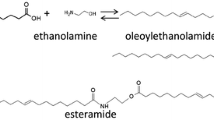
Abstract
Organic syntheses in aqueous solutions are being developed because water is an environmentally friendly, inexpensive, non-toxic and non-flammable solvent. The common method for the synthesis of α-acyloxycarboxamides is the one-pot three-component condensation of a carboxylic acid, an aldehyde and an isocyanide, entitled the Passerini reaction. This reaction is usually performed in organic solvents such as dichloromethane or toluene. Herein we report a novel protocol for the synthesis of α-acyloxycarboxamides in aqueous solution under mild reaction conditions, for which one of the reactants, the carboxylic acid, is a micelle- or vesicle-forming compound. The reaction is carried out successfully with up to 93% yield in an aqueous solution without catalyst or surfactant addition. Our findings showed that the fatty acid used as a substrate accelerates the reaction due to its self-assembly properties. This environmentally benign protocol has several advantages such as high yields, mild reaction conditions and easy workup. Moreover, it allows to synthesize α-acyloxycarboxamides that are inaccessible under standard conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
α-Acyloxycarboxamides are usually synthesized by the Passerini multi-component reaction from a carboxylic acid, an aldehyde and an isocyanide in a one-pot reaction without isolating reaction intermediates. α-Acyloxycarboxamides are key building blocks for the synthesis of natural products, drugs, γ-lactones (Bos and Riguet 2014), 2-furanones (Bossio et al. 1993), peptides (Szymański and Ostaszewski 2008), peptidomimetics (Szymański et al. 2007) and enantiomerically pure α-amino acids (Szymański and Ostaszewski 2006). The Passerini reaction is usually performed in aprotic organic solvents such as dichloromethane or toluene, which are toxic and carcinogenic (Koszelewski et al. 2007). There are several reported examples of the Passerini reactions performed in water (Pirrung and Sarma 2004; Vessally et al. 2011; Taran et al. 2014) or water–ethanol mixtures (Deobald et al. 2012; Dos Santos et al. 2017). Moreover, we found that the presence of cationic bilayer (vesicle) forming surfactants, which themselves do not take part in the reaction, can enhance the Passerini reaction yield. The positive effect of cationic surfactants like dioctadecyldimethylammonium bromide on the Passerini reaction is probably due to two main effects: an increase of the solubility of the reacting molecules in the hydrophobic part of the vesicular aggregates and electrostatic attractions between the cationic surface of the vesicles and the carboxylate ions (Paprocki et al. 2015, 2016). Beneficial influence of water–surfactant systems was also shown for other multi-component reactions such as the Mannich reaction (Ghadami and Jafari 2015), the Ugi reaction (Madej et al. 2017), the Kinugasa reaction (McKay et al. 2009) or the Betti bases synthesis (Kumar et al. 2010). Also, synthetic protocols conducted in various environmental sustainable solvents, e.g. glycerol (Gubta et al. 2016), water (Bagul et al. 2017) or deep eutectic solvent (Azizi and Dezfooli 2016), attract more and more attention.
Despite the benefits of aqueous–surfactant systems, one has to admit that the added surfactants are usually synthesized from non-renewable sources and are not biodegradable; this makes the entire processes less friendly to the environment. There is still demand for clean, efficient and high yielding routes to the large-scale synthesis of α-acyloxycarboxamides. Therefore, we demonstrated that the use of an amphiphilic reactant has a positive effect on the Passerini reaction. The elaborated protocol can be especially beneficial from the point of view of green chemistry issues, since water is used as a reaction medium in the absence of non-renewable surfactants.
Experimental section
1H NMR and 13C NMR spectra were recorded with Varian 200 MHz and Bruker 400 MHz spectrometers, with tetramethylsilane used as an internal standard or with the residual chloroform signal. Elemental analysis was performed on a Vario EL III (Elementor) elemental analyser. High-resolution mass spectrometry (HRMS) spectra were recorded on a Mariner (PerSeptiveBiosystems) and Synapt G2-Si High Definition apparatus. Dynamic light-scattering measurements were performed using a Zetasizer Nano ZS apparatus. The fluorescence measurements were recorded in quartz cuvettes in an F7000 spectrofluorometer (Shimadzu). p-Methoxybenzylisocyanide (3a) was synthesized from p-methoxybenzylamine in a two-step synthesis according to the published procedure (Paprocki et al. 2015). All other starting materials for the Passerini reaction were purchased from Sigma-Aldrich or Tokyo Chemical Industry.
General procedure for the synthesis of compounds 4a–4z
A mixture of an aldehyde (0.5 mmol), a carboxylic acid (0.5 mmol) and an isocyanide (0.5 mmol) was stirred at room temperature in 0.1 M phosphate buffer pH = 5 (5 mL). After 24 h, the reaction mixture was extracted with dichloromethane (3 × 10 mL). The combined organic layers were dried with MgSO4, and the solvent was removed under reduced pressure. The crude product was purified by crystallization or column chromatography on silica gel (hexane/ethyl acetate).
1-(4-Methoxybenzylamino)-1-oxotridecan-2-yl acetate 4a
White powder; elemental analysis found: C, 70.48; H, 9.34; N, 3.38. Calc. for C23H37NO4: C, 70.55; H, 9.52; N, 3.58; 1H NMR (400 MHz; CDCl3) δH 0.88(3H, t, J 7.2 Hz, CH3CH2), 1.21–1.35 (18H, br m, 9 × CH2), 1.80–1.91 (2H, m, CH2CH), 2.11 (3H, s, CH3CO), 3.90 (3H, s, CH3O), 4.34–4.45 (2H, m, CH2N), 5.16–5.20 (1H, m, CH), 6.23 (1H, t, J 5.2 Hz, NH), 6.21–6.24 (2H, m, Ph), 7.18–7.20 (2H, m, Ph); 13C NMR (100 MHz; CDCl3) δC 14.1, 21.0, 22.7, 24.7, 29.2, 29.3, 29.4, 29.5, 29.6, 31.9, 31.9, 42.6, 55.3, 74.2, 114.1, 129.0, 129.9, 159.1, 169.6, 169.69; HRMS calcd. for C23H37NO4Na [M + Na]+: 414.2620, found: 414.2614.
1-(4-Methoxybenzylamino)-1-oxotridecan-2-yl benzoate 4b
White powder; 1H NMR (400 MHz; CDCl3) δH 0.87 (3H, t, J 7.2 Hz, CH3CH2), 1.19–1.36 (16H, br m, 8 × CH2), 1.36–1.48 (2H, m, CH2CH2), 1.97–2.03 (2H, m, CH2CH), 3.78 (3H, s, CH3O), 4.35–4.48 (2H, m, CH2N), 5.44–5.47 (1H, m, CH), 6.30 (1H, br s, NH), 6.83–6.86 (2H, m, Ph), 7.16–7.18 (2H, m, Ph),7.44–7.48 (2H, m, Ph), 7.74–7.61 (1H, m, Ph), 8.03–8.05 (2H, m, Ph); 13C NMR (100 MHz; CDCl3) δC 14.1, 22.6, 24.9, 29.2, 29.3, 29.4, 29.5, 29.6, 31.9, 32.0, 42.7, 55.3, 74.7, 114.1, 128.6, 128.9, 129.7, 130.0, 133.5, 159.1, 165.4, 169.8; HRMS calcd. for C28H39NO4Na [M + Na]+: 476.2777, found: 476.2781.
1-(4-Methoxybenzylamino)-1-oxotridecan-2-ylcaprylate 4c
White powder; 1H NMR (400 MHz; CDCl3) δH 0.86–0.90 (6H, m, 2 × CH3CH2), 1.17–1.39 (24H, br m, 12 × CH2), 1.61–1.65 (4H, m, 2 × CH2CH2), 1.82–1.90 (2H, m, CH2CH), 2.35 (2H, t, J 7.6 Hz, COCH2CH2), 3.80 (3H, s, CH3O), 4.38–4.41 (2H, m, CH2N), 5.20–5.23 (1H, m, CH), 6.20 (1H, br s, NH), 6.85–6.88 (2H, m, Ph), 7.17–7.18 (2H, m, Ph); 13C NMR (100 MHz; CDCl3) δC 14.00, 22.2, 22.3, 24.9, 24.9, 28.9, 29.2, 29.3, 29.4, 29.5, 29.6, 31.6, 31.9, 34.3, 42.7, 55.3, 74.1, 114.1, 129.0, 169.8, 172.5; HRMS calcd. for C29H49NO4Na [M + Na]+: 498.3559, found: 498.3558.
Results and discussion
In the course of our studies on the effect of added micelle- or vesicle-forming surfactants on the Passerini reaction in aqueous media, we considered the case where one of the reactants has amphiphilic properties and through this could influence the reaction in a positive way, for example, by enhancing the reaction yield. To test whether this can be achieved, we performed the Passerini reactions with three different acids: a water-soluble acetic acid (1a) and two insoluble ones: benzoic acid (1b) and caprylic acid (= octanoic acid) (1c) in water. From previous work, it is well known that acid 1c self-assembles into micelles or vesicles in aqueous solution, depending on concentration and pH value (Hargreaves and Deamer 1978; Walde et al. 1994). The three carboxylic acids (1a–c) were reacted separately with dodecylaldehyde (2a) and p-methoxybenzylisocyanide (3a) at room temperature for 24 h. The concentration of each reactant was 100 mM. The results are shown in Table 1.

In distilled water, the reaction yields strongly depended on the used carboxylic acid. For the reaction carried out with acetic acid, the yield of product 4a was only 38%, which may be caused by the high solubility of acetic acid (1a), while the reaction partners 2a and 3a are water-insoluble, what hinders contact between the reacting molecules. For the water-insoluble benzoic acid (1b), the reaction yield of product 4b was 53%. This reaction takes place “on water”, since all reactants used are insoluble in water, what results in the observed significant increase in the reaction yield, if compared to acetic acid. The highest yield (77%, product 4c) was obtained for the reaction carried out with caprylic acid (1c).
Since 1c is known to self-assemble in aqueous solution in a pH- and concentration-dependent manner, we decided to investigate the influence of the pH value on the reaction yield with caprylic acid (1c) at the carefully elaborated conditions of 100 mM of 1c, 2a and 3a. The pH was adjusted by using phosphate buffer solutions (PB, 100 mM), prepared by mixing solutions of orthophosphoric acid (H3PO4), sodium dihydrogen phosphate (NaH2PO4) and disodium hydrogen phosphate (Na2HPO4). The results presented in Fig. 1 show that pH = 5 was optimal for this model reaction, providing product 4c in 79% yield. For the reaction carried out at pH = 3, the yield of 4c was a bit lower (71%), which may be caused by acid-catalysed hydrolysis of the isocyanide (Mayer et al. 2012). For the reactions carried out at pH values above 6, the desired product 4c was obtained in lower yields than at pH 4–6 (69% for pH = 7 and 61% for pH 8), which may be due to base-catalysed hydrolysis of the ester bond in product 4c. However, we have not isolated the products of hydrolysis.
To confirm the presence of aggregates (polymolecular assemblies) in the reaction medium, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was used as fluorescent probe. DiI has weak fluorescence in water (Loew 1988), but in the presence of micelles or vesicles exhibits fluorescence (Klauner and Wolf 1980). We performed three separate experiments with DiI: first, only with buffer solution (pH = 5); second, with buffer solution and 1c (100 mM); and third, with buffer solution and reactants 1c, 2a and 3a. All fluorescence spectra were recorded at an excitation wavelength (λex) of 550 nm. When DiI (1 mM) was added to a pH = 5 phosphate buffer solution, there was no detectable fluorescence. However, when caprylic acid (100 mM) and DiI (1 mM) were added to the buffer solution, fluorescence in range 550–700 nm was observed, indicating the presence of aggregates (micelles and/or vesicles). Also when all reactants (1c, 2a and 3a) and DiI were present in the sample, fluorescence was again observed, which is a clear evidence for the presence of polymolecular assemblies in the reaction mixture. Moreover, the dynamic light-scattering measurements confirmed the presence of aggregates in the solution of caprylic acid (100 mM) in phosphate buffer and in the mixture of 1c, 2a and 3a (100 mM) in phosphate buffer pH = 5.
Next, we carried out the Passerini reaction with longer chain fatty acids, which are also known to form micelles and/or vesicles (Hargreaves and Deamer, 1978; Walde et al. 1994): lauric acid (1d), stearic acid (1e) and oleic acid (1f). The obtained yields of products 4d–f under pH = 5 conditions are shown in Table 2, together with the results obtained with acetic acid (1a), benzoic acid (1b) and caprylic acid (1c). Moreover, we have performed the Passerini reaction with 1a–c in dichloromethane and without solvent, to compare them with those obtained in aqueous solution.
The Passerini reaction carried out with acetic acid (1a) resulted in product 4a with 46% yield, what is slightly higher than in distilled water (38%). Product 4b was obtained with the same yield as in distilled water (53%). With the fatty acids (1c–f), the corresponding products (4c–f) were obtained with significantly higher isolated yields, up to 89%, than for acids 1a (product 4a), and 1b (product 4b). These results verify that micelle- and vesicle-forming fatty acids are able to promote the Passerini reaction, in which they are simultaneously a part of the reactants. The reactions which were carried out with the three different acids in dichloromethane resulted in products 4a-c with yields between 54 and 66% in the case of acetic acid (product 4a), 66% for benzoic acid (product 4b) or caprylic acid (product 4c). Reactions performed without solvent (entries 4–6) resulted in products 4a-c with yields maintained at 53–67%. The obtained results proved that the Passerini reactions in micellar or vesicular systems formed by the carboxylic acid substrate lead to the formation of products with similar or higher yield than under “standard conditions” (dichloromethane as solvent or neat).
Further, we applied the elaborated procedure for the Passerini reaction with different aldehydes (2b–j) and isocyanides (3a–g), in phosphate buffer solution pH = 5 (see Table 3). The results obtained with isovaleraldehyde (2b), 3a and acids 1a–f are in line with the data presented above and with the general concept of this work. The reaction carried out with acetic acid (1a) gave product 4g with low yield (21%). The reaction carried out with water-insoluble benzoic acid (1b), which takes place “on water”, gave 4h in 63% yield. For the reactions carried out with aggregates forming long-chain fatty acids yielded products 4i–l in high yields (75–90%). This proves again the advantageous effect of aggregates formed from one of the reactants itself on the reaction yield.

Reactions carried out with caprylic acid 1c, isocyanide 3a and different aldehydes (2c–j) provided appropriate products 4m–t with moderate to high yields. Application of the two aliphatic aldehydes, acetaldehyde (2c) and propionaldehyde (2d), resulted in products 4m and 4n with 73 and 61% yields, respectively. Reactions carried out with different aromatic aldehydes resulted in products 4o–t with yields between 59 and 93%, without any obvious correlation between substituent type in the phenyl ring and the reaction yield. Reactions carried out with 1c, 2b and different isocyanides 3b–g resulted in products 4u–z with low to moderate yields. It is evident that the reactions performed with benzyl isocyanides 3b, 3c resulted in much higher yields than in the case of the aliphatic isocyanides 3d–f. Moreover, the utility of the newly developed protocol was proven by the reaction carried out with 4-nitrophenyl isocyanide (3g). Under optimized conditions, in phosphate buffer pH = 5, product 4z was obtained with 43% yield, while the same reaction performed under standard conditions in dichloromethane did not occur.
Conclusion
During our studies, we found that the synthesis of α-acyloxycarboxamides from a carboxylic acid, an aldehyde and an isocyanide in aqueous reaction medium is efficiently promoted by the hydrophobic environment which is formed by one of the engaged reactants. This procedure avoids usage of organic solvents, which is highly desirable from an environmental point of view. Additionally, this type of micellar or vesicular reaction mixture allows reducing the amount of waste, because the addition of non-renewable surfactants is not required. Moreover, the yield of the reactions carried out with micelle- or vesicle-forming fatty acids in water was higher than the yield of the same reaction carried out under standard conditions in an organic solvent. This leads to the conclusion that aqueous solutions should be used for the Passerini reactions, where one of the reactants acts as a surfactant. Moreover, this phenomenon increases the reactivity of selected isocyanides. The obtained results are especially important in the context of green chemistry, because no organic solvents and extra surfactant addition are necessary; only equimolar ratios of reactants were applied. Mild reaction conditions and operational simplicity of the developed protocol offer the environmental sustainable and cost-effective large-scale industrial synthesis of α-acyloxycarboxamides.
References
Azizi N, Dezfooli S (2016) Catalyst-free synthesis of imidazo [1,2-a] pyridines via Groebke multicomponent reaction. Environ Chem Lett 14:201–206. https://doi.org/10.1007/s10311-0150541-3
Bagul SD, Rajput JD, Bendre RS (2017) Synthesis of 3-carboxycoumarins at room temperature in water extract of banana peels. Environ Chem Lett 15:725–731. https://doi.org/10.1007/s10311-017-0654-z
Bos M, Riguet E (2014) Synthesis of chiral γ-lactones by one-pot sequential enantioselective organocatalytic michael addition of boronic acids and diastereoselective intramolecular Passerini reaction. J Org Chem 79:10881–10889. https://doi.org/10.1021/jo501908z
Bossio R, Marcaccini S, Pepino R, Torroba T (1993) Studies on isocyanides and related compounds: a novel synthetic route to furan derivatives. Synthesis 8:783–785. https://doi.org/10.1055/s-1993-25940
Deobald AM, Correa AG, Rivera DG, Paixao MW (2012) Organocatalytic asymmetric epoxidation and tandem epoxidation/Passerini reaction under eco-friendly reaction conditions. Org Biomol Chem 10:7681–7684. https://doi.org/10.1039/C2OB26247A
Dos Santos DA, Deobald AM, Cornelio VE, Avila RMD, Cornea RC, Bernasconi GCR, Paixao MW, Vieira PC, Correa AG (2017) Asymmetric synthesis and evaluation of epoxy-α-acyloxycarboxamides as selective inhibitors of cathepsin L. Bioorg Med Chem 25:4620–4627. https://doi.org/10.1016/j.bmc.2017.06.048
Ghadami M, Jafari AA (2015) Efficient synthesis of Mannich bases by sonication in sodium dodecyl sulphate micellar media. Environ Chem Lett 13:191–196. https://doi.org/10.1007/s10311-015-0495-5
Gubta S, Khanna G, Khurana JM (2016) A facile eco-friendly approach for the one-pot synthesis of 3,4-dihydro-2H-naphto[2,3-e][1,3]oxazine-5,10-diones using glycerol as green media. Environ Chem Lett 14:559–564. https://doi.org/10.1007/s10311-016-0570-6
Hargreaves WR, Deamer DW (1978) Liposomes from ionic, single-chain amphiphiles. Biochemistry 17:3759–3768. https://doi.org/10.1021/bi00611a014
Klauner RD, Wolf DE (1980) Selectivity of fluorescent lipid analogs for lipid domains. Biochemistry 19:6199–6203. https://doi.org/10.1021/bi00567a039
Koszelewski D, Redzej A, Ostaszewski R (2007) The study on efficient hydrolases immobilization for the kinetic resolution of the α-acetoxyamides. J Mol Catal B-Enzym 47:51–57. https://doi.org/10.1016/j.molcatb.2007.03.007
Kumar A, Gupta MK, Kumar M (2010) Non-ionic surfactant catalyzed synthesis of Betti base in water. Tetrahedron Lett 51:1582–1584. https://doi.org/10.1016/j.tetlet.2010.01.056
Loew LM (1988) Spectroscopic membrane probes. CRC Press, Boca Raton, pp 193–220
Madej A, Paprocki D, Koszelewski D, Żądło-Dobrowolska A, Brzozowska A, Walde P, Ostaszewski R (2017) Efficient Ugi reactions in an aqueous vesicle system. RSC Adv 7:33344–33354. https://doi.org/10.1039/C7RA03376A
Mayer AMS, Aviles E, Rodriguez AD (2012) Marine sponge Hymeniacidon sp. amphilectane metabolites potently inhibit rat brain microglia thromboxane B2 generation. Bioorg Med Chem 20:279–282. https://doi.org/10.1016/j.bmc.2011.10.086
McKay CS, Kennedy DC, Pezacki JP (2009) Studies of multicomponent Kinugasa reactions in aqueous media. Tetrahedron Lett 50:1893–1896. https://doi.org/10.1016/j.tetlet.2009.02.035
Paprocki D, Koszelewski D, Walde P, Ostaszewski R (2015) Efficient Passerini reactions in an aqueous vesicle system. RSC Adv 5:102828–102835. https://doi.org/10.1039/C5RA22258C
Paprocki D, Koszelewski D, Żądło A, Walde P, Ostaszewski R (2016) Environmentally friendly approach to α-acyloxy carboxamides via a chemoenzymatic cascade. RSC Adv 6:68231–68237. https://doi.org/10.1039/C6RA13078J
Pirrung MC, Sarma KD (2004) Multicomponent reactions are accelerated in water. J Am Chem Soc 126:444–445. https://doi.org/10.1021/ja038583a
Szymański W, Ostaszewski R (2006) Multicomponent diversity and enzymatic enantioselectivity as a route towards both enantiomers of α-amino acids—a model study. Tetrahedron Asymmetry 17:2667–2671. https://doi.org/10.1016/j.tetasy.2006.09.014
Szymański W, Ostaszewski R (2008) Toward stereocontrolled, chemoenzymatic synthesis of unnatural peptides. Tetrahedron 64:3197–3203. https://doi.org/10.1016/j.tet.2008.01.103
Szymański W, Zwolińska M, Ostaszewski R (2007) Studies on the application of the Passerini reaction and enzymatic procedures to the synthesis of tripeptide mimetics. Tetrahedron 63:7647–7653. https://doi.org/10.1016/j.tet.2007.05.044
Taran J, Ramazani A, Joo SW, Slepokura K, Lis T (2014) Synthesis of novel α-(acyloxy)-α-(quinolin-4-yl)acetamides by a three-component reaction between an isocyanide, quinoline-4-carbaldehyde, and arenecarboxylic acids. Helv Chim Acta 97:1088–1096. https://doi.org/10.1002/hlca.201300378
Vessally E, Ramazani A, Yaaghubi E (2011) Green synthesis and characterization of novel α-acyloxycarboxamides through three-component reaction between pyridine carbaldehydes, cyclohexyl isocyanide, and benzoic acid derivatives. Monatsh Chem 142:1143–1147. https://doi.org/10.1007/s00706-011-0536-0
Walde P, Wick R, Fresta M, Mangone A, Luisi PL (1994) Autopoietic self-reproduction of fatty acid vesicles. J Am Chem Soc 116:11649–11654. https://doi.org/10.1021/ja00105a004
Acknowledgements
We gratefully acknowledge the financial support of the Polish National Science Center project Harmonia No 2014/14/M/ST5/00030 and the European Community COST action CM1304 for providing a stimulating environment which led to this collaborative research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paprocki, D., Wilk, M., Madej, A. et al. Catalyst-free synthesis of α-acyloxycarboxamides in aqueous media. Environ Chem Lett 17, 1011–1016 (2019). https://doi.org/10.1007/s10311-018-0797-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0797-5