Abstract
The rising activities of global agriculture and forestry industries are producing huge amounts of lignocellulosic waste, which needs to be well recycled. The management of this waste involves environmental, social, economic and political challenges. Lignocellulosics have been commonly used for construction materials and energy production, thus achieving positive social and environmental impacts. Lignocellulosics represent also a promising feedstock for the production of carriers for enzyme and cell immobilization. Immobilization is a technique in which the biocatalyst is fixed on the surface of an insoluble matrix, allowing to recover the biocatalyst after reaction. The support must have specific characteristics such as inertness, physical strength, stability, renewability and low cost. These characteristics are fulfilled by lignocellulosic materials. Here, we review the applications of lignocellulosic biomass for fermentation, remediation of contaminated water and soil, synthesis of solvents and fine chemicals, juices clarification, and production of fructooligosaccharides. Recycling lignocellulosic waste for the immobilization of enzymes and cells allow to reduce environmental issues. Processes using immobilized cells and enzymes give high rates of solvent productivity, of 1.44–1.67 g/Lh, activity retention, around 90%, and stability, above five cycles of reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Generally, biocatalytic processes can be simpler to operate than chemical processes; they are very competitive due to their environmental profiles. Processes such as heterogeneous catalysis, flow chemistry, continuous processing, green solvents, catalyst immobilization, and recycling are some of the main relevant and emerging technologies that hold great potential for clean and selective production processes (Gericke et al. 2015). Biocatalysis has been widely accepted in different economic sectors, owing to its substrate specificity and green chemistry characteristics by which transformation reactions are carried out under mild conditions of temperature, pressure and pH (Datta et al. 2012). Biocatalysis is an energetically efficient and sustainable technique that produces the lowest waste and can be used in a variety of scientific and industrial applications. However, the use of biocatalysis is restricted because of its long operational time, stability, difficult recovery and reuse of the catalyst. These issues can be overcome through the immobilization (Sheldon and Van Pelt 2013). Immobilization of cells and enzymes is a biotechnological technique in which these biocatalysts are fixed in a suitable matrix that limits their movement to increase their stability and reusability. Immobilization has been widely researched, and a lot of reviews focusing on techniques to immobilization of enzymes, and in the use of organic and inorganic carriers to immobilization have been published (Velasco-Lozano et al. 2016; Bilal and Iqbal 2019; Thangaraj and Solomon 2019). Even, works in immobilization of cells (Bouabidi et al. 2018) on organic waste as eggshell (Salleh et al. 2016), and recently trends in immobilization onto novel materials such as magnetic nanoparticles have been published (Mehta et al. 2016; Vaghari et al. 2016; Khoshnevisan et al. 2017). These studies have demonstrated that when the carrier fulfills with physical, chemical, electrical, or mechanical characteristics, the activity and stability of the biocatalysts can improve across a broader range of operating conditions (Chapman et al. 2018).
Spite the fact that the immobilization has several advantages as functionality for use in continuous processes and easy separation of the enzyme from the medium of reaction, and reuse of the enzyme. The cost of the carrier and its additional preparation materials and methods is a disadvantage that limits in some cases the use of carriers. So, it is important that the immobilized systems provide positive economic benefits that can overcome and balanced the additional costs associated with the materials used as carriers (Chapman et al. 2018).
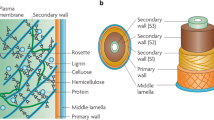
One interesting option that contributes to reducing immobilization costs is the use of natural supports from lignocellulosic materials (Fig. 1) that are cheaper than other kind of supports and are widely available in the world. Lignocellulosic carriers also have desirable physical and mechanical properties, and they are environmentally friendly, renewable, biodegradable and nontoxic (Moniruzzaman and Goto 2016), which make their use and industrial scale implementation easier (Velasco-Lozano et al. 2016; Thangaraj and Solomon 2019). In fact, several authors have evaluated the use of lignocellulosic biomass as a carrier to immobilization. They have demonstrated the success of these systems in several biotechnological, pharmaceutical and environmental fields. However, there are no reviews that summarize these efforts and let the academic community to know about the exclusive use of lignocellulosic biomass as carrier for immobilization. For that, taking into account the above-mentioned considerations, this review makes a special emphasis on the use of lignocellulosic biomass to immobilization of enzyme and cells. Besides describing the most commonly used enzymes and cells and their uses, this review provides descriptions of raw materials, pretreatments and the methods used to activate the carrier according to the method of immobilization.
Lignocellulosic biomass
Globally, billions of metric tons of biomass are produced directly from agricultural activities including crop-based and agro-industrial residues and by-products. Tons of dry biomass (DM) has been used for feed (58%), bioenergy (heat and electricity, 16%), food (14%), materials (10%) and biofuels (1%) (Schröder et al. 2018). As improper management of large amounts of biomass causes environmental problems due to difficult disposal or reuse, enormous efforts are being made to explore innovative alternatives that add value to these residues and simultaneously reduce the problems that are associated with their final disposal. The huge amounts of lignocellulosic biomass can potentially be used to obtain heat power, and various values added biotechnological products, such as hydrogen, alcohol, olefins, gasoline, diesel, methane, oils, specialty chemicals, bioethanol, biodiesel, biobutanol, biohythane and others (Anwar et al. 2014; Akalın et al. 2017; Pérez-Rodríguez et al. 2018). Also, lignocellulosic waste has been used for the development of nanomaterials to biofuel production (Srivastava et al. 2017). In addition, after different conditioning treatments, lignocellulosic biomass can be used for the production of immobilized cell and enzyme systems. Immobilization on lignocellulosic wastes also allows reducing the disadvantages of using soluble enzymes.
Biomass can be classified in two ways according to the origin, function and final products. (1) Categorization based on types of biomass existing in nature, according to ecology or type of vegetation, which in turn divides into wood and woody biomass, herbaceous biomass, aquatic biomass, animal and human waste biomass, and biomass mixtures. (2) Categorization based on the use and application of biomass as feedstock (Tursi 2019). Herbaceous biomass is split into two main groups: energy crops, that have been exploited only in the bioenergy sector, and agricultural residues such as by-products of food, fibers or food industries (Tursi 2019). Typically, cell wall of agricultural lignocellulosic biomass is composed by cellulose (40–50% w/w), hemicellulose (10–25%) and lignin (20–30%), plus a small number of extractive solids and ashes (Anwar et al. 2014), although the content ratio of the biopolymers depends on the feedstock.
Cellulose is a natural semicrystalline polysaccharide that is widely abundant and present in the form of fibers of different sizes, from 0.5 to several mm. It is composed of β-d-glucopyranose subunits linked by highly resistant β-1,4-glycosidic bonds that can reach several thousand glucose units in length, with the levels together forming the spiral mode of a three-layer cell wall structure that forms the cellulose fiber (Anwar et al. 2014; Saha et al. 2016). Cellulose chains aggregate together in the form of elementary fibrils approximately 3 nm in diameter and can aggregate into larger micro- or macrofibrils. Cellulose nanofibers have been isolated from natural lignocellulosic sources, such as wood, banana peel, eucalyptus, kenaf, corn stalk, pine, jute, palm, grass, etc. (Alemdar and Sain 2008; Jonoobi et al. 2009; Uetani and Yano 2011; Besbes et al. 2011; Chen et al. 2011; Deepa et al. 2011; Jahan et al. 2011; Duan et al. 2013; Benhamou et al. 2014; Nakagaito et al. 2015; Zhu et al. 2016; Bondancia et al. 2017). Different processes, such as alkaline chemical pulping, bleaching, enzymatic hydrolysis and TEMPO oxidation, are used for chemical pretreatments of raw material. These processes are applied to separate fiber by dissolving lignin and hemicellulose (Rahmati et al. 2010; Chen et al. 2011; Jahan and Rahman 2012; Pouyet et al. 2013; Khalil et al. 2014b; Boufi et al. 2016). Other methods, such as high-pressure homogenization, microfluidization, microgrinding, high-intensity ultrasonication, electrospinning and steam explosion, are used as isolation treatments (Kim and Park 2006; Spence et al. 2011; Khalil et al. 2014a, 2016; Sulaiman et al. 2015a). Cellulose fibers, along with packaging, paper and coating, electronics, membranes, nanocomposites, textile and other industrial applications, have been widely used in various fields (Reddy and Yang 2005; Erdman et al. 2016; Khalil et al. 2016).
Techniques of immobilization
The selection of one adequate technique for immobilization plays a very important role in determining the activity of the biocatalyzer and the characteristics of the system. Factors such as cost of the immobilization procedure, enzyme deactivation and regeneration, toxicity of reagents, and final properties of the system must be considered for process specification and for selecting the immobilization technique (Mohamad et al. 2015). The immobilization of biocatalysts can be separated into two categories: chemical and physical methods. In the chemical method, covalent or ionic bonds form, while the physical method employs techniques that mainly involve hydrophobic or van der Waals interactions. The most commonly applied techniques for immobilization of cells and enzymes are adsorption, entrapment or inclusion, covalent binding and cross-linking. Systems with immobilized cells are less vulnerable to changes in the environmental condition, such as pH modification, and offer protection against toxic compounds.
Adsorption
The adsorption process is assessed by the direct submersion of the support in a medium containing an enzyme or cell. The attachment of the enzyme or the adhesion of microbial cells on surfaces is mainly carried out through van der Waals forces, ionic and hydrophobic interactions and hydrogen bonds. The adsorbed enzymes are protected from aggregation, proteolysis and interaction with hydrophobic interfaces (Datta et al. 2012). However, under industrial conditions involving high reactant and product concentrations, physical adsorption is generally too weak to keep enzyme fixed on the support (Sheldon 2007). Even so, immobilization of enzymes and cells by adsorption on lignocellulosic supports has been used successfully for the synthesis of products such as bioethanol, xylitol, hydrogen, fructooligosaccharides (FOS), acetone, butanol and others (see Tables 1 and 2). In solid supports with low loads of enzymes or cells, there is an excess of surface area, which means that the biocatalyst tends to maximize its contact with the support and spreads across the surface, which leads to its inactivation. To increase the adsorption efficiency, the tendency of the biocatalyst to leach must be suppressed, although it could be prevented by covalent binding (Sheldon and Woodley 2018).
Covalent binding
Covalent binding is a technique that has been researched extensively for coupling biocatalysts to insoluble matrices. Covalent links can form between functional groups of the support and functional groups of the amino acid residues in the biocatalyzer. Normally, in this technique, the enzyme is coupled with the previous activation of the functional groups on the support, thus making it a stronger electrophilic for a posterior reaction with some nucleophilic groups in the biocatalyzer such as –NH2 from lysine or arginine, –COOH from aspartic and glutamic acid, –OH from serine and threonine and, –SH from cysteine. The reaction is followed by the elimination of excess biomolecules and reagents (Sassolas et al. 2012). At the end, the enzyme is tightly fixed on the support, the lixiviation is reduced, and the product is not contaminated with the enzyme. Covalent immobilization involves several reaction procedures, such as a peptide bond, diazotization, amino bond, Schiff’s bases formation, thiol-disulfide, amidation reaction and alkylation (Sulaiman et al. 2015b). Generally, covalent immobilization is preferred in aqueous solutions and when there are factors of denaturation in the medium (Hanefeld et al. 2009). Enzyme immobilization by covalent binding has several advantages, e.g. little leakage of enzyme from the support during catalysis because of the tight binding, and typically improves the stability of the system (Varavinit et al. 2001). Some authors have used natural supports, such as cashew apple bagasse, wood cellulignin and spent grain, for successful immobilization through the covalent binding of Candida antarctica lipase, Candida rugosa lipase and trypsin (see Table 1), among others.
Cross-linking
The immobilization of enzymes by cross-linking is executed by simple precipitation of the enzyme from an aqueous solution, such as the physical aggregates of protein molecules, by the addition of salts, water miscible organic solvents or nonionic polymers (Homaei et al. 2013a). This technique is also applicable to the preparation of cross-linked enzyme aggregates containing two or more enzymes for use in one-pot, multistep syntheses (Sheldon et al. 2009). The most commonly used cross-linker is glutaraldehyde because it is inexpensive and easy to obtain in commercial quantities, although other cross-linkers, such as dextran polyaldehyde, are used when glutaraldehyde exhibits poor results (Sheldon and Van Pelt 2013). Few works have been published in relation to immobilization on lignocellulosic material by cross-linking. Some of those studies are mainly focused on the immobilization of enzymes, such as invertase and β-d-fructofuranosidase for the hydrolysis of sucrose and the stabilization and production of fructooligosaccharides, respectively (see Table 1).
Entrapment or inclusion
Direct immobilization of biocatalysts by physical entrapment into hydrogels or inside of fibers is applicable for purified enzymes and crude cell extracts. There are two types of material that are commonly used for enzyme immobilization by entrapment: hydrophobic materials and hydrophilic materials; the latter is useful because of its ability to keep large amounts of enzyme and its capacity for activity retention There are different methods of entrapment, such as temperature-induced gelation, polymerization by a chemical/photochemical reaction, and ionotropic gelation of macromolecules with multivalent cations (Asgher et al. 2014). In cell immobilization by entrapment, they are captured within a support matrix, which offers protection of cells from external aggressions (Bouabidi et al. 2018).
Carriers
Matrix characteristics play an important role in obtaining an efficient immobilized system. The differences in the morphological and physical–chemical characteristics such as pore size, hydrophilic/hydrophobic balance and surface chemistry of the support can affect enzyme immobilization, its catalytic properties, the stability and yield of the system (Mohamad et al. 2015). So, the performance of immobilized biocatalyst is tightly regulated by the material properties of the carriers (Verma et al. 2019). These are basic properties for the correct selection of an immobilization carrier: resistance to microbial and chemical degradation, the availability of a reactive functional group, preservation of physical integrity during the process and recycles, insolubility and inertness toward the reaction medium, a high diffusion coefficient and a superficial area. In addition, the carrier must be available in high quantities and must be affordable (Santos et al. 2005; Silva et al. 2007). For selection of the support material, factors to consider are the following: the nature of the bonds as covalent, noncovalent or different types of covalent or noncovalent bonds, the amount of bonds that form between the enzyme and the support, and the degree of confinement of enzymatic molecules in the support. The microenvironment and the condition of the system (pH, temperature) also have great importance (Cao 2005; Mohamad et al. 2015). In this regard, the optimal supports should have characteristics such as a hydrophobic or hydrophilic character, surface charges, surface functionalization, good chemical and mechanical stability, high surface area, porosity and the proper particle size (Hanefeld et al. 2009).
Biocatalytic production holds great potential for clean and selective production processes. Enzymes are readily biodegradable, and generally after their use in industrial production, they have lower or null toxicity (Raman and Henning 2013). The materials that are used in the manufacture of the immobilization support can be natural polymers, such as alginate, chitosan and chitin, collagen, carrageenan, gelatin, cellulose, starch, pectin and Sepharose, synthetic polymers, such as ion exchange resins, polyvinyl chloride, polyurethane microparticles, polyvinyl alcohol and polyaniline, and inorganic materials, like zeolites, ceramics, Celite, silica, glass, activated carbon and charcoal (Datta et al. 2012; Morin-Crini et al. 2019). Recently, several types of carriers have been developed, such as magnetic nanoparticles, e.g., microspheres of various biomaterials and copolymers with magnetic particles; novel nanoparticles, e.g., gold nanoparticles, polyurethane microspheres-gold nanoparticles, palladium nanoparticles and zeolite-gold nanoparticles, and nonmagnetic nanoparticles, e.g., nanoparticles, nanotubes, nanoporous matrices and nanofibers (Ansari and Husain 2012).
Immobilization of enzymes and cells
Enzymes immobilization
Enzymes are readily biodegradable and generate lower global waste during the obtainment of products and at the end of processes. Additionally, enzymatic process meets the demands of green chemistry for industrial processes (Velasco-Lozano et al. 2018). Enzymatic catalysis has been implemented in a broad range of industries in recent years due to its specificity, fast action and often savings in raw materials, chemicals and/or water compared to those of traditional processes, and they are perceived to work under environmentally friendly conditions (Raman and Henning 2013).
In enzyme immobilization, the mobility of an enzyme is limited by the means of electrostatic or material barriers that separate it from the reaction medium, but the enzyme is still able to interact with target molecules from reagents and products (Rangel et al. 2011). Some advantages of enzyme immobilization are: (1) the product can be separated easily from the medium reaction; (2) stability of the reaction (under different pH and temperature conditions, solvents, and controlled impurities and contaminants) is improved; and (3) their stability could be enhanced depending on the enzyme, the method of immobilization and the type of support (Kim and Park 2006; Khalil et al. 2014a). Consequently, the use of immobilized enzymes greatly simplifies the design of the reactor and the control of the reaction (Mateo et al. 2007). The cost of the carrier is one of the most important criteria by which to consider processes at the industrial level; this, in combination with interest in the reuse of by-products, has led to a search for cheap and widely available carriers (Silva et al. 2007). Taking into account the above-mentioned conditions and criteria, lignocellulosic biomass meets the requirements for the selection of an optimal carrier in the immobilization process.
Wood from sawdust and shavings, coproducts from the rice industry, such as husk, straw, and hull ash carbon from rice bran, and derivatives from fruit processing, such as coconut fibers, cashew apple and bagasse, are waste that are commonly used in the immobilization of enzymes. System enzyme-waste has been used in processes such as the decolorization of aqueous solutions, pulp delignification, dyes and contaminated water or soil remediation, detergent formulations, the synthesis of fine chemicals, and pharmaceuticals. Some of the most used enzymes to develop biocatalytic systems waste-enzyme are:
Lipase
Nowadays, lipases have gained a lot of importance in the area of organic synthesis. However, the inactivation of lipase by organic solvents is often a problem (Kajiwara et al. 2019). Immobilization of lipase has allowed obtaining more thermostable and stable systems, as the reported by De Souza et al. (2016) to immobilization of lipase by covalent binging on cashew apple. In that case, the catalytic system was most stable in the presence of organic solvent for the production of (R)-indanol than the soluble lipase. Techniques as mass balance, chemical composition (FTIR), coupling yield (hydrolysis of olive oil), and catalytic activity in nonaqueous medium allow validating the selection of active biocatalysts (Gomes et al. 2005). Some authors have reported the biocompatibility of residues as wood cellulignin, coconut fibers, corn stalks, and spent grain to immobilization of lipase (Gomes et al. 2005; Brígida et al. 2008; Lv et al. 2013). Gomes et al. (2005) reported a biocatalytic system composed of wood cellulignin-lipase with similar or better characteristics than the natural and synthetic polymers, such as chitin and styrene–divinylbenzene copolymer. Lignocellulosic biomass as spent grain was modified with magnetic nanoparticles, and this novel technique allowed obtaining a biocompatible magnetic carrier for lipase immobilization. This system proposed by Pospiskova and Safarik (2012) presented stability much higher than the free enzyme. Biocatalytic system kept at least 80% of the enzymatic activity during 30 days, while the free enzyme just kept 8% of its initial activity after the same period of evaluation. These studies show that lignocellulosic materials are an interesting tool for immobilization of lipases. They have shown good stability and reusability, being a promising low-cost biocatalytic applicable in fields as food technology and biotechnology.
Laccase
Laccase has large number of potential applications in biotechnological fields due to its different biological roles as lignin degradation, pathogenicity, detoxification morphogenesis and sporulation. For that, it is very used to prevent fruit juices & wine decoloration, detoxification of environmental effluents, oxidation of synthetic dye, among others (Singh and Gupta 2020). Clarification of juice has been widely studied, being immobilization of enzymes a novel alterative for the reuse of the enzyme. Bezerra et al. (2015) studied the use of coconut fibers (CF) as carrier to immobilization of laccase. They found that the combination of CF-laccase with thermal decompression and alkaline treatment, both allow producing and active and stable system, capable of efficiently oxidizing phenolic compounds to apple juice clarification. Also, Da Silva et al. (2012) reported that the catalytic systems conformed by spent grain-laccase was a successful biocatalyst to pulp delignification, dye decolorization and contaminated water. The authors, after kinetic parameters examination, found that the immobilized laccase on spent grain after acid and alkaline treatments had a much higher affinity and velocity for ABTS than the laccase immobilized on spent grain without chemical treatments. This is likely due that the alkaline digestion opens the structure of the remaining cell walls in the raw material, increasing the availability of functional groups for immobilization (Brányik et al. 2004). Laccase can be also immobilized by adsorption to degradation of pharmaceutically active compounds, as the reported by Torán et al. 2017 (Torán et al. 2017). The authors worked with pallet wood in a fluidized bed-reactor, and they achieved a favorable removal of ibuprofen, ketoprofen and naproxen from flocculated hospital wastewater after 49 days of operation. These results show that immobilized laccase can be used to confront the pollutants and environmental challenges, and others fields of waste management.
Other enzymes
Invertase is an enzyme that catalyzes the hydrolysis of the glycoside bond of the sucrose molecule to obtain an equimolar mixture of monosaccharides (Gonçalves et al. 2015). Much of the biochemistry processes that involve invertase are focused on yeast fermentations and processes for conversion of starch to sugar. The use of lignocellulosic materials has played an interesting role to immobilization of invertase to hydrolysis sucrose and production of fructooligosaccharides. Gonçalves et al. 2015 tested a wide variety of lignocellulosic waste with low-cost and biodegradable characteristics, such as hydrophilic cotton, filter paper, multipurpose cloth, sugar cane bagasse, string, or gauze as carrier to immobilization of invertase. They obtained higher yield of immobilization by using filter paper and string. In addition, the authors found that all the carriers tested were more stable than the free enzyme, indicating a good enzyme stabilization and a good production of stable derivatives. Also, biocompatibility of trypsin was tested in spent grain and corn cob powder, to establish an efficient protocol to immobilization and to production of bioactive peptides, respectively (Rocha et al. 2011; Bassan et al. 2016). Authors found that the retention of activity depends of the grade of purity of the enzyme and that it is possible to obtain hydrolyzates from cheese whey and catalytic systems with higher activities. Table 1 summarizes some of the lignocellulosic materials that have been reported as support to the immobilization of enzymes.
Cells immobilization
Immobilization of cells has been a well-known practice in the alcoholic beverage industry since the end of the last century. The immobilization of cells can be done by adsorption or entrapment, but immobilization by passive adhesion to surfaces is more preferred due to limited problems related to diffusion (Survase et al. 2012). Cells immobilization has many advantages, such as a large number of easily available catalysts, which lead to an eventual increase in the efficiency and productivity of the process, the formation or production of secondary metabolites coupled to growth, the application of anchorage-dependent cells that grow only when they are attached to surfaces, and the ease of catalytic system recovery from the medium reaction (Varavinit et al. 2001; Homaei et al. 2013b). In addition, when cells are immobilized by adsorption, they offer direct contact between nutrients and the immobilized cells, which minimizes the diffusion problems (Zhang et al. 2009). Also, the systems developed offer other advantages, such as lower susceptibility to contamination by other microorganisms, less vulnerability to the toxic substances that are present in the bulk phase and protection against pH changes, which makes the cells more tolerant to environmental changes (Harde et al. 2014; Zur et al. 2016). However, the immobilization of cells presents disadvantages, such as limited volumetric activity, difficulty in predicting changes in cellular growth, and physiological and metabolic activity mass transfer limitation due to cellular growth in the catalyst matrix (Agudelo et al. 2012b; Bornscheuer et al. 2012). System cell-waste has been used mainly in alcoholic fermentation of several carbohydrate substrates like glucose, molasses, xylose, raisin extracts, to obtain solvents such as butanol, ethanol, acetone, acetic acid and butyric acid. Some of the most used cells to develop biocatalytic systems waste-cells are:
Saccharomyces
Strains of Saccharomyces cerevisiae have been used for many years to produce fermented foods and beverages. It is the most used microorganism for the production of bioethanol from biomass (Favaro et al. 2019). Traditional ethanol fermentation is produced by free or immobilized cells. In the process with free cells, the batch bioreactor is filled with the culture medium and cells, at the end of fermentation; the fermented medium is removed from the bioreactor. While fermentation with free cells implies a decrease in productivity due to the process of filling and emptying of the bioreactor, fermentation with immobilized cells allows obtaining enhanced biological stability, higher biomass concentrations and higher process productivity (Vučurović and Razmovski 2013; Harde et al. 2014). For that, in a continuous fermentation process, cell immobilization is preferred because it facilitates the purification of extracellular products (Li et al. 2014). The use of lignocellulosic materials for Saccharomyces Cerevisiae immobilization has been demonstrated to be an interesting alternative to ethanol production, as reported by Genisheva et al. (2011) to wine elaboration by using grape skins and corn cobs. They found that the amount of cells immobilized into untreated grape skins and corn cobs was higher than that obtained to the same treated materials. Authors noted that the immobilization did not occur in a homogeny form on the material structure. It is because rough and porous structures favored cells adhesion. However, this not implies that the ethanol concentration has to be proportional to the concentration of immobilized cells, but it has the advantage that the reuse of the support provides a higher ethanol productivity (Genisheva et al. 2011).
Clostridium
Clostridium is widely used as a natural producer of biofuels and bulk chemicals. Some authors have reported the use of lignocellulosic materials to immobilization of Clostridium (see Table 2). Loyarkat et al. (2015) evaluated the production of solvents by using palm tree wastes to Clostridium beijerinckii immobilization. They found that the oil palm fronds were the most suitable support for cell immobilization and that the operation of two-stage fermentation was optimal for production of solvents with a high yield. On the other hand, Survase et al. (2012) worked with several agricultural residues to immobilization of Clostridium acetobutylicum. They obtained a process cost-effective, promoting a successful concept of wood-based bio-refinery, in which pulp produced in the process of making sugar mixture was used as carrier to cells immobilization. As can be seen, one of the most attractive properties of using lignocellulosic biomass as support to immobilization of cells is that in some cases, the materials can be sent back to the hydrolysis process, as in the case of sugar production, which minimizes waste generation (Zhang et al. 2009).
Other microorganisms
Although Saccharomyces cerevisiae is the preferred microorganism to ethanol production, its growth is slightly reduced around 35 °C. However, authors have evaluated the production of ethanol at temperature from 40 to 45 °C by using immobilized cells with the ability to assimilate sugar under high temperatures (Du Le et al. 2013). In another way, ethanol production by yeast cells immobilization has some technical barriers, which can be overcome with a correct selection of porous materials to immobilization (Gajula et al. 2011). Other systems’ cell-waste has been evaluated with biotechnological and bioremediation purposes, as the continuous production of hydrogen and fructooligosaccharides, and mesotrione biodegradation. Table 2 summarizes some of the lignocellulosic materials that have been reported as support to the immobilization of cells.
Immobilization on cellulose nanofibers
Nanocellulose or cellulose nanofibers (CNF) have several properties, such as a high aspect ratio, a low density, a very low coefficient of thermal expansion and a high tensile strength. Moreover, they exhibit some excellent properties for enzyme immobilization, including a high number of functional groups on the surface support. Depending on the production process, CNF can present different forms, such as suspensions, powders, films, hydrogels and aerogels or nanofibrous membranes (Nechyporchuk et al. 2015; Mishra et al. 2018). Nanocellulose plays an important role in biotechnologies fields, mainly in few emerging areas, including enzyme immobilization, flexible electronics, modeling of cellulosic, among others. Enzyme immobilization on cellulose derivatives, such as cellulose acetate and carboxymethyl cellulose, has been widely studied (Santos et al. 2005; Gajula et al. 2011), although it has not been efficiently studied on CNF without modifications. For instance, Sulaiman et al. (2015a) studied the development of a matrix for covalent binding immobilization of cyclodextrin glucanotransferase from B. macerans on CNF from kenaf by using an alkaline process and high-intensity ultrasonication as the raw material pretreatment. On the other hand, Mahmoud et al. (2009) studied the development of a matrix for the covalent binding immobilization of cyclodextrin glycosyl transferase and alcohol oxidase on a nanocomposite of cellulose nanocrystal from flax/gold nanoparticles; finally, Sathishkumar et al. (2014) immobilized laccase on cellulose nanofibers by covalent binding to simulate a dye effluent treatment.
Lignocellulosic waste pretreatment
Generally, biomasses can have physical and/or chemical modifications. A pretreatment step is required to increase the affinity between the carrier and the biocatalyst, thereby improving the effectiveness of immobilization. Physical pretreatments are carried out by size reduction, washing and simple sterilization using higher temperature and pressure to remove impurities and dirt (Jørgensen and Pinelo 2017). While chemical treatments involve the use of the reagents such as sodium hydroxide, sulfuric acid, hydrochloric acid, and hydrogen peroxide, among others. HCl is usually used to activate the surface of the carrier by removing the wax and destroy hemicellulose (Zhang et al. 2009). On the other hand, hydrolysis with alkaline solution is used to remove lignin by saponification of intermolecular ester bonds (hemicelluloses-lignin) (Bassan et al. 2016). In addition, a combination of high temperatures with organic solvents promotes intramolecular hydrolysis of lignin, disruption of intermolecular ester bonds, and promotes the hydrolysis of glycoside bonds of hemicelluloses (Bassan et al. 2016). In fact, the use of these treatments makes the support more porous and increased the superficial area, which creates an environment that improves immobilization. Tables 1 and 2 show some of the pretreatments used to raw material conditioning.
Functional group activation
Different chemical agents react with functional groups on the support; however, the treatments that are used require high care and selection of the appropriate parameters to carry out the reaction in the system. In a covalent interaction, it is important to use chemical coupling agents, such as ligands and spacer arms, since these can improve binding efficiency and minimize the steric hindrance to provide greater mobility. Cellulose fibers or nanofibers present great potential for covalent immobilization due to their good mechanical properties, and they are rich in hydroxyl functional groups that can be activated or modified by the use of specific reagents (Sulaiman et al. 2015b). Several protocols of activation by using agents such as 1,4-butanodiol diglycidyl ether, carbonyldiimidazole, cyanogen bromide, and 3-aminopropyltriethioxysilane, have been reported. These have been used for insertion by chemical coupling of a reactive hydroxyl group, which leads to the formation of activated groups, such as amino, carboxyl and epoxy groups, that can react with amine groups from enzyme (Gomes et al. 2005; Schaubroeck et al. 2010; Eldin Mohy et al. 2011; Wang et al. 2011; Sun et al. 2012; Elschner and Heinze 2015; Homaei 2015; Manoel et al. 2015; Zhang et al. 2016; Lou et al. 2017; Sharifi et al. 2018; Jiménez-Meneses et al. 2019). Also, lignocellulosic supports are converted into anion exchangers through contact with reagents as polyethyleneimine (Gonçalves et al. 2015). As is well known, glutaraldehyde has been widely used as a cross-linker for stabilization of the enzyme and for changing the binding of functional groups of cellulose. It can also act as a ligand agent or spacer arm between the support and the enzyme, thereby preventing steric constraints (Rocha et al. 2011; Gunda et al. 2014; Bezerra et al. 2015; De Souza et al. 2016; Zhang et al. 2016). Table 3 summarizes some of the reaction mechanisms of the interaction of chemical coupling agents.
Conclusion
In search of developing environmentally friendly systems and aiming to solve the problem of waste storage and management, the use of lignocellulosic biomass as a support for immobilization is a very competitive alternative. Lignocellulosic materials encompass the characteristics an ideal matrix. Physical and chemical biomass modifications offer an ideal environment for immobilization. Immobilization on lignocellulosic materials is attracting the interest of the scientific community in different fields, such as chemical, pharmaceutical, biotechnology, food industry and environmental. In this context, lignocellulosic biocatalysts constitute a green route for the development of more sustainable bioprocesses.
References
Agudelo LM, Salazar U, Peñuela M (2012a) Continuous production of ethanol in packed bed-bioreactors with immobilized yeast cells on lignocellulosic waste. Dyna 174:107–113
Agudelo LM, Salazar U, Peñuela M (2012b) Yeast immobilization in lignocellulosic wastes for ethanol production in packed bed bioreactor. Rev Fac Ing Univ Antioquia 62:66–76
Akalın MK, Tekin K, Karagöz S (2017) Supercritical fluid extraction of biofuels from biomass. Environ Chem Lett 15:29–41. https://doi.org/10.1007/s10311-016-0593-z
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues—wheat straw and soy hulls. Bioresour Technol 99:1664–1671. https://doi.org/10.1016/j.biortech.2007.04.029
Ansari SA, Husain Q (2012) Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol Adv 30:512–523. https://doi.org/10.1016/j.biotechadv.2011.09.005
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Asgher M, Shahid M, Kamal S, Iqbal HMN (2014) Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B Enzym 101:56–66. https://doi.org/10.1016/j.molcatb.2013.12.016
Barouni E, Petsi T, Kolliopoulos D et al (2016) Immobilized rennin in TC/SG composite in cheese production. Food Chem 200:76–82. https://doi.org/10.1016/j.foodchem.2016.01.009
Bassan J, de Souza Bezerra T, Peixoto G et al (2016) Immobilization of trypsin in lignocellulosic waste material to produce peptides with bioactive potential from whey protein. Materials (Basel) 9:357. https://doi.org/10.3390/ma9050357
Benhamou K, Dufresne A, Magnin A et al (2014) Control of size and viscoelastic properties of nanofibrillated cellulose from palm tree by varying the TEMPO-mediated oxidation time. Carbohydr Polym 99:74–83. https://doi.org/10.1016/j.carbpol.2013.08.032
Besbes I, Rei M, Boufi S (2011) Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: preparation, characteristics and reinforcing potential. Carbohydr Polym 86:1198–1206. https://doi.org/10.1016/j.carbpol.2011.06.015
Bezerra TMDS, Bassan JC, Santos VTDO et al (2015) Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochem 50:417–423. https://doi.org/10.1016/j.procbio.2014.12.009
Bilal M, Iqbal HMN (2019) Naturally-derived biopolymers: potential platforms for enzyme immobilization. Int J Biol Macromol 130:462–482. https://doi.org/10.1016/j.ijbiomac.2019.02.152
Bondancia TJ, Mattoso LH, Marconcini JM, Farinas CS (2017) A new approach to obtain cellulose nanocrystals and ethanol from eucalyptus cellulose pulp via the biochemical pathway. Am Inst Chem Eng 33:1085–1095. https://doi.org/10.1002/btpr.2486
Bornscheuer UT, Buchholz Klaus, Kasche Volker (2012) Biocatalysts and enzyme technology, 2nd edn. Wiley, Hoboken
Bouabidi ZB, El-Naas MH, Zhang Z (2018) Immobilization of microbial cells for the biotreatment of wastewater: a review. Environ Chem Lett 17:241–257. https://doi.org/10.1007/s10311-018-0795-7
Boufi S, González I, Delgado-aguilar M et al (2016) Nanofibrillated cellulose as an additive in papermaking process: a review. Carbohydr Polym 154:151–166. https://doi.org/10.1016/j.carbpol.2016.07.117
Brányik T, Vicente AA, Machado Cruz J, Teixeira J (2001) Spent grains—a new support for brewing yeast immobilisation. Biotechnol Lett 23:1073–1078. https://doi.org/10.1023/A:1010558407475
Brányik T, Vicente A, Oliveira R, Teixeira J (2004) Physicochemical surface properties of brewing yeast influencing their immobilization onto spent grains in a continuous reactor. Biotechnol Bioeng 88:84–93. https://doi.org/10.1002/bit.20217
Brígida AIS, Pinheiro ADT, Ferreira ALO, Gonçalves LRB (2008) Immobilization of Candida antarctica lipase B by adsorption to green coconut fiber. Appl Biochem Biotechnol 146:173–187. https://doi.org/10.1007/s12010-007-8072-4
Brígida A, Pinto D, Silveira M et al (2009) Immobilization of Candida antarctica lipase B on NaOCl/NaOH treated green coconut fiber by adsorption. New Biotechnol 25:S135. https://doi.org/10.1016/j.nbt.2009.06.450
Cao L (2005) Immobilised enzymes: science or art? Curr Opin Chem Biol 9:217–226. https://doi.org/10.1016/j.cbpa.2005.02.014
Chapman J, Ismail AE, Dinu CZ (2018) Industrial applications of enzymes: recent advances, techniques, and outlooks. Catalysts 8:20–29. https://doi.org/10.3390/catal8060238
Chen W, Yu H, Liu Y et al (2011) Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr Polym 83:1804–1811. https://doi.org/10.1016/j.carbpol.2010.10.040
Da Silva AM, Tavares APM, Rocha CMR et al (2012) Immobilization of commercial laccase on spent grain. Process Biochem 47:1095–1101. https://doi.org/10.1016/j.procbio.2012.03.021
Datta S, Christena LR, Rajaram YRS (2012) Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3:1–9. https://doi.org/10.1007/s13205-012-0071-7
De Castro HF, De Lima R, Roberto C (2001) Rice straw as a support for immobilization of microbial lipase. Biotechnol Prog. https://doi.org/10.1021/bp010099t
De Souza TC, Fonseca TDS, Jessyca A et al (2016) Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: application to the chemoenzymatic production of (R)-Indanol. J Mol Catal B Enzym 130:58–69. https://doi.org/10.1016/j.molcatb.2016.05.007
Deepa B, Abraham E, Mathew B et al (2011) Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour Technol 102:1988–1997. https://doi.org/10.1016/j.biortech.2010.09.030
Dey G, Nagpal V, Banerjee R (2002) Immobilization of alpha-amylase from Bacillus circulans GRS 313 on coconut fiber. Appl Biochem Biotechnol 102–103:303–313. https://doi.org/10.1385/ABAB:102-103:1-6:303
Dhabhai R, Chaurasia SP, Dalai AK (2012) Efficient bioethanol production from glucose-xylose mixtures using co-culture of Saccharomyces cerevisiae immobilized on canadian pine wood chips and free Pichia stipitis. J Biobased Mater Bioenergy 6:594–600. https://doi.org/10.1166/jbmb.2012.1253
Di S, Yan N (2009) Adsorption and inactivation behavior of horseradish peroxidase on cellulosic fiber surfaces. J Colloid Interface Sci 338:410–419. https://doi.org/10.1016/j.jcis.2009.07.005
Du Le H, Thanonkeo P, Le VVM (2013) Impact of high temperature on ethanol fermentation by Kluyveromyces marxianus immobilized on banana leaf sheath pieces. Appl Biochem Biotechnol 171:806–816. https://doi.org/10.1007/s12010-013-0411-z
Duan L, Wang H, Yu W (2013) Preparation and characterization of cellulose nanofibers from jute using blender combined with chemical pretreatments. Adv Mater Res 651:408–413. https://doi.org/10.4028/www.scientific.net/AMR.651.408
Eldin Mohy MS, Seuror EI, Nasr MA, Tieama HA (2011) Affinity covalent immobilization of glucoamylase onto ρ-benzoquinone-activated alginate beads: II. Enzyme immobilization and characterization. Appl Biochem Biotechnol 164:45–57. https://doi.org/10.1007/s12010-010-9113-y
Elschner T, Heinze T (2015) Cellulose carbonates: a platform for promising biopolymer derivatives with multifunctional capabilities. Macromol Biosci 15:735–746. https://doi.org/10.1002/mabi.201400521
Erdman P, Kulpinski K, Olejnik A (2016) Application of nanocomposite cellulose fibers with luminescent properties to paper functionalization. Cellulose 23:2087–2097. https://doi.org/10.1007/s10570-016-0943-9
Favaro L, Jansen T, van Zyl WH (2019) Exploring industrial and natural Saccharomyces cerevisiae strains for the bio-based economy from biomass: the case of bioethanol. Crit Rev Biotechnol 39:800–816. https://doi.org/10.1080/07388551.2019.1619157
Gajula C, Chandel AK, Konakalla R et al (2011) Fermentation of groundnut shell enzymatic hydrolysate for fuel ethanol production by free and sorghum stalks immobilized cells of Pichia stipitis NCIM 3498. Int J Chem React Eng. https://doi.org/10.1515/1542-6580.2514
Genisheva Z, Mussatto SI, Oliveira JM, Teixeira JA (2011) Evaluating the potential of wine-making residues and corn cobs as support materials for cell immobilization for ethanol production. Ind Crops Prod 34:979–985. https://doi.org/10.1016/j.indcrop.2011.03.006
Gericke D, Ott D, Matveeva VG et al (2015) Green catalysis by nanoparticulate catalysts developed for flow processing? Case study of glucose hydrogenation. RSC Adv 5:15898–15908. https://doi.org/10.1039/C4RA14559C
Gomes FM, Silva GS, Pinatti DG et al (2005) Wood cellulignin as an alternative matrix for enzyme immobilization. Appl Biochem Biotechnol 121–124:255. https://doi.org/10.1385/ABAB:121:1-3:0255
Gonçalves HB, Jorge JA, Guimarães LHS (2015) Immobilization of Fusarium graminearum β-d-fructofuranosidase using alternative cellulosic supports: stabilization and production of fructooligosaccharides. Food Sci Biotechnol 24:1429–1435. https://doi.org/10.1007/s10068-015-0183-z
Gregolin G, Suelen G, Ruiz P (2014) Biosorption potential of synthetic dyes by heat-inactivated and live Lentinus edodes CCB-42 immobilized in loofa sponges. World J Microbiol Biotechnol 30:3229–3244. https://doi.org/10.1007/s11274-014-1750-9
Gunda NSK, Singh M, Norman L et al (2014) Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl Surf Sci 305:522–530. https://doi.org/10.1016/j.apsusc.2014.03.130
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38:453–468. https://doi.org/10.1039/b711564b
Harde SM, Bankar SB, Ojamo H et al (2014) Continuous lignocellulosic ethanol production using Coleus forskohlii root hydrolysate. Fuel 126:77–84. https://doi.org/10.1016/j.fuel.2014.02.046
Homaei A (2015) Enhanced activity and stability of papain immobilized on CNBr-activated sepharose. Int J Biol Macromol 75:373–377. https://doi.org/10.1016/j.ijbiomac.2015.01.055
Homaei AA, Sariri R, Vianello F, Stevanato R (2013) Enzyme immobilization: an update. J Chem Biol 6:185–205. https://doi.org/10.1007/s12154-013-0102-9
Jahan MS, Rahman MM (2012) Effect of pre-hydrolysis on the soda-anthraquinone pulping of corn stalks and Saccharum spontaneum (kash). Carbohydr Polym 88:583–588. https://doi.org/10.1016/j.carbpol.2012.01.005
Jahan MS, Saeed A, He Z, Ni Y (2011) Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 18:451–459. https://doi.org/10.1007/s10570-010-9481-z
Jiménez-Meneses P, Bañuls MJ, Puchades R, Maquieira Á (2019) Novel and rapid activation of polyvinylidene fluoride membranes by UV light. React Funct Polym 140:56–61. https://doi.org/10.1016/j.reactfunctpolym.2019.04.012
Jonoobi M, Harun J, Shakeri A et al (2009) Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 4:626–639. https://doi.org/10.15376/biores.4.2.626-639
Jørgensen H, Pinelo M (2017) Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod Biorefin 11:150–167. https://doi.org/10.1002/bbb
Kajiwara S, Komatsu K, Yamada R et al (2019) Modification of lipase from Candida cylindracea with dextran using the borane-pyridine complex to improve organic solvent stability. J Biotechnol 296:1–6. https://doi.org/10.1016/j.jbiotec.2019.02.009
Khalil HPSA, Davoudpour Y, Islam MN et al (2014a) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99:649–665. https://doi.org/10.1016/j.carbpol.2013.08.069
Khalil HPSA, Hossain MS, Rosamah E et al (2014b) High-pressure enzymatic hydrolysis to reveal physicochemical and thermal properties of bamboo fiber using a supercritical water fermenter. BioResources 9:7710–7720. https://doi.org/10.15376/biores.9.4.7710-7720
Khalil HPSA, Davoudpour Y, Saurabh CK et al (2016) A review on nanocellulosic fibres as new material for sustainable packaging: process and applications. Renew Sustain Energy Rev 64:823–836. https://doi.org/10.1016/j.rser.2016.06.072
Khoshnevisan K, Vakhshiteh F, Barkhi M et al (2017) Immobilization of cellulase enzyme onto magnetic nanoparticles: applications and recent advances. Mol Catal 442:66–73. https://doi.org/10.1016/j.mcat.2017.09.006
Kim TG, Park TG (2006) Surface functionalized electrospun biodegradable nanofibers for immobilization of bioactive molecules. Biotechnol Prog 22:1108–1113. https://doi.org/10.1021/bp060039t
Kumar N, Das D (2001a) Electron microscopy of hydrogen producing immobilized E. cloacae IIT-BT 08 on natural polymers. Int J Hydrogen Energy 26:1155–1163. https://doi.org/10.1016/S0360-3199(01)00061-1
Kumar N, Das D (2001b) Continuous hydrogen production by immobilized Enterobacter cloacae IIT-BT 08 using lignocellulosic materials as solid matrices. Enzyme Microb Technol 29:280–287. https://doi.org/10.1016/S0141-0229(01)00394-5
Kumar A, Swarnalatha S, Kamatchi P et al (2009) Immobilization of proteolytic enzyme on highly porous activated carbon derived from rice bran. J Porous Mater 16:439–445. https://doi.org/10.1007/s10934-008-9216-9
Li T, Chen X, Chen J et al (2014) Open and continuous fermentation: products, conditions and bioprocess economy. Biotechnol Biotechnol Equip 9:1503–1511. https://doi.org/10.1002/biot.201400084
Liu J, Chen S, Ding J et al (2015) Sugarcane bagasse as support for immobilization of Bacillus pumilus HZ-2 and its use in bioremediation of mesotrione-contaminated soils. Appl Microbiol Biotechnol 99:10839–10851. https://doi.org/10.1007/s00253-015-6935-0
Lou R, Yu W, Song Y et al (2017) Fabrication of stable galactosylated alginate microcapsules via covalent coupling onto hydroxyl groups for hepatocytes applications. Carbohydr Polym 155:456–465. https://doi.org/10.1016/j.carbpol.2016.08.098
Loyarkat S, Cheirsilp B, Prasertsan P (2015) Two-stage repeated-batch fermentation of immobilized Clostridium beijerinckii on oil palm fronds for solvents production. Process Biochem 50:1167–1176. https://doi.org/10.1016/j.procbio.2015.04.016
Lv JS, Liu XY, Xu JX et al (2013) Preparation and properties of adsorption material from corn stalks core when used for enzyme immobilization and the subsequent activities of the adsorbed enzymes. Ind Crops Prod 50:787–796. https://doi.org/10.1016/j.indcrop.2013.08.068
Mahmoud DAR (2007) Immobilization of invertase by a new economical method using wood sawdust waste. Aust J Basic Appl Sci 1:364–372
Mahmoud KA, Male KB, Hrapovic S, Luong JHT (2009) Cellulose nanocrystal/gold nanoparticle composite as a matrix for enzyme immobilization. ACS Appl Mater Interfaces 1:1383–1386. https://doi.org/10.1021/am900331d
Manoel EA, dos Santos JCS, Freire DMG et al (2015) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57. https://doi.org/10.1016/j.enzmictec.2015.02.001
Mateo C, Palomo JM, Fernandez-lorente G et al (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. https://doi.org/10.1016/j.enzmictec.2007.01.018
Mehta J, Bhardwaj N, Bhardwaj SK et al (2016) Recent advances in enzyme immobilization techniques: metal-organic frameworks as novel substrates. Coord Chem Rev 322:30–40. https://doi.org/10.1016/j.ccr.2016.05.007
Mishra RK, Mishra P, Verma K et al (2018) Electrospinning production of nanofibrous membranes. Environ Chem Lett 17:767–800. https://doi.org/10.1007/s10311-018-00838-w
Mohamad NR, Marzuki NHC, Buang NA et al (2015) An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip 29:205–220. https://doi.org/10.1080/13102818.2015.1008192
Moniruzzaman M, Goto M (2016) Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem Eng J. https://doi.org/10.1016/j.bej.2016.01.021
Morin-Crini N, Lichtfouse E, Torri G, Crini G (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17:1667–1692. https://doi.org/10.1007/s10311-019-00904-x
Mussatto SI, Aguilar CN, Rodrigues LR, Teixeira JA (2009) Fructooligosaccharides and B-fructofuranosidase production by Aspergillus japonicus immobilized on lignocellulosic materials. J Mol Catal B Enzym 59:76–81. https://doi.org/10.1016/j.molcatb.2009.01.005
Nakagaito AN, Ikenaga K, Takagi H (2015) Cellulose nanofiber extraction from grass by a modified kitchen blender. Mod Phys Lett B 29:1–4. https://doi.org/10.1142/S0217984915400394
Nechyporchuk O, Belgacem MN, Bras J (2015) Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2016.02.016
Pérez-Rodríguez N, García-Bernet D, Domínguez JM (2018) Faster methane production after sequential extrusion and enzymatic hydrolysis of vine trimming shoots. Environ Chem Lett 16:295–299. https://doi.org/10.1007/s10311-017-0668-5
Plessas S, Bekatorou A, Koutinas AA et al (2007) Use of Saccharomyces cerevisiae cells immobilized on orange peel as biocatalyst for alcoholic fermentation. Bioresour Technol 98:860–865. https://doi.org/10.1016/j.biortech.2006.03.014
Pospiskova K, Safarik I (2012) Magnetically modified spent grain as a low-cost, biocompatible and smart carrier for enzyme inmobilisation. J Sci Food Agric 93:1598–1602. https://doi.org/10.1002/jsfa.5930
Pouyet F, Lachenal D, Das S, Chirat C (2013) Minimizing viscosity loss during totally chlorine-free bleaching of hardwood kraft pulp. BioResources 8:238–249. https://doi.org/10.15376/biores.8.1.238-249
Rahmati H, Ebrahimi P, Sedghi M (2010) Effect of cooking conditions and oxygen-delignification on Bambusa tulda kraft pulping. Indian J Chem Technol 17:74–77
Raman K, Henning P (2013) Environmental assessment of enzyme use in industrial production—a literature review. J Clean Prod 42:228–240. https://doi.org/10.1016/j.jclepro.2012.11.005
Ramírez-Montoya LA, Hernández-Montoya V, Montes-Morán MA, Cervantes FJ (2015) Correlation between mesopore volume of carbon supports and the immobilization of laccase from Trametes versicolor for the decolorization of Acid Orange 7. J Environ Manage 162:206–214. https://doi.org/10.1016/j.jenvman.2015.07.035
Rangel SX, García J, Orrego CE (2011) Inmovilización de lipasa de candida antarctica sobre soportes de quitosano-gelatina. Rev Colomb Química 40:149–164
Reddy N, Yang Y (2005) Properties and potential applications of natural cellulose fibers from cornhusks. Green Chem 7:190–195. https://doi.org/10.1039/b415102j
Riansa-ngawong W, Suwansaard M, Prasertsan P (2012) Application of palm pressed fiber as a carrier for ethanol production by Candida shehatae TISTR5843. Electron J Biotechnol 15:1–12. https://doi.org/10.2225/vol15-issue6-fulltext-1
Rocha C, Goncalves MP, Teixeira JA (2011) Immobilization of trypsin on spent grains for whey protein hydrolysis. Process Biochem 46:505–511. https://doi.org/10.1016/j.procbio.2010.10.001
Rodríguez-couto S (2014) Decolouration of industrial metal-complex dyes in successive batches by active cultures of Trametes pubescens. Biotechnol Rep 4:156–160. https://doi.org/10.1016/j.btre.2014.10.006
Saha P, Chowdhury S, Roy D et al (2016) A brief review on the chemical modifications of lignocellulosic fibers for durable engineering composites. Polym Bull 73:587–620. https://doi.org/10.1007/s00289-015-1489-y
Salleh S, See YS, Serri NA et al (2016) Synthesis of butyl butyrate in 93% yield by Thermomyces lanuginosus lipase on waste eggshells. Environ Chem Lett 14:189–194. https://doi.org/10.1007/s10311-016-0553-7
Santos JC, Pinto IRG, Carvalho W et al (2005) Sugarcane bagasse as raw material and immobilization support for xylitol production. Appl Biochem Biotechnol 121–124:673–683. https://doi.org/10.1385/ABAB:122:1-3:0673
Sassolas A, Blum LJ, Leca-Bouvier BD (2012) Immobilization strategies to develop enzymatic biosensors. Biotechnol Adv 30:489–511. https://doi.org/10.1016/j.biotechadv.2011.09.003
Sathishkumar P, Kamala-kannan S, Cho M et al (2014) Enzymatic Laccase immobilization on cellulose nanofiber: the catalytic efficiency and recyclic application for simulated dye effluent treatment. J Mol Catal B Enzym 100:111–120. https://doi.org/10.1016/j.molcatb.2013.12.008
Schaubroeck D, De Baets J, Desmet T et al (2010) Surface modification of an epoxy resin with polyamines via cyanuric chloride coupling. Appl Surf Sci 256:6269–6278. https://doi.org/10.1016/j.apsusc.2010.04.003
Schröder P, Beckers B, Daniels S et al (2018) Intensify production, transform biomass to energy and novel goods and protect soils in Europe—a vision how to mobilize marginal lands. Sci Total Environ 616–617:1101–1123. https://doi.org/10.1016/j.scitotenv.2017.10.209
Sharifi M, Robatjazi SM, Sadri M, Mosaabadi JM (2018) Covalent immobilization of organophosphorus hydrolase enzyme on chemically modified cellulose microfibers: statistical optimization and characterization. React Funct Polym 124:162–170. https://doi.org/10.1016/j.reactfunctpolym.2018.01.019
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307. https://doi.org/10.1002/adsc.200700082
Sheldon RA, Van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev Chem Soc Rev 42:6223–6235. https://doi.org/10.1039/c3cs60075k
Sheldon RA, Woodley JM (2018) Role of biocatalysis in sustainable chemistry. Chem Rev 118:801–838. https://doi.org/10.1021/acs.chemrev.7b00203
Sheldon RA, Schoevaart R, Van Langen LM (2009) Cross-linked enzyme aggregates (CLEAs): a novel and versatile method for enzyme immobilization (a review). Biocatal Biotransform 23:141–147. https://doi.org/10.1080/10242420500183378
Silva SS, Mussatto SI, Santos JC et al (2007) Cell immobilization and xylitol production using sugarcane bagasse as raw material. Appl Biochem Biotechnol 141:215–227. https://doi.org/10.1007/BF02729063
Singh D, Gupta N (2020) Microbial Laccase : a robust enzyme and its industrial applications. Biologia (Bratisl). https://doi.org/10.2478/s11756-019-00414-9
Spence KL, Venditti RA, Pawlak JJ (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18:1097–1111. https://doi.org/10.1007/s10570-011-9533-z
Srivastava N, Srivastava M, Manikanta A et al (2017) Nanomaterials for biofuel production using lignocellulosic waste. Environ Chem Lett 15:179–184. https://doi.org/10.1007/s10311-017-0622-6
Sulaiman S, Mokhtar MN, Naim MN et al (2015a) Study on the preparation of cellulose nanofibre (CNF) from kenaf bast fibre for enzyme immobilization application. Sains Malays 44:1541–1550
Sulaiman S, Mokhtar MN, Naim MN et al (2015b) A review: potential usage of cellulose nanofibers (CNF) for enzyme immobilization via covalent interactions. Appl Biochem Biotechnol 175:1817–1842. https://doi.org/10.1007/s12010-014-1417-x
Sun L, Liang H, Yuan Q et al (2012) Study on a carboxyl-activated carrier and its properties for papain immobilization. J Chem Technol Biotechnol 87:1083–1088. https://doi.org/10.1002/jctb.3714
Survase SA, Van Heiningen A, Granström T (2012) Continuous bio-catalytic conversion of sugar mixture to acetone-butanol-ethanol by immobilized Clostridium acetobutylicum DSM 792. Appl Microbiol Biotechnol 93:2309–2316. https://doi.org/10.1007/s00253-011-3761-x
Thangaraj B, Solomon PR (2019) Immobilization of lipases—a review. Part II: carrier materials. ChemBioEng Rev 6:167–194. https://doi.org/10.1002/cben.201900017
Torán J, Blánquez P, Caminal G (2017) Comparison between several reactors with Trametes versicolor immobilized on lignocellulosic support for the continuous treatments of hospital wastewater. Bioresour Technol 243:966–974. https://doi.org/10.1016/j.biortech.2017.07.055
Torres JA, Nogueira FGE, Silva MC et al (2017) Novel eco-friendly biocatalyst: soybean peroxidase immobilized onto activated carbon obtained from agricultural waste. RSC Adv 7:16460–16466. https://doi.org/10.1039/c7ra01309d
Tripathi A, Sami H, Jain SR et al (2010) Improved bio-catalytic conversion by novel immobilization process using cryogel beads to increase solvent production. Enzyme Microb Technol 47:44–51. https://doi.org/10.1016/j.enzmictec.2010.03.009
Tursi A (2019) A review on biomass: importance, chemistry, classification, and conversion. Biofuel Res J 6:962–979. https://doi.org/10.18331/BRJ2019.6.2.3
Uetani K, Yano H (2011) Nanofibrillation of wood pulp using a high- speed blender. Biomacromolecules 12:348–353. https://doi.org/10.1021/bm101103p
Vaghari H, Jafarizadeh-Malmiri H, Mohammadlou M et al (2016) Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol Lett 38:223–233. https://doi.org/10.1007/s10529-015-1977-z
Varavinit S, Chaokasem N, Shobsngob S (2001) Covalent immobilization of a glucoamylase to bagasse dialdehyde cellulose. World J Microbiol Biotechnol 17:721–725. https://doi.org/10.1023/A:1012984802624
Velasco-Lozano S, López-Gallego F, Mateos-Díaz JC, Favela-Torres E (2016) Cross-linked enzyme aggregates (CLEA) in enzyme improvement—a review. Biocatalysis 1:166–177. https://doi.org/10.1515/boca-2015-0012
Velasco-Lozano S, da Silva ES, Llop J, López-Gallego F (2018) Sustainable and continuous synthesis of enantiopure l-amino acids by using a versatile immobilised multienzyme system. ChemBioChem 19:395–403. https://doi.org/10.1002/cbic.201700493
Verma ML, Kumar S, Das A et al (2019) Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ Chem Lett. https://doi.org/10.1007/s10311-019-00942-5
Vučurović V, Razmovski R (2013) Alcoholic fermentation by yeast immobilized on maize stem disks filled with Ca-alginate. Rom Biotechnol Lett 18:8873–8882
Wang A, Zhang F, Chen F et al (2011) A facile technique to prepare cross-linked enzyme aggregates using p-benzoquinone as cross-linking agent. Korean J Chem Eng 28:1090–1095. https://doi.org/10.1007/s11814-010-0476-0
Yu J, Yue G, Zhong J et al (2010) Immobilization of Saccharomyces cerevisiae to modified bagasse for ethanol production. Renew Energy 35:1130–1134. https://doi.org/10.1016/j.renene.2009.11.045
Zhang Y, Ma Y, Yang F (2009) Continuous acetone–butanol–ethanol production by corn stalk immobilized cells. J Ind Microb Biotechnol 36:1117–1121. https://doi.org/10.1007/s10295-009-0582-3
Zhang D, Hegab HE, Lvov Y et al (2016) Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus. https://doi.org/10.1186/s40064-016-1682-y
Zhu H, Luo W, Ciesielski PN et al (2016) Wood-derived materials for green electronics, biological devices, and energy applications. Chem Rev 116:9305–9374. https://doi.org/10.1021/acs.chemrev.6b00225
Zur J, Wojcieszynska D, Guzik U (2016) Metabolic responses of bacterial cells to immobilization. Molecules 21:958. https://doi.org/10.3390/molecules21070958
Acknowledgements
The authors gratefully acknowledge COLCIENCIAS, for the doctoral scholarship awarded in the frame of the Colombian National Program for Doctoral Formation 727-2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Restrepo, Y.A., Orrego, C.E. Immobilization of enzymes and cells on lignocellulosic materials. Environ Chem Lett 18, 787–806 (2020). https://doi.org/10.1007/s10311-020-00988-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-00988-w