Abstract
The dead biomass of Aspergillus niger was studied as biosorbent for removing Zn(II) and Cr(VI) from wastewater. The surface characteristics of the biomass were evaluated based on the point of zero charge (PZC), identification of adsorption sites and structure by scanning electron microscopy. The adsorption capacity was determined by kinetic studies and adsorption equilibrium. The results showed that the PZC is between pH 4.4 and 4.6. At pH values below the PZC, the adsorption of anions occurs by electrostatic attraction. For pH values above the PZC, the surface is negatively charged, and cations are removed. The adsorption of metals by the dead biomass fits the pseudo-first and pseudo-second-order models. Moreover, the equilibrium followed the Langmuir and Freundlich models when the adsorbate concentrations were lower than 50 mg/L, whereas it followed only the Freundlich model at concentrations above 50 mg/L. The biosorption process was characterized by the prevalence of chemical forced between the functional groups present on the biomass surface and the metallic ions. Adsorption capacities of Zn(II) and Cr(VI) were 3.833 and 4.997 mg/L, respectively. Therefore, the biomass of A. niger shows potential application as a biosorbent in the removal of these metal ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium and zinc are among the most toxic metals that are found in effluents of mining, as well as electroplating, anti-corrosive and leather industries. Tri- and hexavalent chromium are pollutants, however, chromium (VI) has greater potential pollution and is more toxic than chromium (III), and is carcinogenic, mutagenic, teratogenic and bioaccumulate in the food chain (WHO 1998). Zinc in small concentrations is essential to the metabolism of cells, but at concentrations above 5 mg/L is toxic to humans, causing cumulative poisoning, cancer, brain damage, skin irritation, anaemia, stomach pain, etc. (Agrawal et al. 2004; Sousa 2007).
For a long time, the removal of toxic metals from industrial wastewaters was carried out through physical–chemical processes. However, from the 1970s, the biological processes for removing such ions for adsorption, known as biosorption, were widely used (Sag et al. 1998; Nagda et al. 2006). This is because the lower sludge production and lower operational costs than those of physical–chemical treatment. Many microorganisms, including bacteria, algae and fungi have the capacity of removing heavy metals from the environment (Vilar et al. 2007; Lee and Chang 2011; Javaid et al. 2011; Akar et al. 2012). This ability, as well as the mechanisms of accumulation, varies with the microbial species or strain, and occur due to binding of the metal ions to functional groups present in the cell wall or on the membrane surface of microorganisms by: complexation on the cell surface, ion exchange, physical adsorption or microprecipitation (Veglio and Beolchini 1997; Gupta et al. 2000). The biosorption of the metal ions is affected by factors other than the specific properties of the surface of the cell wall such as physical–chemical properties of the medium, pH, temperature, presence of nutrients, adsorbent dosage and equilibrium time (Kapoor et al. 1999; Pagnanelli et al. 2003; Das Nilanjana et al. 2008; Bayramoglu et al. 2009; Wang et al. 2010; Fomina and Gadd 2014).
The most important factor responsible for biosorption by fungi is the cell wall area and properties, which contains functional groups that serve as active sites for metal ions (Bowman and Free 2006; Ramrakhiani et al. 2011). Filamentous fungi present hyphae that are able to form pellets when subjected to ideal conditions of orbital shaking (Liao et al. 2007). This pellets comprised long and fibrous hyphae, randomly arranged in an entangled net, with high biomass density and surface area that improves mass transfer and diffusion of the surrounding metal ions solution into pellets (Moreira et al. 1996). Therefore, the ions have more chances of contact with the active sites inside the pellet, increasing the capacity of biosorption (Fu and Viraraghavan 2002; Mungasavalli et al. 2007; Arunakumara and Xuecheng 2008; Borràs et al. 2008). Among the filamentous fungi, Aspergillus niger can be used for producing pelletised biomass due to the composition of its cell wall that contains carboxyl, amine, and phosphate groups, which are responsible for the adsorption of metals (Hajahmadi et al. 2015).
The objective of this work is to evaluate the use the dead biomass of pelletised A. niger for removing Cr(VI) and Zn(II) from synthetic industrial wastewaters by biosorption, and to study the equilibrium and kinetics of the process.
Materials and methods
Pellets production
The fungal species used in this study was A. niger isolated from the effluent of a Brazilian oil refinery (Petróleo Brasileiro SA). The isolation was performed by plating and identified on the basis of the classical taxonomic keys. After identification, the fungus A. niger was subcultured on plates containing Sabouraud dextrose agar (65 g/L) and 1 mL/L of nutrient solution (EDTA, 10.0 g/L; ZnSO4·7H2O, 4.4 g/L; MnCl2·4H2O, 1.0 g/L; CoCl2·6H2O, 0.3 g/L; CuSO4·5H2O, 0.3 g/L; (NH4)6·Mo7O24·4H2O, 0.2 g/L; CaCl2·2H2O, 1.5 g/L; FeSO4·7H2O, 1.0 g/L). The plates were incubated for 3 days at 30 ± 2 °C for sporulation. Following, the spores were removed from plates and used for producing a Tween 80 suspension solution, which was stored in test tubes.
The pellets were produced by cultivating the suspension of spores in a culture medium comprised of 8.0 g/L of the previously mentioned nutrient solution and 0.05 g/L of chloramphenicol (antibiotic). Subsequently, this medium was autoclaved for 15 min at 121 ± 2 °C. A volume of 100 mL of the autoclaved culture medium and 2.0 × 106 spores of A. niger were added to 10 Erlenmeyer flasks of 250 mL that were sealed with hydrophobic cotton and incubated at 28 °C for 72 h under orbital shaking (120 rpm). The pellets, with diameter in the range of 3–5 mm, were washed with distilled water, autoclaved during 30 min at 121 °C and dried in an oven during 24 h at 50 °C. Next, the biomass was macerated and used for determining the adsorption sites, point of zero charge (PZC) and for the adsorption experiments. For scanning electron microscopy, the pellets were merely washed and then sieved in order to avoid changes in the fungal structure.
The determination of the adsorption sites of metal ions was carried out using the Fourier Transform Infrared Spectroscopy (FTIR) (Mukhopadhya 2008). The morphological characterization of the pellets, before and after contact with a solution of 50 mg/L of Zn(II) during 12 h, was carried out by using a scanning electron microscope (Model DSM 940 A, Zeiss).
Biosorption tests
The two stock solutions containing 1000 mg/L of Zn (II) and Cr(VI) were prepared using reagent grade Zn(NO3)2 and K2Cr2O7. The tests solution comprised of different concentrations of the stock solution and a buffer (C8H5KO4). The Initial pH was varied from 2.5 to 6.0 using HCl 0.1 mol/L or NaOH 0.1 mol/L.
The optimal conditions for biosorption of Cr(VI) and Zn(II) were determined in batch tests based on the adsorption equilibrium at different pH values, on the initial concentration and on the equilibrium time. Each condition was evaluated independently for Zn(II) and for the Cr(VI).
The biosorption capacity, which is the amount of adsorbed material per mass of adsorbent (Q), was calculated using the Eq. (1) (Hameed et al. 2007). The removal efficiency of metals was calculated based on Eq. (2).
where Q is the biosorption capacity (mg/g), C 0 is the initial adsorbate concentration (mg/L), C e is the equilibrium (final) adsorbate concentration (mg/L), V is the working volume of each batch test (L), m is the mass of adsorbent (g), % removal is the removal efficiency in %.
The determination of Zn(II) remaining in the solutions was performed by flame atomic-absorption spectrophotometry (Fast Sequential Atomic Absorption Spectrometer-Model AA240FS-Varian). The determination of the residual Cr(VI) was carried out by molecular absorption spectrophotometry (UV–Vis) using 1.5-diphenylcarbazide as chromogenic agent. The determination of Zn(II) and Cr(VI) followed the procedure described in Eaton et al. (2005).
The effect of pH on the biosorption of Zn(II) and Cr(VI) was evaluated for values between 2.5 and 6.0 under orbital shaking condition (120 rpm) during 24 h. Each flask contained 20 g/L of biomass and 50 ml of the stock solution (Zn(II) or Cr(VI)). After 24 h, the volume is the flask was filtered and the residual concentration of the metal was determined. The effect of the concentration of biosorbent was evaluated for values between 0 and 40 g/L. The stock solution Zn(II) at pH 5.0 or Cr(VI) at pH 2.5. The point of zero charge (PZC) was determined using the method of Oliveira and co-authors (Oliveira et al. 2008), adapted from Valdes and co-authors (Váldes et al. 2002), based on the pH curve as a function of mass.
All the analytical determinations were based on Standard Methods for the Examination of Water and Wastewater (Eaton et al. 2005). All necessary care with the preparation of solutions and handling of the fungus were taken according to the standard operating procedure established in the laboratory where the work was done.
Biosorption kinetics
The biosorption kinetics of Zn(II) and Cr(VI) were determined in two batch experiments comprised of 20 flasks. Each flask contained 10 or 20 mg of biosorbent, and 50 ml of the stock solution Zn(II) at pH 5.0 or Cr(VI) at pH 2.5. The tests were performed under orbital shaking condition (120 rpm) during a time ranging from 10 to 1440 min. The results of the tests were evaluated based on a pseudo-first-order model (Eq. 3) and a pseudo-second-order model (Eq. 4) (Shek et al. 2009).
where, Q e is the adsorbate concentration in dry biomass at equilibrium (mg/g); Q t is the adsorbate concentration in dry biomass at time “t” (mg/g); K 1 is the rate constant of pseudo-first-order reactions (min−1) and K 2 is the rate constant of pseudo-second-order reactions (g/mg min).
Adsorption isotherms
The adsorption isotherms were generated based on the results of adsorption capacity (Q e) and equilibrium concentration of adsorbent (C e). The tests were performed in batch experiments, where the flasks contained 50 ml of a solution of Zn (II) or Cr(VI) with concentrations ranging between 10 and 1000 mg/L. The biosorbent mass was 20 g for the tests with zinc and 10 g for the tests with chromium. The flasks were sealed with hydrophobic cotton and incubated at 28 °C for 1440 min under orbital shaking (120 rpm). The equilibrium adsorption isotherms were described using the classical models of Langmuir (Eq. 5) and Freundlich (Eq. 6) (Ruthven 1984; Cooney 1999).
where Q max is the maximum adsorption capacity of the adsorbent for metal (mg/g), b is the affinity of the adsorbent by the metal (L/mg), P is an indicator of the adsorption capacity of the adsorbent (L/mg) and n is a constant related to the strength of adsorption (Bingol et al. 2009).
The determination of the models that best fit the experimental data of Cr(VI) and Zn(II) biosorption were based on the normalized percent deviation (P) (Crisafully et al. 2008). The main characteristics of Languir isotherm can be described by the dimensionless separation factor, or the equilibrium parameter, that is expressed by Eq. (7) (Bingol et al. 2009).
where R L is the separation factor, b is the Langmuir constant (L mg−1), and C 0 is the initial concentration (mg L−1) R L indicates the adsorption process to be either favourable (0 < R L < 1), favourable (R L > 1), linear (R L = 1) or irreversible (R L < 0) (Bingol et al. 2009; Chowdhury et al. 2011).
Results and discussion
FTIR spectroscopy of the adsorption site
The infrared spectra of the A. niger biomass, before and after the biosorption of the Cr(VI) and Zn(II), are shown in Fig. 1. The bands in the spectra were assigned to different functional groups and bonds based on their respective wavelengths (Mungasavalli et al. 2007; Pandey et al. 2008, 2009; Sun et al. 2009; Amini et al. 2009; Akar et al. 2009). The classification of the functional groups according to their characteristic wavelength is presented in Table 1.
The band at 3406 cm−1 represents the asymmetric stretching of the NH2 groups (Bai and Abraham 2002; Aftab et al. 2013), which are present in proteins and chitin contained in ketamines fractions in the cell wall of fungi (Bai and Abraham 2002; Fischer et al. 2006; Sun et al. 2009), and have been identified as functional groups that may be involved in the biosorption of Cr(VI) (Park et al. 2005; Lameiras et al. 2008). The curve at the bottom of this band indicates the presence of hydroxyl (–OH) groups, which are also present in ketamines of the fungal cell wall, as well as in glucose molecules (Bai and Abraham 2002; Fischer et al. 2006; Sun et al. 2009). Mungasavalli et al. (2007) had also observed bands in this region in their studies on A. niger biomass for the biosorption of chromium. These authors observed that the stretching of the OH group also occurs due to the presence of carboxylic acids (–COOH), which can be identified from very broad bands in the range of 3430–3400 cm−1.
The band at 2925 cm−1 represents CH stretching in the carboxyl groups of aliphatic compounds (Saygideger et al. 2005). The band at 2135 cm−1 is characteristic of C≡C groups in alkynes (Dudley and Fleming 1995).
Other major bands at 1647 and 1558 cm−1 were also identified, representing primary and secondary amides, respectively; and at 3406 cm−1 for the amine stretching –NH and hydroxyl group (–OH). All the functional groups corresponding to these bands were responsible for the sequestration of different metals by the biomass (Mungasavalli et al. 2007). The weak-intensity band observed at 1406 cm−1 was identified as symmetric deformation band of a carboxylate anion (–COO–). The band at the wavelength of 2368 cm−1 is characteristic of the presence of CO2. This band was not taken into consideration in this study because it is associated with sample contamination due to CO2 in the environment.
Phosphate (-PO4 3−) is identified in the spectrum of absorption bands at 1080 and 1035 cm−1 (Mungasavalli et al. 2007). These groups are present mainly in glycoproteins of the cell membrane of fungi and are important for the biosorption of cations, since they exhibit a negative charge at a pH above 3 (Sanghi et al. 2009).
The functional groups like amides (–NH2), phosphate (PO4 3−), carboxyl (–COOH), and hydroxyl (–OH) are responsible for the sequestration of metals, such as Cr, Th, Mo, V, Ni, by fungal biomass (Tobin et al. 1984). Another band at 601 cm−1 in the spectrum indicates the presence of amines and amides, and represents the angular deformation of the NH bond out of plane. The region between 700 and 900 cm−1 is regarded as the fingerprint zone of microorganisms (Fischer et al. 2006).
The presence of all these absorption bands confirms the ability of the fungus in removing metals from solution, since the metal ions in solution bind to these functional groups, either through complex formation or electrostatic attraction. Amine groups are mainly responsible for the removal of Cr(VI) as chromate, predominantly in the forms of Cr2O7 2−, HCrO4−, and Cr2O4−, from acidic solutions (Bai and Abraham 2002; Mungasavalli et al. 2007; Congeevaram et al. 2007). The negatively charged chromate ion is electrostatically attracted to the positively charged protonated amine present in the cell wall of A. niger. However, metal cations are removed primarily by carboxyl and hydroxyl groups (Deepa et al. 2006).
The presence of amine, carboxyl, and phosphate groups suggests that the biosorbent is composed primarily of chitin and glucan. The cell wall of the fungus can be considered as a mosaic of different functional groups that act as coordination complexes in the presence of metal ions (Sanghi et al. 2009).
Comparing the FTIR spectra before and after biosorption of Zn(II) and Cr(VI) (Fig. 1), it can be observed a decrease in intensity of all absorption bands. The effect of the biosorption was more intense at 3406, 1647, 1080 and 1035 cm−1, identified as hydroxyl, amines, carboxyl and phosphates groups, respectively. It has been reported that the hydroxyl groups are primary involved in the biosorption of metal cations, such as Zn(II). These functional groups have negative charges that interact with the positive charges of the metal ion, promoting biomass-metal bond (Pandey et al. 2008; Amini et al.,2009).
Bands in the low wavelength region (<800 cm−1) were identified in the biomass spectra after biosorption of Zn(II). According to Akar et al. (2009), rounded bands in this region can be attributable to the interaction of Zn(II) and nitrogen-containing bioligands. There is a substantial decrease in the intensity of the bands in 1647 cm−1 (amide) and 3406 cm−1 (ν NH amine) after biosorption of Cr(VI) by biomass, which is an indication that these groups may be responsible for the biosorption of chromate ions in the biomass (Bai and Abraham 2002).
Morphological characterization of biomass by scanning electron microscopy
The surface morphology of the fungal pellet is shown in the scanning electron micrograph in Fig. 2. The pellets of A. niger have long hyphae and abundant fibrous surface structures. On the basis of the arrangement of hyphae, it is possible to infer that the pellets have avoided and heterogeneous morphology, which can thus facilitate the diffusion of substrates (Papagianni 2004). This is because the tangles formed by the hyphae create empty spaces within the pellet and increase its specific surface area (Bayramoglu and Arica 2008). These surface properties can be considered as the core factors responsible for the metal ions removal. Similar morphological characteristics were also observed in other fungi such as Penicillium chrysogenum and Lentinos edodes. In studies on metal ions removal using the mycelia of A. niger, Amini et al. (2008) observed that the inner surface of the mycelium appeared to have multi-layered walls, which was not observed in the micrographs obtained in our study (Fig. 3).
Figure 3 shows the micrograph of a pellet of A. niger after contact with a solution of 50 mg/L of Zn(II) at pH 5.0. A comparison of the micrographs obtained before the biosorption of zinc (Fig. 2) and after the experiment (Fig. 3) reveals an increase in the thickness of the hyphae and a deformation on the surface. This phenomenon occurred probably due to the binding of Zn(II) to the functional groups on the biomass, such as carboxyl and hydroxyl ions, which were identified by performing infrared spectroscopy. Moreover, the experiment was carried out at pH 5, which induces a negative charge on the surface of the biomass, facilitating the biosorption capacity of the metal cations.
Adsorption tests of the ions Cr(VI) and Zn(II)
The effect of pH
Figure 4 shows the results of the effect of pH on the removal of Zn(II) and Cr(VI) by the dead biomass of A. niger. Changes in pH of the solution showed significant differences on the biosorption of the metals. The removal efficiency of Zn(II) increased with the pH until 5.0, achieving 24.9 %. At pH >5, Zn biosorption percentage decreased by 18 %, probably due to precipitation of the metal species in the salt form or metal species for hydration at alkaline pH (Fan et al. 2008). According to Carrott et al. (1996), at pH values below 7.0 there are the two kinds of zinc ion: Zn(II) that predominates and Zn(OH)+ in small quantities. Therefore, in this work, the optimum pH for removal of zinc was the predominant species Zn(II). The pH increase caused a decrease in the removal efficiency of Cr(VI), and the optimum value was 2.5, achieving 98.7 %. For pH values between 2.0 and 6.0, the predominant species of chromium are HCrO4−and Cr2O7 2−. According to Bingol et al. (2009), the ionic species HCrO4− is predominant in values ranging from pH 2.0 to 4.0. These ions are converted to Cr2O7 2− at increasing pH until values around 8.0; and from this point, the CrO4 2− prevails. At pH values below 2.0 the polymerized species of chromium oxides are formed (Cr3O10 2− and Cr4O13 2−) (Karthikeyan et al. 2005; Kumar et al. 2008).
PZC is defined as the pH in which the surface of the fungal biomass has a neutral charge and remains constant independent of initial pH, behaving as a buffer. In this work, the convergence curves of pH as a function of the mass indicates that the PZC values are between 4.4 and 4.6 (Fig. 5). The removal of metal ions depends on the charge on the surface of the adsorbent. The surface charge is neutral in the PZC and ion adsorption is minimal. For pH values below the PZC, the surface is positively charged and anion adsorption takes place by electrostatic attraction. Above the PZC, the surface is negatively charged and the cations are removed from the reaction solution as they are adsorbed onto the surface of the biomass. The pH optimum for the removal of Zn(II), 5.0, by dead biomass of A. niger, found in this work are close to those found by Sag et al. (1998) who studied the removal of Zn(II) by dead biomass of Rhizopus arrhizus and found optimum pH between 4.0 and 5.0.
The PZC study shows that at pH 5.0 the surface of the biomass is negatively charged and promotes the adsorption of Zn(II). At pH 6.0 the percentage removal of Zn (II) decreases to 18 % (Fig. 4). Javaid et al. (2011) also observed that at pH higher than 5, the biosorption of Zn(II) by fungal biomass decreased. This is probably due to the precipitation of metal species in salt form or to the hydration of the metal species at alkaline pH (Fan et al. 2008). At very low pH (<2.0), with a positive surface charge, metal cations and protons compete for binding sites, thus reducing the biosorption capacity of Zn(II) (Fig. 4) (Sari and Tuzen 2009). Yun-Guo et al. (2006) emphasize the existence of competition between H+ and the metal cation for the adsorption site.
According to Fig. 5, at pH 2.5 the surface is positively charged and adsorption of anionic species occurs. However, in this work, the biosorption tests with Cr(VI) were carried out in solutions with pH ranging from 2.5 to 6.0 to avoid the formation of H2CrO4. At pH below 2.0 the predominant form is the molecular (H2CrO4) and Cr(VI) will be adsorbed as anion. The interaction will initially take place between the oppositely charged sites of the biosorbent with the species in solution. And at pH above 6, the overall surface charge of the biomass becomes increasingly negative, which will hinder the biosorption of anions in the active sites on the surface of the biomass due to competition with the OH− ions (Gupta and Babu 2009). Therefore, it is more likely that HCrO4 − ionic species has been preferentially adsorbed by the biomass. Javaid et al. (2011) found the same optimum pH (2.5) for removal of Cr(VI) using Pleurotus ostreatus biomass.
The effect of the adsorbent concentration
Figure 6 shows the removal efficiency of Zn(II) and Cr(VI) as a function of the adsorbent concentration. It was observed that higher removals of Zn(II) (36 %) and Cr(VI) (99.1 %) were achieved using 20 and 10 g adsorbent/L, respectively. Higher concentrations did not caused any increase in the removal efficiency, indicating saturation of the adsorption sites in the biomass. Better performance in metals removal at a higher biomass dosage is attributed to the higher number of available adsorption sites. But this phenomenon only occurs until a threshold point in the adsorbance rate curve, from where it starts declining with increasing biosorbent concentration. This is because the fungal biomass became swelled up and, at high concentration, the biomass active sites became very close to each other, causing an electrostatic interaction among these sites, which generates a shield, known as “shell effect”, that hinders further occupation by the metal ions. The same phenomenon was observed by Romera et al. (2007) and Al-Homaidan et al. (2014). Therefore, the amount of adsorbent significantly influenced the extent of metals biosorption.
Adsorption kinetics
The effect of the contact time
In the biosorption systems, the time required for reaching equilibrium is a function of several factors such as number and types of functional groups involved in biosorption, size and shape of the particles, biomass condition (live or dead), as well as the metal ion to be adsorbed (Wang and Qin 2006).
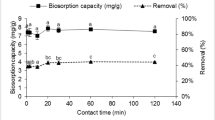
Figure 7 shows the removal efficiency of Zn(II) and Cr(VI) as a function of the contact time. Most of the adsorption occurred within the first 10 min of contact and equilibrium was reached after 180 min of the experiment. The high adsorption rate observed at the beginning of the experiment can be explained by the higher availability of empty active sites that are able to adsorb metal ions. However, it was not possible to distinguish which type of mechanism (physisorption or chemisorption) was prevalent at the very beginning of the experiment, i.e. within the first 10 min, as no samples were taken during this period. Therefore, only the data obtained after 10 min will be considered for discussion on the effect of the contact time. After this rapid step, the biosorption occurred slowly, especially in the outer surface layer of the biosorbent (Chen et al. 2005). With the gradual occupation of these sites the adsorption rate decreases along the experimental time.
Kinetic models
Table 2 shows the values of biosorption capacity (Q), reaction rate constant (k) and correlation coefficients (R 2) obtained by the linear regression of the experimental data, using the pseudo-first-order and the pseudo-second-order models. It can be observed that the values of biosorption capacity for the pseudo-first-order model (Q 1) and for the pseudo-second-order model (Q 2) are consistent with the experimental values of biosorption capacity (Q exp). The curve of contact time of the ions is shown in Fig. 7 and it indicates that there are two phases: the first, very quick, which was not possible to classify according to the involved forces, followed by chemical adsorption represented by the slow phase. Jinshao et al. (2010) observed that biosorption of Cr(VI) using microbial biomass also occurred in two steps, quick phase followed by a slow phase. The physical mechanisms involve weaker forces such as the electrostatic forces, while the chemical mechanisms involve reactions that share electrons through valence forces or ion exchange between adsorbent and adsorbate (Rangabhashiyam and Selvaraju 2015; Mohan et al. 2006).
The determination of the model that best fits the experimental data of Cr(VI) e Zn(II) biosorption was based on the normalized percent deviation (P), given by Eq. (8) (Kapoor and Yang 1989; Ncibi 2008; Crisafully et al. 2008). The main advantage of this error function is the minimization of the fractional error distribution throughout the studied biosorbent concentration range (Kapoor and Yang 1989; Ncibi 2008). According Ayranci and Hoda (2005), a model fits the experimental data when the value of P is <5, and the smaller the P value, the better the fit of the calculated results to the experimental data.
where P is the normalized percent deviation (%), Q exp and Q m are the experimental and modelled biosorption capacities, respectively, and N is the number of observations.
The kinetic parameters obtained from experimental (Q exp) and modeled data (Q 1 and Q 2) and the normalized percent deviations are shown in Table 2. The of pseudo-second-order model for biosorption of Cr(VI) and Zn (II) show better correlation coefficient (R 2) than pseudo-first-order models. However, using the normalized percent deviations for the error evaluation, we found that the kinetics of the pseudo-second-order model (Q 2) is more adapted to the biosorption of Zn(II), while the pseudo-model first-order model (Q 1) is more suitable for Cr(VI). The results obtained for Cr(VI) are contradictory with the majority of the investigations, i.e. the kinetics of the pseudo-second-order is that best describes the biosorption process of this metal (Mungasavalli et al. 2007; Khambhaty et al. 2009).
The experimental Q and P values (Table 2) indicates that the complete Cr(VI) biosorption process follows a pseudo-first-order model, while the Zn(II) biosorption follows a pseudo-second-order model. However, according to Shek et al. (2009), the reaction of pseudo-first-order (Lagergren) is generally more suitable for early stages of the adsorption process, i.e. the first 30 min of contact between adsorbent and adsorbate, as physical processes occur first. After this high adsorption rate, the pseudo-second-order reaction (Freundlich) fits better, as chemical reaction occurs slower. In this study, most of the metal ions were rapidly adsorbed by the biomass within the first 10 min of contact, and then the remaining was absorbed slowly until the end of the assay. Probably, the mechanism of absorbance is a complex process including physical and chemical forces, as proposed by (Khambhaty et al. 2009; Liu and Shen 2008; Vilar et al. 2007).
Adsorption isotherms
Generally, the equilibrium is described by an isotherm equation whose parameters express the surface properties and affinity of the adsorbent at fixed pH and temperature (Ghaedi et al. 2013). Figure 8a, b show the curves obtained by the experimental adsorption isotherms of Zn (II) and Cr(VI), respectively, using the isotherms model of Langmuir and Freundlich. Table 3 presents the Langmuir and Freundlich constants (b and 1/n, respectively) obtained from the isotherms, as well as the correlation coefficients and the maximum biosorption capacity (Q max) by the live and dead biomass. The adsorption capacity (Q max) was obtained by the Langmuir model: 3833 mg Zn(II)/g with pH 5.0 after 24 h at 28 ± 2 °C and 4.997 mg Cr(VI)/g with pH 2.5 after 24 h at 28 ± 2 °C. When low concentrations of adsorbate (<50 mg/L) are used, the adsorption of Zn (II) and Cr(VI) can be represented by both the Langmuir isotherm model and the Freundlich model. At adsorbate concentrations above 50 mg/L the Freundlich model prevailed. This is an indication that adsorption occurs at the surface of heterogeneous adsorption sites and the sites with higher bond strength are occupied first (Davis et al. 2003).
The isotherm of Zn(II) showed a sigmoidal shape and it fits in the class of isotherm type S. This type of curve shows low initial adsorption, and increases as the number of adsorbed molecules increases. This phenomenon occurs probably due to a cooperative adsorption between the adsorbent molecules (Gilles et al. 1974). The experimental isotherm of Cr(VI) was classified as L-4 by Giles et al. (1974), representing a multilayer adsorption.
The dead biomass of A. niger had a higher affinity for Cr(VI) than for Zn(II) and values of correlation coefficients reported in Table 3 for the adsorption of Zn (II) and Cr(VI) by biomass are generally above of 0.90 indicating good correlation of the data with the two isotherm models. However, analysing the Fig. 8a, b, it is found that at concentrations lower than 50 mg/L is dominated by two isotherm models, and concentrations >50 mg/L, the prevailing model Freundlich indicating that adsorption occurs at the surface of heterogeneous adsorption sites and the sites with higher bond strength are occupied first (Davis et al. 2003). The R L values found for biosorption of Zinc (II) and Cr(VI) by the dead biomass of A. niger are all in the range between 0 and 1. This is a indication that the biosorption is favorable.
Biosorption capacity of fungal biomass varies according to the different metals, fungus species and experimental condition, e.g. pH, biomass concentration, metal concentration and the use of a pre-treatment for enhancing biomass surface ions (Fomina and Gadd 2014). Other types of materials such as olive oil mill solids residues and potato peels were used as biosorbent for removal of Zn(II) and Cr(VI) solutions, with adsorption capacity of 5.6 and 3.2 mg/g, respectively (Hawari et al. 2009; Mutongo et al. 2014), which are similar to the values found in this work.
Conclusions
The biosorption of Zn(II) and Cr(VI) in biomass of A. niger was influenced by pH conditions and the initial concentration of metal and biosorbent. The pH directly influences the surface charge of the biosorbent. The Zn(II) showed higher biosorption capacity at pH 5 in the form of Zn2+, whereas Cr(VI) showed higher biosorption capacity at pH 2.5 as an anion. Optimal biosorbent dosages were 20 and 10 g/L for Zn(II) and Cr(VI), respectively. The biosorption kinetics of Cr(VI) follows the pseudo-first-order model, whilst the kinetics of Zn(II) follows the pseudo-second-order model. A biosorption is favoured by the fibrous structure of the pellets surface, as well as the presence of functional groups such as hydroxyl, carboxylic, phosphates and amine. At biosorbent concentrations lower than 50 mg/L, the biosorption curves of Zn(II) and Cr(VI) fit to the Langmuir and Freundlich isotherm models, whilst at higher concentrations only the Freundlich model represents the biosorption process. Therefore, the use of A. niger biomass as biosorbent for removal of Zn(II) and Cr(VI) in wastewater is feasible.
References
Aftab K, Akhtar K, Jabber A, Bukhari II, Noreen R (2013) Physico-chemical study for zinc removal and recovery onto native/chemically modified Aspergillus flavus NA9 from industrial effluent. Water Res 47:4238–4246
Agrawal A, Sahu KK, Pandey BD (2004) Removal of zinc from aqueous solutions using sea nodule residue. Colloids Surf A Physicochem Eng Asp 237:133–140
Akar ST, Gorgulo A, Anilan B, Kayanak Z, Akar T (2009) Investigation of the biosorption characteristics of lead(II) ions onto Symphoricarpus albums: batch and dynamic flow studies. J Hazard Mater 165:126–133
Akar ST, Aslan S, Alp T, Aslan D, Akar T (2012) Biosorption potential of the waste biomaterial obtained from Cucumis melo for removal for the removal of Pb2+ ions from aqueous media: equilibrium, kinetic, thermodynamic and mechanism analysis. Chem Eng J 185–186:82–90
Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, Elgaaly G, Moubayed NMS (2014) Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab J Chem 7:57–62
Amini M, Younesi H, Bahramifar N, Lorestani AAZ, Ghorbani F, Daneshi A, Sharifzadeh M (2008) Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J Hazard Mater 154(1–3):694–702
Amini M, Younesi H, Bahramifar N (2009) Biosorption of nickel(II) from aqueous solution by Aspergillus niger: response surface methodology and isotherm study. Chemosphere 75:1483–1491
Arunakumara KKIU, Xuecheng Z (2008) Heavy metal bioaccumulation and toxicity with special reference to microalgae. J Ocean Univ China 7(1):60–64
Ayranci E, Hoda N (2005) Adsorption kinetics and isotherms of pesticides onto activated carbon-cloth. Chemosphere 60:1600–1607
Bai RS, Abraham TE (2002) Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Res 36:1224–1236
Bayramoglu G, Arica MY (2008) Removal of heavy mercury(II), cadmium(II) and zinc(II) metal ions by live and heat inactivated Lentinus edodes pellets. Chem Eng J 143:133–140
Bayramoglu G, Gursel I, Tunali Y, Arica MY (2009) Biosorption of phenol and 2-chlorophenol by funalia trogii pellets. Bioresour Technol 100:2685–2691
Bingol A, Aslan BA, Cakicia A (2009) Biosorption of chromate anions from aqueous solution by a cationic surfactant-modified lichen (Cladonia angiformis (L.)). J Hazard Mater 161:747–752
Borràs E, Blánquez P, Sarrà M, Caminal G, Vicent T (2008) Trametes versicolor pellets production: low-cost medium and scale-up. Biochem Eng J42(1):61–66
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. Bioessays 28:799–808
Carrott PJM, Carrott RMML, Nabais JMV, Ramalho PJP (1996) Influence of surface ionization on the adsorption of aqueous zinc species by activated carbons. Carbon 35(3):403–410
Chen XC, Wang YP, Lin Q, Shi JY, Wu WX, Xu Y, Chen YXD (2005) Biosorption of copper(II) and zinc(II) from aqueous Polution by Pseudomonas putida Z1. Colloids Surf B Interfaces 46(2):101–107
Chowdhury ZZ, Zain SM, Khan RA, Ahmed AA (2011) Equilibrium kinetics and isotherm studies of Cu (II) adsorption from waste water onto alkali activated oil palm ash. Am J Appl Sci 8(3):230–237
Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K (2007) Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater 146:270–277
Cooney DO (1999) Adsorption design for wastewater treatment. CRC Press, Boca Raton
Crisafully R, Milhome MAL, Cavalcante RM, Silveira ER, De Keukeleire D, Nascimento RF (2008) Removal of some polycyclic aromatic hydrocarbons from petrochemical wastewater using low-cost adsorbents of natural origin. Bioresour Technol 99:4515–4519
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Deepa KK, Sathishkumar M, Binupriya AR, Murugesan GS, Swaminathan K, Yun SE (2006) Sorption of Cr(VI) from dilute solutions and wastewater by live pretreated biomass of Aspergillus flavous. Chemosphere 62:833–840
Dudley HW, Fleming I (1995) Spectroscopic methods in organic chemistry. Mc Graw-Hill, London
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (2005) Standard methods for the examination of water and wastewater. American Public Health Association—APHA, Washington, D.C
Fan T, Liu Y, Feng B, Zeng G, Yang C, Zhou M, Zhou H, Tan Z, Wang X (2008) Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: isotherms, kinetics and thermodynamics. J Hazard Mater 160:655–661
Fischer G, Braun S, Thissen R, Dott W (2006) FT-IR spectroscopy as a tool for rapid identification and intra-species characterization of airborne filamentous fungi. J Microbiol Methods 64:63–67
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Fu Y, Viraraghavan T (2002) Dye biosorption sites in Aspergillus niger. Bioresour Technol 82(2):139–145
Ghaedi M, Hajati S, Karimi F, Barazesh B, Ghezelbash G (2013) Equilibrium, kinetic and isotherm of some metal ion biosorption. J Ind Eng Chem 19:987–992
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I: theoretical. J Colloids Interface Sci 47:755–765
Gupta S, Babu BV (2009) Removal of toxic metal Cr(VI) from aqueous solutions using sawdust as adsorbent: equilibrium, kinects and regeneration studies. Chem Eng J 150:352–365
Gupta R, Ahuja P, Khan S, Saxena RK, Mohapatra H (2000) Microbial biosorbents: meeting challenges of heavy metal pollution in aqueous solutions. Curr Sci 78(8):967–973
Hajahmadi Z, Younesi H, Bahramifar N, Khakpour H, Pirzadeh K (2015) Multicomponent isotherm for biosorption od Zn(II), Co(II) and Cd(II) from ternary mixture onto pretreat dried Aspergillus niger biomass. Water Resour Ind 11:71–80
Hameed BH, Ahmad AA, Aziz N (2007) Isotherms, kinetic and thermodynamics of acid dye adsorption on activated palm ash. Chem Eng J 133:195–203
Hawari A, Rawajfih Z, Nsour N (2009) Equilibrium and thermodynamic analysis of zinc ions adsorption by olive mill solid residues. J Hazard Mater 168:1284–1289
Javaid A, Bajwa R, Shafique U, Anwar J (2011) Removal of heavy metals by adsorption on Plerotus ostreatus. Biomass Bioenergy 35:1675–1682
Kapoor A, Yang RT (1989) Correlation of equilibrium adsorption data of condensable vapours on porous adsorbents. Gas Sep Purif 3:187–192
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using fungus Aspergillus niger. Bioresour Technol 70:95–104
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater 124(1–3):192–199
Khambhaty Y, Mody K, Basha S, Jha B (2009) Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem Eng J 145:489–495
Kumar R, Bishnoi NR, Garima BK (2008) Biosorption of Chromium (VI) from aqueous solutions and electroplating wastewater using fungal biomass. Chem Eng J 135:202–208
Lameiras S, Quintelas C, Tavares T (2008) Biosorption of Cr(VI) using a bacterial biofilm supported on granular activated carbon and on zeolite. Bioresour Technol 99:801–806
Lee Y, Chang S (2011) Biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresour Technol 102:5297–5304
Liao W, Liu Y, Frear C, Chen S (2007) A new approach of pellet formation of a filamentous fungus—Rhizopus oryzae. Bioresour Technol 98:3415–3423
Liu Y, Shen L (2008) A general rate law equation for biosorption. Biochem Eng J 38:390–394
Mohan D, Singh KP, Singh VK (2006) Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J Hazard Mater 135(1–3):280–295
Moreira MT, Sanromain A, Feijo G, Lema JM (1996) Control of pellet morphology of filamentous fungi in fluidized bed bioreactors by means of a pulsing flow: application to Aspergillus niger and Phanerochaete chrysosporium. Enzyme Microbiol Technol 19(4):261–266
Mukhopadhya YM (2008) Role of surface properties during biosorption of copperby pretreated Aspergillus niger biomass. Colloids Surf A Physicochem Eng Asp 329:95–99
Mungasavalli DP, Viraraghavan T, Jin YC (2007) Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: batch and column studies. Colloids Surf A Physicochem Eng Asp 301:214–222
Mutongo F, Kuipa O, Kuipa PK (2014) Removal of Cr(VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg Chem Appl 2014:1–7. doi:10.1155/2014/973153
Nagda GK, Ghole VS, Diwan AM (2006) Tendu leaves refuse as a biosorbent for COD removal from Molasses Fermentation based Bulk drug industry effluent. J Appl Sci Environ Manag 10(3):15–20
Ncibi MC (2008) Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J Hazard Mater 153:207–212
Nilanjana D, Vimala R, Karthika P (2008) Biosorption of heavy metals—an overview. Indian J Biotechnol 7:159–169
Oliveira WE, Franca AS, Oliveira LS, Rocha SD (2008) Untreated coffee huusks as biosorbents for the removal of heavy metals from aqueous solutions. J Hazard Mater 152:1073–1081
Pagnanelli F, Esposito A, Toro L, Veglio F (2003) Metal speciation and pH effect on Pb, Cu, Zn and Cd biosorption onto Sphaerotilus natans: Langmuir-type empirical model. Water Res 37:627–633
Pandey PK, Verma Y, Choubey S, Pandey M, Chandrasekhar K (2008) Biosorptive removal of cadmium from contaminated groundwater and industrial effluents. Bioresour Technol 99(10):4420–4427
Pandey PK, Choubey S, Verma Y, Pandey M, Chandrashekhar K (2009) Biosorptive removal of arsenic from drinking water. Bioresour Technol 100:634–637
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Park D, Yun YS, Park JM (2005) Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 60:1356–1364
Ramrakhiani L, Majumder R, Khowala S (2011) Removal of hexavalent chromium by heat inactivated fungal biomass of Termitomyces clypeatus: surface characterization and mechanism of biosorption. Chem Eng J 171:1060–1068
Rangabhashiyam S, Selvaraju N (2015) Evaluation of the biosorption potential of a novel Caryota urens inflorescence waste biomass for the removal of hexavalent chromium from aqueous solutions. J Taiwan Inst Chem Eng 47:59–70
Romera E, González F, Ballester A, Blázquez ML, Munoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol 98:3344–3353
Ruthven DM (1984) Principles of adsorption and adsorption process. Wiley, New York
Sag Y, Kaya A, Kutsal T (1998) The simultaneous biosorption of Cu(II) and Zn on Rhizopus arrhizus: application of the adsorption models. Hydrometallurgy 50:297–314
Sanghi R, Sankararamakrishnan N, Dave BC (2009) Fungal bioremediation of chromates: conformational changes of biomass during sequestration, binding, and reduction of hexavalent chromium ions. J Hazard Mater 169:1074–1080
Sari A, Tuzen M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J Hazard Mater 164(2–3):1004–1011
Saygideger S, Gulnaz O, Istifli ES, Yucel N (2005) Adsorption of Cd(II), Cu(II) and Ni(II) ions by Lemna minor L.: effect of physicochemical environment. J Hazard Mater B 126:96–104
Shek TH, Ma A, Lee VKC, Mckay G (2009) Kinetics of zinc ions removal from effluents using ion exchange resin. Chem Eng J 146:63–70
Sousa FW (2007) Adsorção de metais tóxicos em efluente aquoso usando a casca de coco verde tratado. Dissertação de Mestrado em Engenharia Civil—área de concentração em Saneamento Ambiental. Universidade Federal do Ceará, Fortaleza
Sun XF, Wang SG, Zhang XM, Chen PJ, Li XM, Gao BY, Ma Y (2009) Spectroscopic study of Zn2+ and Co2+ binding to extracellular polymeric substances (EPS) from aerobic granules. J Colloid Interface Sci 335:11–17
Tobin JM, Cooper DG, Neufeld RJ (1984) Uptake of metal ions by Rhizopus arrhizus biomass. Appl Environ Microbiol 47:821–824
Váldes H, Sánchez-Polo M, Rivera-Utrilla J (2002) Effect of ozone treatment on surface properties of activated carbon. Langmuir 18:2111–2116
Veglio F, Beolchini F (1997) Removal biosorption: a review. Hydrometallurgy 44:301–316
Vilar VJ, Botelho CMS, Boaventura RAR (2007) Chromium and zinc uptake by algae Gelidium and agar extraction algal waste: kinetics and equilibrium. J Hazard Mater 149:643–649
Wang XS, Qin Y (2006) Removal of Ni(II), Zn(II) and Cr(VI) from aqueous solution by Alternanthera philoxeroides biomas. J Hazard Mater B 138:582–588
Wang J, Hua X, Liu Y, Xie S, Bao Z (2010) Biosorption of uranium (VI) by immobilized Aspergillus fumigatus beads. J Environ Radioact 1001:504–508
WHO (1998) Environmental health criteria 61: chromium. http://www.inchem.org/documents/ehc/ehc/ehc61.htm
Ye J, Yin H, Mai B, Hui P, Qin H, He B, Zhang N (2010) Biosorption of chromium from aqueous solution and electroplating wastewater using mixture of Candida lipolytica ans dewatered sewage sludge. Bioresour Technol 101:3893–3902
Yun-Guo L, Ting F, Guan-Ming Z, Xin L, Quing T, Fei Y, Ming Z, Wei-Hua X, Yu H (2006) Removal of cadmium and zinc ions from aqueous solutions by living Aspergillus niger. Trans Nonferr Metals Soc China 16:681–686
Acknowledgments
The authors are grateful to the Improvement Coordination of Higher Education Personal and National Council of Technological and Scientific Development Universal Edict proc. 470628/2006-5 for partial financial support to realization of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vale, M.d., do Nascimento, R.F., Leitão, R.C. et al. Cr and Zn biosorption by Aspergillus niger . Environ Earth Sci 75, 462 (2016). https://doi.org/10.1007/s12665-016-5343-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5343-9