brønsted acid ionic liquid-catalyzed transesterification of castor oil. ACS Omega 3:15455–15463. https://doi.org/10.1021/acsomega.8b02021
Patle DS, Shrikhande S, Rangaiah GP (2020) Process development, design and analysis of microalgal biodiesel production aided by microwave and ultrasonication (Ch-10). In: Adrian A, Rangaiah GP (eds) Process systems engineering for biofuels development. Wiley, New York
Pavithra KG, Kumar PS, Jaikumar V, Vardhan KH, Rajan PSS (2020) Microalgae for biofuel production and removal of heavy metals: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01046-1
Peng L, Fu D, Chu H et al (2020) Biofuel production from microalgae: a review. Environ Chem Lett 18:285–297. https://doi.org/10.1007/s10311-019-00939-0
Perez-Garcia O, Bashan Y (2015) Microalgal heterotrophic and mixotrophic culturing for bio-refining: from metabolic routes to techno-economics. In: Prokop A, Bajpai RK, Zappi ME (eds) Algal biorefineries. Springer International Publishing, Cham, pp 61–131
Prabakaran P, Ravindran AD (2011) A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett Appl Microbiol 53(2):150–154. https://doi.org/10.1111/j.1472-765X.2011.03082.x
Pruvost J, Cornet J-F, Pilon L (2016) Large-scale production of algal biomass: photobioreactors. In: Bux F, Chisti Y (eds) Algae biotechnology. Springer International Publishing, Cham, pp 41–66
Ramos-Tercero EA, Domenicali G, Bertucco A (2014) Autotrophic production of biodiesel from microalgae: an updated process and economic analysis. Energy 76:807–815. https://doi.org/10.1016/j.energy.2014.08.077
Rashid U, Anwar F, Moser BR, Ashraf S (2008) Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass Bioenergy 32(12):1202–1205
Ren H, Liu B, Ma C, Zhao L, Ren N (2013) A new lipid-rich microalga Scenedesmus sp strain R-16 isolated using Nile red staining: effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol Biofuels 6:143
Raskar HD, Avhad DN, Rathod VK (2014) Ultrasound assisted production of daunorubicin: process intensification approach. Chem Eng Process 77:7–12. https://doi.org/10.1016/j.cep.2013.12.008
Rodionova MV, Poudyal RS, Tiwari I et al (2017) Biofuel production: challenges and opportunities. Int J Hydrogen Energy 42:8450–8461. https://doi.org/10.1016/j.ijhydene.2016.11.125
Safari J, Javadian L (2015) Ultrasound assisted the green synthesis of 2-amino-4H-chromene derivatives catalyzed by Fe3O4-functionalized nanoparticles with chitosan as a novel and reusable magnetic catalyst. Ultrason Sonochem 22:341–348. https://doi.org/10.1016/j.ultsonch.2014.02.002
Salama E-S, Kurade MB, Abou-Shanab RAI et al (2017) Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew Sustain Energy Rev 79:1189–1211. https://doi.org/10.1016/j.rser.2017.05.091
Salim S (2013) Harvesting microalgae by bio-flocculation and autoflocculation. Ph.D. Thesis, Wageningen University
Sancheti SV, Gogate PR (2017) A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason Sonochem 36:527–543. https://doi.org/10.1016/j.ultsonch.2016.08.009
Santos FFP, Rodrigues S, Fernandes FAN (2009) Optimization of the production of biodiesel from soybean oil by ultrasound assisted methanolysis. Fuel Process Technol 90:312–316. https://doi.org/10.1016/j.fuproc.2008.09.010
Sara M, Brar SK, Blais JF (2016) Comparative study between microwave and ultrasonication aided in situ transesterification of microbial lipids. RSC Adv 6:56009–56017. https://doi.org/10.1039/C6RA10379K
Sarve AN, Varma MN, Sonawane SS (2016) Ultrasound assisted two-stage biodiesel synthesis from non-edible Schleichera triguga oil using heterogeneous catalyst: Kinetics and thermodynamic analysis. Ultrason Sonochem 29:288–298. https://doi.org/10.1016/j.ultsonch.2015.09.016
Sati H, Mitra M, Mishra S, Baredar P (2019) Microalgal lipid extraction strategies for biodiesel production: a review. Algal Res 38:101413. https://doi.org/10.1016/j.algal.2019.101413
Sawarkar AN (2019) Cavitation induced upgrading of heavy oil and bottom-of-the-barrel: a review. Ultrason Sonochem 58:104690. https://doi.org/10.1016/j.ultsonch.2019.104690
Schmidt CW (2007) Biodiesel: cultivating alternative fuels. Environ Health Perspect 115:A86–A91
Scragg AH, Morrison J, Shales SW (2003) The use of a fuel containing Chlorella vulgaris in a diesel engine. Enzyme Microbial Technol 33:884–889. https://doi.org/10.1016/j.enzmictec.2003.01.001
Sharma S, Rangaiah GP (2013) Multi-objective optimization of a bio-diesel production process. Fuel 103:269–277. https://doi.org/10.1016/j.fuel.2012.05.035
Singh RN, Sharma S (2012) Development of suitable photobioreactor for algae production—a review. Renew Sustain Energy Rev 16:2347–2353. https://doi.org/10.1016/j.rser.2012.01.026
Sinha S, Agarwal AK, Garg S (2008) Biodiesel development from rice bran oil: Transesterification process optimization and fuel characterization. Energy Conv Manag 49(5):1248–1257
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenerg 53:29–38. https://doi.org/10.1016/j.biombioe.2012.12.019
Son Y, Lim M, Khim J (2009) Investigation of acoustic cavitation energy in a large-scale sonoreactor. Ultrason Sonochem 16:552–556. https://doi.org/10.1016/j.ultsonch.2008.12.004
Srivastava A, Prasad R (2000) Triglycerides-based diesel fuels. Renew Sustain Energy Rev 4:111–133. https://doi.org/10.1016/S1364-0321(99)00013-1
Srivastava RK, Shetti NP, Reddy KR, Aminabhavi TM (2020) Biofuels, biodiesel and biohydrogen production using bioprocesses: a review. Environ Chem Lett 18:1049–1072. https://doi.org/10.1007/s10311-020-00999-7
Solecki M, Scodel A, Epstein B (2013) Advanced biofuel market report, San Francisco, California, https://www.fuelinggrowth.org/wp-content/uploads/2013/09/E2-Biofuel-Market-Report-2013.Final_.pdf, Accessed Jan 2020
Suganya T, Kasirajan R, Renganathan S (2014) Ultrasound-enhanced rapid in situ transesterification of marine macroalgae Enteromorpha compressa for biodiesel production. Bioresour Technol 156:283–290. https://doi.org/10.1016/j.biortech.2014.01.050
Sun A, Davis R, Starbuck M et al (2011) Comparative cost analysis of algal oil production for biofuels. Energy 36:5169–5179. https://doi.org/10.1016/j.energy.2011.06.020
Sun XM, Ren LJ, Zhao QY, Ji XJ, Huang H (2018) Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol Biofuels 11:272. https://doi.org/10.1186/s13068-018-1275-9
Suri C, Takenaka K, Yanagida H et al (2002) Chaotic mixing generated by acoustic streaming. Ultrasonics 40:393–396. https://doi.org/10.1016/S0041-624X(02)00150-6
Thanh LT, Okitsu K, Sadanaga Y et al (2010) Ultrasound-assisted production of biodiesel fuel from vegetable oils in a small scale circulation process. Biores Technol 101:639–645. https://doi.org/10.1016/j.biortech.2009.08.050
Thompson LH, Doraiswamy LK (1999) Sonochemistry: science and engineering. Ind Eng Chem Res 38:1215–1249. https://doi.org/10.1021/ie9804172
Toma M, Vinatoru M, Paniwnyk L, Mason TJ (2001) Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem 8:137–142. https://doi.org/10.1016/S1350-4177(00)00033-X
Tomasevic AV, Siler-Marinkovic SS (2003) Methanolysis of used frying oil. Fuel Process Technol 81(1):1–6
Tran D-T, Chang J-S, Lee D-J (2017) Recent insights into continuous-flow biodiesel production via catalytic and non-catalytic transesterification processes. Appl Energy 185:376–409. https://doi.org/10.1016/j.apenergy.2016.11.006
Valachovic P, Pechova A, Mason TJ (2001) Towards the industrial production of medicinal tincture by ultrasound assisted extraction. Ultrason Sonochem 8:111–117. https://doi.org/10.1016/S1350-4177(00)00066-3
Velasquez-Orta SB, Lee JGM, Harvey AP (2013) Evaluation of FAME production from wet marine and freshwater microalgae by in situ transesterification. Biochem Eng J 76:83–89. https://doi.org/10.1016/j.bej.2013.04.003
Vilkhu K, Mawson R, Simons L, Bates D (2008) Applications and opportunities for ultrasound assisted extraction in the food industry:a review. Innov Food Sci Emerg Technol 9:161–169. https://doi.org/10.1016/j.ifset.2007.04.014
Vo HNP, Ngo HH, Guo W et al (2019) A critical review on designs and applications of microalgae-based photobioreactors for pollutants treatment. Sci Total Environ 651:1549–1568. https://doi.org/10.1016/j.scitotenv.2018.09.282
Wahidin S, Idris A, Yusof NM et al (2018) Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers Manage 171:1397–1404. https://doi.org/10.1016/j.enconman.2018.06.083
Wang X, Liu X, Zhao C et al (2011) Biodiesel production in packed-bed reactors using lipase–nanoparticle biocomposite. Biores Technol 102:6352–6355. https://doi.org/10.1016/j.biortech.2011.03.003
Weissman JC, Goebel RP, Benemann JR (1988) Photobioreactor design: mixing, carbon utilization, and oxygen accumulation. Biotechnol Bioeng 31:336–344. https://doi.org/10.1002/bit.260310409
West A, Posarac D, Ellis N (2008) Assessment of four biodiesel production processes using HYSYS.Plant. Biores Technol 99:6587–6601. https://doi.org/10.1016/j.biortech.2007.11.046
Wileman A, Ozkan A, Berberoglu H (2012) Rheological properties of algae slurries for minimizing harvesting energy requirements in biofuel production. Biores Technol 104:432–439. https://doi.org/10.1016/j.biortech.2011.11.027
Yan S, Kim M, Salley SO, Simon Ng KY (2009) Oil transesterification over calcium oxides modified with lanthanum. Appl Catal A: Gen 360(2):163–170
Yellapu SK, Kaur R, Tyagi RD (2017) Detergent assisted ultrasonication aided in situ transesterification for biodiesel production from oleaginous yeast wet biomass. Bioresour Technol 224:365–372. https://doi.org/10.1016/j.biortech.2016.11.037
Yin Z, Zhu L, Li S et al (2020) A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions. Biores Technol 301:122804. https://doi.org/10.1016/j.biortech.2020.122804
Zhang J, Chen S, Yang R, Yan Y (2010) Biodiesel production from vegetable oil using heterogenous acid and alkali catalyst. Fuel 89:2939–2944. https://doi.org/10.1016/j.fuel.2010.05.009
Zhang X, Yan S, Tyagi RD et al (2014) Ultrasonication aided in-situ transesterification of microbial lipids to biodiesel. Biores Technol 169:175–180. https://doi.org/10.1016/j.biortech.2014.06.108
Zhang X, Yan S, Tyagi RD et al (2016) Ultrasonication aided biodiesel production from one-step and two-step transesterification of sludge derived lipid. Energy 94:401–408. https://doi.org/10.1016/j.energy.2015.11.016
Zheng H, Yin J, Gao Z, Huang H, Ji X, Dou C (2011) Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl Biochem Biotechnol 164(7):1215–1224
Acknowledgements
Authors gratefully acknowledge the funding received from DST-SERB through the research Grant ECR/2016/001866 to carry out this work. Authors also thank Mr. Varun Kaushal (VIT Vellore) for his help in data compilation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patle, D.S., Pandey, A., Srivastava, S. et al. Ultrasound-intensified biodiesel production from algal biomass: a review. Environ Chem Lett 19, 209–229 (2021). https://doi.org/10.1007/s10311-020-01080-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01080-z