Abstract
Highly porous activated carbon (HPAC) was used as carrier matrix for immobilization of acid protease (AP). Immobilization of acid protease on mesoporous activated carbon (AP-HPAC) performs as best enzyme carrier. At pH 6.0, 250 mg acid protease g−1 HPAC was immobilized. The optimum temperature for both free and immobilized AP activities were 50 °C. After incubation at 50 °C, the immobilized AP maintained about 50% of its initial activity, while the free enzyme was completely inactivated. When testing the reusability of AP-HPAC combination immobilized system, a significant catalytic efficiency was maintained along more than five consecutive reaction cycles. The highly porous nature of the carbon permits significant higher loadings of enzyme, which results in a higher enzyme-support strength and increased stability. The changes in the AP, HPAC and AP-HPAC were confirmed by Fourier Transform Infrared spectroscopy (FT-IR). Furthermore, scanning electron microscopy (SEM) allowed us to observe that the morphology of the surface of HPAC and the AP-HPAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Immobilization of free enzymes on a variety of water-insoluble supports facilitates product and enzyme separation, improves biocatalysts stability, paves the way for their reuse and application in continuous operations, with a positive consequence on the process economy [1]. Supports suitable for practical applications should maintain a high level of enzyme activity while preventing a possible leaching out during the reaction [2]. Though numerous techniques had been used to immobilize enzymes, several approaches have been explored for the preparation of immobilized enzymes because of its advantages over enzymes in bulk solution [3, 4].
Thus, immobilization leads to ease of recoverability and reusability of the enzymes [5]. In fact, for large-scale production, the process would be more economical if the immobilized enzyme could be reused [6]. Moreover, these systems may increase the stability of enzymes to thermal treatments and extremes of pH [7]. Immobilization of enzymes has been carried out by entrapment, ionic interaction, covalent attachment, encapsulation and adsorption onto hydrophobic or hydrophilic surfaces. Among these methods, adsorption has been considered as a simple and an economical mechanism for immobilization [8]. Moreover, enzyme immobilization by adsorption has the benefit of wide applicability and may provide relatively small perturbation of the enzymes native structure and function, which contributes to the maintenance of enzyme activity [9]. Various solid matrices such as cellulose, polyamide nonwoven materials [10], glass beads [11], polymer [12], magnetite [13] and zeolite [14] have been considered for immobilization of protease. The studies concluded that enzymes in inorganic carriers are more stable than that attached with organic polymers. Previous researchers proved that porous materials have favoring features for immobilization compared to non porous materials owing to their pore size, large surface area, pore volume and opened structures [15–17]. Therefore, in the present investigation the feasibility for immobilization of protease onto inorganic highly porous activated carbon matrix has been explored.
The acid proteases are a family of enzymes involved in a number of important biological processes. As the acid proteases have been extensively used in biotechnology and pharmaceutical industry [18], the acid protease immobilization appears to be a very important one. Beside the numerable properties of protease, it was considered essential to investigate the potential of its immobilization on porous activated carbon.
2 Experimental
2.1 Synthesis of the high porous activated carbon (HPAC)
High porous activated carbons were prepared in two sequential steps: pre-carbonization and chemical activation. For the pre-carbonization process, rice husk was packed in an air-tight crucible and heated to 400 °C for 4 h. The pre-carbonized carbon has been treated with hydrofluoric acid at 80 °C in order to render it as highly porous in nature. It was further activated using chemical activation method with the ortho phosphoric acid in the ratio 1:4.5 and followed by heating at 800 °C, at a heating rate of 5–7 °C/min using a programmer and maintained at the final temperature for 1 h before cooling.
2.2 Characterization of the HPAC
The surface area and pore size distribution were derived from the N2 adsorption–desorption isotherms. The N2 adsorption–desorption isotherms of activated carbons were measured using an automatic adsorption instrument (Quantachrome Corp. Nova-1,000 gas sorption analyzer). Prior to measurement, HPAC was degassed at 150 °C overnight. The nitrogen adsorption–desorption data was recorded at liquid nitrogen temperature 77 K. The surface area of the activated carbons was calculated using BET equation, which is the most widely used model for determining the specific surface area (m2/g). The pore size distribution was determined using BJH method. In addition, the t-plot method [19] was applied to calculate the micropore volume and external surface area (mesoporous surface area).
The C, H, N content of the different heat-treated carbons were determined using CHNS 1108 model Carlo–Erba analyzer.
The X-ray diffraction (XRD) experiments were performed with a Philips X’pert diffractometer for 2θ values from 10 to 80° using Cu Kα radiation at a wavelength of λ = 1.54060 Å. The other experimental conditions included 1/2° divergence slits, a 5-s residence time at each step, and intensity measured in counts.
2.3 Acid protease (AP) immobilization on HPAC
The acid protease was produced from tannery solid wastes and extracted using ammonium sulphate precipitation as described by our previous observations [20]. The HPAC particles were added to the enzyme solution (100, 150, or 200 U enzymes per 1 mL phosphate buffer at pH 6.0) and the immobilization reaction was carried out in a shaking water bath for 4 h. Particles were separated and the unbounded enzyme was removed. The immobilized enzyme was used freshly and stored at 4 °C between reuses.
2.4 Determination of immobilized protein amount
Hundred milligrams protease/mL of phosphate buffer at pH 6.0, added to the solutions of the mesoporous activated carbon particles (100 mg/mL), and stirred for 24 h at low temperature to avoid microbial growth. The surplus of non-adsorbed enzyme is then removed by centrifugal. The amount of non-adsorbed enzyme was determined spectroscopically from the eluate, which in turn gives the amount of bound enzyme.
2.5 Protein assay
The amount of proteins immobilized on a substrate was determined on a sample prepared in parallel to that used for enzymatic analysis. Proteins were detached from HPAC by acid hydrolysis. Samples were incubated overnight at room temperature with automatic stirring in a solution of 50 mM phosphate buffer and 2 N HCl [21].
2.6 Protease activity assay
Protease activity was determined using casein as the substrate. Enzyme solution (0.5 mL) was added to 3.0 mL of substrate solution (0.6% casein in 20 mM Phosphate buffer, pH 6.0) and the mixture was incubated at 37 °C for 20 min. The reaction was terminated by the addition of 3.0 mL of trichloro acetic acid mixture, and the mixture was kept at room temperature for 30 min followed by filtration through Whatman filter paper No. 1. The absorbance of the filtrate was measured at 280 nm. One unit of protease activity is defined as the amount of enzyme required to liberate 1μg of tyrosine per min under optimized conditions.
2.7 Determination of kinetic parameters
To determine the maximum reaction rate (Vmax) and the Michaelis–Menten constant (Km), the activity assay was applied for different substrate (caesin) concentrations. The caesin solutions (0.05/0.1/0.2/0.3/0.4/0.5 g/L) were prepared in phosphate buffer (pH 6.0) and kept in a water bath at 37 °C for 5 min, and then the immobilized protease or free enzyme solution was added to the test tubes and shaken for different incubation times.
2.8 Determination of the storage stability of free and immobilized AP
The activity of the free and immobilized enzyme was measured after stored in phosphate buffer (50 mM, pH 6.0) at 4 °C for 40 days. The remaining percentage of immobilized enzyme activity was calculated in each determination.
2.9 FT-IR studies
A Perkin-Elmer infrared spectrometer was used for the investigation of the surface functional groups. The samples were mixed with KBr of spectroscopic grade and made into pellets at a pressure of about 1 MPa. The pellets were about 10 mm in diameter and 1 mm in thickness. The samples were scanned in the spectral range of 4,000–400 cm−1.
2.10 Scanning Electron Microscopy
The surface morphology of HPAC and acid protease immobilized HPAC (AP-HPAC) were determined using Leo-Jeol Scanning Electron Microscope at the magnification of 2,500–10,000×. The carbon samples were coated with gold by a gold sputtering device for the clear visibility of the surface morphology.
3 Results and discussion
3.1 Characterization of the carbon
The Characterization of the HPAC such as surface area, pore size, elemental composition was given in Table 1. The XRD analysis of HPAC contains only the amorphous structure of carbon as a hump at 2θ angle of 22.5° (Results not shown). It was observed that the crystalline phases were absent [22, 23].
3.2 Immobilization of acid protease on the high porous activated carbon (AP-HPAC) support
Table 2 shows the activity parameters of the free and immobilized protease under the optimum reaction conditions of pH 6.5 and temperature 50 °C. The amount of bound protein was 250 mg/g, without any negative effect on the expressed activity. In comparison with the free enzyme, the immobilized protease under its optimum reaction condition retains 75% of its activity.
3.3 Effect of reaction pH and temperature on enzyme activity
The effect of pH on the activity of the free and the immobilized protease was assayed in the pH range of 5.0–7.5 at temperature 50 °C. The immobilized enzyme was stable from pH 5.5 to 7.0, whereas more fluctuations in the activity of free enzymes around the mentioned pH (Fig. 1). The enzyme molecules possess a multiplicity of ionizable groups. The state of these ionizable groups depend much on the extracellular pH. Therefore, the microenvironment of the immobilized enzyme on the carbon particles might have been buffered and immobilized enzyme was less affected from the acidity of the solution. The immobilized protease revealed acceptable pH stabilities over a broad range of experiments.
The effect of temperature on the activity of free and immobilized acid protease was tested in the temperature range of 20–80 °C at pH 6.0. It was found that the optimum temperatures for free and immobilized acid protease were obtained at 50 °C (Fig. 2). The rate of enzymatic reactions increases with increase in reaction temperature upto optimum and decreases thereafter. The denaturation of protein leads to disarrangement in the native structures and its active sites at temperatures above 50 °C, which led the enzyme inactive.
3.4 Evaluation of kinetic parameters
The activities of free and immobilized enzymes for casein concentrations were plotted in Lineweaver–Burk graph, from which maximal activities (Vmax) and Michealis–Menten constants (Km) values were calculated (Fig. 3). Lineweaver-Burk plots of the free and immobilized acid protease gave Km (Michaelis constant) of 10 mg mL−1 and 12 mg mL−1, respectively, with caesin. The Vmax (the maximum reaction rate) of the free and immobilized acid protease were 3.6 and 4.1 U mg−1 protein, respectively (Table 3). The apparent Michealis–Menten constant for protease was increased after immobilization. The increase in Km value after immobilization could also be by multiple fixation of the enzyme by the effect of binding forces leading to an increase in the flexibility of the enzyme molecule, which is reflected by an increase in catalytic activity. The other possibility of increasing Km value may be due to the unaltered secondary structure of the immobilized enzyme [24]. The increase in Km value and increase in Vmax after enzyme immobilization have also been reported by other investigators [25].
3.5 Storage stability
For storage and application of the immobilized enzyme systems in the physiological environment, it is important to examine the storage stability. Enzymes are not stable during storage in solutions and their activities decrease gradually by the time. The storage stability was investigated by measuring the enzyme activities at certain time intervals and the results are given in Fig. 4. After 10 days, there were tiny differences between the activities of the immobilized enzyme and the free enzyme. There was a significant decrease in the activity of the immobilized and free enzyme over 25 days period, but immobilized enzyme provided a prominence advantage in stability over free enzyme, especially at longer durations.
3.6 Operational stability of immobilized enzymes
Operational stability of free and immobilized enzymes are presented in Table 4. The activities of acid protease were much higher on HPAC than free enzyme. Also, one can see in table 4 that, using HPAC as a support, the activity of acid protease was greater up to 15 times of enzyme use. Only at 20 cycles of use, did the activity of protease show a lower value. The activities of these enzymes were constant (100%) up to 10 cycles of use. It is worth stating that even after using these enzymes for 20 cycles, their activities retained more than 60% of the initial values, respectively, which may be considered good. Although various researchers stated that immobilized enzymes could be reused up to 50% of their initial activity [26, 27], in our case it would be important to keep their activity near 80%. No report concerning the operational stability of acid protease was found in the literature.
Other positive aspects of immobilization of enzymes on solid supports have been mentioned by Paolo et al. [28], such as high concentration and even distribution of the protease enzyme, enhancing its operational stability. The immobilization imparts stability to proteins by restricting the movement of the protein molecule by attachment via chemical bonds to activated carbon [29]. The various domains are therefore held in the correct orientation to retain activity at least over an extended period of time when compared with enzymes in free solution.
3.7 Reusability of HPAC supports
The HPAC matrix can be reused by washing with hot distilled continuously until the pH of the water reaches 7.0. The washed HPAC sample was dried at 110 °C overnight. The dried HPAC can be activated using phosphoric acid treatment.
3.8 Fourier transform infrared spectra (FT-IR)
The infrared spectra of activated carbon has a wide band at about 3,400–3,425 cm−1 due to O–H stretching mode of hexagonal groups and adsorbed water. The position and symmetry of this band at lower wave numbers indicate the presence of strong hydrogen bonds. The C–H stretching for activated carbon is observed at 2,925 cm−1 due to aliphatic groups. The peak at about 470, 795 and 1,100 cm−1 confirmed the presence of phosphate bridges arises due to the phosphoric acid activation. The peak centered at 1,583 cm−1 corresponds to the asymmetric stretching of carboxylic functional group present in HPAC. The presence of carboxylic functional group present in HPAC is useful for the effective immobilization. The FT-IR spectrum of protease contains two broad bands near 3,200–3,400 cm−1 and is due to N–H stretching frequencies. This can be assigned to primary amines, as there are two bands owing to N–H asymmetric and N–H symmetric stretching. A band near 1,600 and 1,620 cm−1 corresponds to N–H inplanar bending while two sharp bands at 1,400 and 1,135 cm−1 correspond to C–N stretching frequencies. A sharp band at 613 cm−1 corresponds to the C–H out of plane bending. Amide I and amide II in protease enzyme are centered at 1,660 and 1,528 cm−1 respectively. In particular, in the first region, bands ranging from 1,725 to 1,660 cm−1 are assigned to β-sheet and from 1,660 to 1,623 cm−1 are allocated to α- helix. The FTIR of immobilized enzyme shows a minor shift near 3,000 cm−1, which indicates stronger bonding of protease with the functional groups of activated carbon. The N–H stretching frequency in the region 3,200–3,500 cm−1 is observed in enzyme immobilized activated carbon. The band corresponding to 1,400 and 1,700 cm−1 was due to C–N stretching vibrations in the enzyme immobilized carbons. A large percentage of α-helix structure is observed to have a decrease in band centered at about 1,623 and 1,725 cm−1. An increase in β-sheet region 1,660 cm−1 with prominent peak is observed in enzyme immobilized carbon (AP-HPAC). This behavior arises from a strong interaction between carbon and enzyme. The infrared spectra of adsorbed enzyme reveals that there is an increase in the amide I band 1,660 cm−1 and amide II band 1,528 cm−1 are masked (Fig. 5).
As the effective immobilization of the enzyme with HPAC takes place through the functional group (carboxylic) of HPAC and amide group of Protease, this matrix has got lots of scope for the immobilization of the other enzymes. The adsorption of enzymes was also based on the magnitude of charges between the HPAC and the enzymes. At acidic pH, the carbon surface is positively charged and at alkaline pH the carbon surface is negatively charged [30].
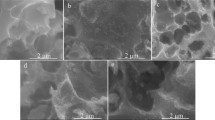
3.9 Scanning electron microscopy (SEM)
Surface morphology of HPAC is shown in Fig. 6a and the micrograph clearly shows the presence of highly porous nature and differences in pore sizes due to chemical activation by hydrofluoric acid. It was made very clear that the opening of the pores in the surface of the rice husk should be due to the extraction of some materials so as to create, upon activation, micro- and mesopores in the carbon components. As a result of the creation of pores, there was an increase in both the surface area and the pore volume, which were stably created in the activated carbon. The surface morphology of the enzyme immobilized HPAC is shown in Fig. 6b. The enzyme is also located at the outer pore surface area of activated carbon. It is seen in the micrograph that the enzymes are well bound to the inner wall of the pores in the carbon matrix.
4 Conclusions
The effectiveness of an immobilization process depends mainly on the carrier matrix used. The immobilization on HPAC carried out at pH 6.0 with 4 h shaking gave rise to the highest immobilization yield. The immobilized AP showed broader pH, temperature tolerance and improved storage stability. The FT-IR analysis of peak position and their relative intensities after adsorption confirmed that the enzyme conformation and orientation remains unvaried. Furthermore, using a scanning electron micrograph, it was observed that the HPAC has large contact sites for enzyme immobilization. This may be evidence for a higher amount of AP loading. The increase in stability and the high activity shown by AP-HPAC would be encouraging for its choice in industrial applications.
References
B.A. Jarzebski, S.S. Katarzyna, B. Jolanta, M.B. Julita, Cat. Today 124, 2 (2007)
N.E. Kotel’nikova, S.A. Mikhailova, E.N. Vlasova, Russian J. Appl. Chem. 80, 322 (2007)
G.A. Vikhoreva, K.P. Khomyakov, I.Yu Sakharov, L.S. Galbraikh, Fibre Chem. 27, 337 (1996)
A.R. Sheldon, Adv. Synth. Catal. 349, 1289 (2007)
B. Zhao, B. Shi, R. Ma, Eng. Life Sci. 5, 436 (2005)
A.S. Rani, M.L.D. Das, S. Satyanarayana, J. Mol. Catal. B 10, 471 (2000)
L. Furegon, A.D.B. Peruffo, A. Curioni, Proc. Biochem. 32, 113 (1996)
B. Al-Duri, Y.P. Yong, J. Biochem. Eng. 4, 207 (2000)
N. Durán, M.A. Rosa, A. D’annibale, L. Gianfreda, Enzyme Microb. Technol. 31, 907 (2002)
K. Moeschel, M. Nouaimi, C. Steinbrenner, H. Bisswanger, Biotech. Bioeng. 82, 190 (2003)
H.J. Chae, M.J. In, E.Y. Kim, Appl. Biochem. Biotech. 73, 195 (1998)
C.J.S.M. Silva, Q. Zhang, J. Shen, A.C. Paulo, Enzyme Microb. Technol. 39, 634 (2006)
P.F. Yang, C.K. Lee, Biochem. Eng. J. 37, 108 (2007)
A.X. Yan, X.W. Li, Y.H. Ye, Appl. Biochem. Biotech. 101, 113 (2002)
K.L. Lie, H.L.C. Lina, T.W. Keng, Clin. Biochem. 35, 181 (2002)
X.S. Zhao, X.Y. Bao, W. Guo, F.Y. Lee, Mat. Today 9, 32 (2006)
J.S. Macedo, L. Otubo, O.P. Ferreira, I.F. Gimenez, I.O. Mazali, L.S. Barreto, Micro. Meso. Mat. 107, 276 (2008)
D. Spelzini, B. Farruggia, G. Pico, J. Chromatograph. B 821, 60 (2005)
S.J. Gregg, K.S.W. Sing, Academic Press, London (1982)
A. Ganesh Kumar, N. Nagesh, T.G. Prabhakar, G. Sekaran, Biores. Technol. 99, 2364 (2008)
L. Blasi, L. Longo, G. Vasapollo, R. Cingolani, R. Rinaldi, T. Rizzello, R. Acierno, M. Maffia, Enzyme Microb. Technol. 36, 818 (2005)
Y. Chang, Y.H. Park, C.R. Park, Carbon 39, 559 (2001)
L. John Kennedy, J. Judith Vijaya, G. Sekaran, Ind. Eng. Chem. Res. 43, 1832 (2004)
S. Jang, D. Kim, J. Choi, K. Row, W. Ahn, J. Porous. Mater. 13, 385 (2006)
L. Zhongli, B. Shuxian, Enzyme Microb.Technol. 40, 1442 (2007)
Y.F. Li, F.Y. Jia, J.R. Li, G. Liu, Y.Z. Li, Biotechnol. Assl. Biochem. 33, 29 (2001)
H. Lin, H. Wang, C. Xue, M. Ye, Enz. Microb. Technol. 31, 588 (2002)
B. Paolo, D.A. Alessandro, G. Carlo, G. Patrizia, N.P. Ana Sofia, J. Mol. Cat. B Enzymatic. 41, 61 (2006)
A. Kilinç, S. Önal, A. Telefoncu, Turk. J. Chem. 26, 311 (2002)
L. John Kennedy, J. Judith Vijaya, K. Kayalvizhi, G. Sekaran, Chem. Eng. J. 132, 279 (2007)
Acknowledgements
The author A. Ganesh Kumar is thankful to Council of Scientific and Industrial Research (CSIR), Central Leather Research Institute (CLRI), Sathyabama University, India, for awarding a Research Fellowship and providing the facilities needed to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganesh Kumar, A., Swarnalatha, S., Kamatchi, P. et al. Immobilization of proteolytic enzyme on highly porous activated carbon derived from rice bran. J Porous Mater 16, 439–445 (2009). https://doi.org/10.1007/s10934-008-9216-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-008-9216-9