Abstract
The real behavior of water organic contaminants such as pesticides and pharmaceuticals is not well known because research experiments usually simplify the conditions by studying the sorption of a pure compound on a single solid. However, in natural waters, biofilms, suspended particles, and sediments are solid substances that coexist, and thus may change the contaminant fate. Therefore, we studied here the sorption of lindane and ciprofloxacin by three single-solid and three double-solid sorbents using batch experiments. We also compared the effect of dissolved organic matter (DOM) between single- and double-solid sorption systems. Results show that the sorption quantity of lindane to the double-solid system of suspended particles and sediments is lower, of 0.99 L/g, than the sum of sorption quantity in the single-solid system, of 1.39 L/g. The sorption quantity of ciprofloxacin is higher, of 2.70 L/g, than the sum of sorption quantity in the single-solid system, of 1.90 L/g. These findings are explained by changes in DOM that suppress or promote sorption. To our best knowledge, this is the first study to present evidence that coexisting river solids modify lindane and ciprofloxacin sorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fate of organic contaminants in the aquatic environment is complex (Kucher and Schwarzbauer 2017; Kalathoor et al. 2015) and controlled by sorption (Moura et al. 2017; Zhang and Dong 2008). Biofilms, suspended particles, and sediments often exist in natural aquatic environments (Fan et al. 2017; Miao et al. 2014). These natural solids play an important role in the sorption of classic and emerging organic contaminants, such as organochlorine pesticides and antibiotics. Biofilms can sorb pentachlorophenol, a chlorinated phenol, through interactions with extracellular polymeric substances (Dong et al. 2017b). Biofilms can also sorb antibiotics, including ofloxacin and norfloxacin (Dong et al. 2017a; Zhang et al. 2018). The sorption capacities of organic contaminants onto suspended particles and sediments are different those onto biofilms due to the different dissolved organic matter (DOM) contents between distinct solid sorbents (Dong et al. 2017a). Most previous studies have focused on sorption onto single-solid sorbents. However, biofilms, suspended particles, and sediments often coexist in the same natural aquatic environment and interactions can occur between them. Biofilms can develop on suspended particles and sediments, while suspended particles and surface sediments become interchangeable with changes in environmental conditions (Dong et al. 2011). The organic contaminants sorbed onto biofilms, suspended particles, and sediments may undergo desorption and redistribution when these sorbents interact (Guo et al. 2015). Thus, the sorption of organic contaminants in a multiple-solid sorption system could be different from those in a single-solid–liquid system, which have not been well investigated yet.
DOM from natural solids modifies the sorption of organic contaminants onto such solids, although it constitutes a small amount of all organic components (Polubesova et al. 2007). When biofilms, suspended particles, and sediments coexist in a sorption system, DOM originating from different solids may interact and affect sorption mechanisms. However, there are few studies on changes and the role of DOM in sorption capacities in multiple-solid sorption systems.
In this study, lindane and ciprofloxacin were selected as model classic and emerging organic contaminants, respectively (Berger et al. 2016; Tijani et al. 2016). The aims were to (a) investigate differences in the sorption capacities of single- and double-solid sorption systems and (b) demonstrate that changes in DOM in a double-solid sorption system could affect the sorption capacities of solid sorbents. To our knowledge, this is the first study to investigate the lindane and ciprofloxacin sorption capacities of coexisting river solids.
Experimental
Sorption experiments
Natural biofilms, suspended particles, and surface sediments were collected from Liao River in Jilin Province, northeastern China. Lindane (purity > 99%) and ciprofloxacin (purity > 98%) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany) and Fluka BioChemika (Buchs, Switzerland), respectively.
Single- and double-solid sorption systems with equal mass were designed as follows: two 10-mL beakers were placed in a 250-mL wide-mouth flask and one type of solid substance was put into one beaker. In the single-solid systems, one solid substance was put into two 10-mL beakers. In the double-solid system, the biofilms, suspended particles, and sediments were paired: biofilms and particles (B + P), biofilms and sediments (B + S), and particles and sediments (P + S), and put into two 10-mL beakers, respectively. The mass of the solids in each beaker was approximately 30 mg. Sorption isotherms were generated from the batch method, and the pH of each system was adjusted to 7.0 ± 0.1. Lindane solutions (200 mL) with concentrations ranging from 5 to 60 μg/L, containing 0.1 mol/L KCl and 100 mg/L NaN3, were added to the 250-mL flasks. The flasks were then sealed with parafilm, stored in darkness, and were then shaken in an air-bath shaker at 20 °C for 24 h. Blank samples without lindane and reference samples without solid substances were equilibrated alongside the other samples. For the ciprofloxacin sorption experiments, the ciprofloxacin concentrations ranged from 10 to 100 mg/L. The other experimental steps followed those of lindane. Each treatment was conducted in triplicate.
Single- and double-solid sorption systems with equal dissolved organic matter (DOM) content were prepared to study the effect of DOM on sorption. To obtain equal DOM content from each solid, the mass ratio of the added biofilms, suspended particles, and sediments was 2.5:10:60 according to the dissolved organic carbon content of the blank samples in the single-solid system with equal mass. Thus, the masses added to each 10-mL beaker in the single-solid systems with equal DOM contents were 1.25 + 1.25, 5 + 5, and 30 + 30 mg for the biofilms, suspended particles, and sediment, respectively. For the double-solid systems, the masses were 2.5 + 10, 2.5 + 60, and 10 + 60 mg for the B + P, B + S, and P + S systems, respectively. The other experimental steps followed those of the equal mass sorption system.
After sorption, all solutions were centrifuged at 1000×g for 10 min. The supernatants were then filtered through a 0.45-μm film. The concentrations of lindane in the filtrates were analyzed using a gas chromatograph (GC-ECD, GC-2014C, Shimadzu, Japan) after 40 mL of the lindane solution filtrates were extracted by C18 solid-phase extraction columns. The concentrations of ciprofloxacin were determined through high-performance liquid chromatography (LC-20AB, Shimadzu, Japan) with an ultraviolet detector and an ODS-SP column (5 μm, 4.6 mm × 250 mm). The amounts of lindane and ciprofloxacin sorbed onto the solids were calculated from the differences between the sorbate concentrations in the solutions before and after sorption.
Ultraviolet–visible absorption measurement of dissolved organic matter
Ultraviolet–visible (UV–Vis) absorption spectra of DOM in the blank samples from the single- and double-solid sorption systems with equal mass were recorded with a spectrophotometer (UV-1800, Shimadzu, Japan) at each 1-nm interval from 200 to 700 nm. A medium scan speed was set, and the background solution was used as a blank sample. The procedure was conducted in triplicate, and mean values were used for discussion.
Data analysis
Linear, Langmuir, and Freundlich models are often used to describe the sorption of organic contaminants (Matott et al. 2017). Therefore, these three models were used to fit the sorption data. These models are expressed by Eqs. 1, 2, and 3, respectively.
where Qe is the amount of sorbate sorbed onto a solid substance (mg/kg for lindane, mg/g for ciprofloxacin); Ce is the sorbate equilibrium concentration (μg/L for lindane, mg/L for ciprofloxacin); KH is the linear partition coefficient, L/g; Qmax is the Langmuir sorption maximum capacity (mg/kg for lindane, mg/g for ciprofloxacin); KL is the Langmuir equilibrium constant (L/g); KF is the Freundlich equilibrium coefficient [(mg/kg)/(μg/L)n for lindane, (mg/g)/(mg/L)n for ciprofloxacin]; and n is the Freundlich nonlinear coefficient.
To compare the sorption capacity of solids characterized by the Langmuir or Freundlich models, the mean single-point distribution coefficients (KM) were calculated from 100 equilibrium concentrations of sorbate from the best-fitting model. The single-point distribution coefficients (KD) were calculated as follows.
where CD is the selected sorbate equilibrium concentration (μg/L for lindane, mg/L for ciprofloxacin); and QD is the sorption amount at the selected equilibrium concentration (mg/kg for lindane, mg/g for ciprofloxacin).
A T test was utilized to compare the sorption capacities between different systems, with a significance level of 0.05. The model fitting and T test were conducted using Origin (version 9.0, OriginLab, USA).
Results and discussion
Differences between the measured and estimated sorption capacities
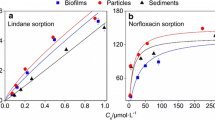
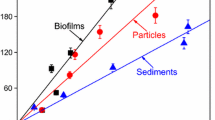
The sorption isotherms of lindane and ciprofloxacin in the single- and double-solid sorption systems with equal mass are described in this section. The sorption parameters and adjusted coefficient of determination values (\(r_{\text{adj}}^{2}\)), fitted by the Linear, Langmuir, and Freundlich models, are listed in Table 1. According to \({\text{r}}_{\text{adj}}^{ 2}\), the isotherm of lindane sorption onto three single- and double-solid sorbents was well fitted by the linear model (\(r_{\text{adj}}^{2} > 0.946\)), suggesting that a linear partition contributed to sorption. For lindane, the hydrophobic partition should be the most important mechanism of its sorption onto natural solids, and the isotherm was linear (Du et al. 2010). Meanwhile, the isotherm of ciprofloxacin onto these sorbents was well fitted by the Langmuir model (\(r_{\text{adj}}^{2} > 0.699\)), indicating that nonlinear sorption mechanisms were involved, such as H-bonding and electrostatic interactions (Cao et al. 2017).
To demonstrate the changes in the sorption capacities of the double-solid systems, the estimated KH values for lindane (KH, E) and KM for ciprofloxacin (KM, E) were calculated from the relevant values in the single-solid system. The KH, E of the B + P sorption system was calculated as follows: KH, E, B+P = (KH, B + KH, P)/2, where KH, B and KH, P were the linear partition coefficients in the biofilm and particle sorption systems, respectively. KM, E was calculated in the same matter as KH, E. The KH, E and KM, E in B + P, B + S, and P + S systems were 1.98 ± 0.12, 1.83 ± 0.11, and 1.39 ± 0.07 L/g for lindane and 2.42 ± 2.15, 2.10 ± 1.63, and 1.90 ± 2.30 L/g for ciprofloxacin, respectively. There was no significant difference between KH, E and KH, and between KM, E and KM (P > 0.05) in the B + P and B + S systems. In contrast, KH, E was significantly greater than KH (0.99 L/g), while KM, E was significantly lower than KM (2.70 L/g) (P < 0.05) in the P + S system. This indicates that the sorption capacities of lindane were lower and those of ciprofloxacin were higher than the estimated capacities in the P + S system. A previous study found that DOM in multiple-solid sorption systems was responsible for changes in sorption characteristics (Guo et al. 2012). Here, we hypothesized that differences between the estimated and measured values in the double-solid systems could be due to the redistribution and transformation of DOM originating from different solids.
Role of dissolved organic matter in sorption capacities in the double-solid sorption systems
To test the hypothesis that changes in DOM could affect sorption in the double-solid system, lindane and ciprofloxacin were sorbed onto single- and double-solid substances with equal DOM content. As different sorbent masses were utilized, the sorption amounts of the sorbates could not be used to compare sorption capacities. Thus, the mean decreased sorbate concentrations (C) at different initial sorbate concentrations are listed in Table 2.
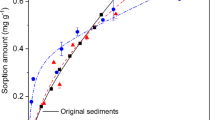
The estimated C values in the double-solid system (CE) were calculated from the sum of the measured C values in the single-solid system to determine the effect of DOM on sorption in a double-solid system. Thus, the values of CE in the B + P, B + S, and P + S sorption systems were 6.52 ± 3.62, 9.54 ± 5.68, and 11.10 ± 6.19 μg/L for lindane, and 11.27 ± 2.93, 12.96 ± 1.73, and 15.35 ± 2.70 mg/L for ciprofloxacin, respectively. There was no significant difference between the estimated and measured values in the double-solid sorption system with equal DOM contents (P > 0.05). This could be due to the weak redistribution and transformation of DOM, which did not affect the lindane and ciprofloxacin sorption capacities of natural solid substances.
To further elucidate changes in DOM due to redistribution and transformation, UV–Vis absorption spectra of DOM from the blank samples of single- and double-solid systems with equal mass were captured (Fig. 1). E250 and E365 represent absorbance at 250 and 365 nm, respectively. The quotient E250/E365 is negatively correlated with the molecular size and aromaticity of DOM (De Haan and De Boer 1987). The values of E250/E365 in the biofilms, particles, sediments, B + P, B + S, and P + S sorption systems were 6.19, 5.75, 4.22, 4.57, 3.52, and 1.56, respectively. Here E250/E365 were lower in the double-solid systems than those in the relevant single-solid systems, indicating that the molecular size and aromaticity of DOM increased when it mixed with solid substances. The transformation of DOM could affect the binding affinities of the organic pollutant to DOM and of DOM to the organic and inorganic matrices, and the sorption affinity of the DOM–organic pollutant complex to the natural solids. The changed DOM could affect the solubility of lindane (Dorado et al. 2003). The increased solubility of lindane would reduce its sorption onto natural solids. In contrast, the decreased solubility of lindane would enhance sorption (Dong et al. 2017b). Furthermore, the changed DOM could have a weak competitive sorption capacity for hydrophobic organic contaminants (Poerschmann and Kopinke 2001). Thus, DOM exhibited complex effects on sorption capacities. The changed DOM could decrease the lindane sorption capacities of solid substances under certain circumstance (such as the coexistence of suspended particles and sediments). Ciprofloxacin can be easily adsorbed onto natural solid substances through cation exchange reactions between its cationic amine moiety (=NH2+) to negatively charged clay minerals, hydrogen bonding and columbic attraction to organic matter, or surface complexation reactions between its carboxyl group (–COO–) to oxide minerals (Tan et al. 2015). The changes in DOM could affect these interactions and enhance the binding affinity of ciprofloxacin to solid substances in the P + S sorption system. In summary, the combined suppressive and promotive effects of DOM resulted in differences between the measured and estimated lindane and ciprofloxacin sorption capacities in double-solid sorption systems.
Conclusion
Here we showed that the coexisting river solids could affect the lindane and ciprofloxacin sorption. The lindane and ciprofloxacin sorption capacities in double-solid system of particles and sediments were lower and higher than the estimated values, respectively. However, there was no significant difference between measured and estimated sorption capacities of both contaminants in the biofilms and particles, and biofilms and sediments systems. These apparent changed or unchanged sorption capacities of the double-solid sorption system were caused by the combination of complex effects of DOM that suppressed and promoted sorption. Thus, the effects caused by DOM should be considered when estimating sorption capacities in different solids systems from those of single-solid sorption systems.
References
Berger M, Löffler D, Ternes T et al (2016) Hexachlorocyclohexane derivatives in industrial waste and samples from a contaminated riverine system. Chemosphere 150:219–226. https://doi.org/10.1016/j.chemosphere.2016.01.122
Cao EJ, Duan WZ, Wang AQ et al (2017) Oriented growth of poly (m-phenylenediamine) on Calotropis gigantea fiber for rapid adsorption of ciprofloxacin. Chemosphere 171:223–230. https://doi.org/10.1016/j.chemosphere.2016.12.087
De Haan H, De Boer T (1987) Applicability of light absorbance and fluorescence as measures of concentration and molecular size of dissolved organic carbon in humic Lake Tjeukemeer. Water Res 21:731–734. https://doi.org/10.1016/0043-1354(87)90086-8
Dong DM, Guo ZY, Hua XY et al (2011) Sorption of DDTs on biofilms, suspended particles and river sediments: effects of heavy metals. Environ Chem Lett 9:361–367. https://doi.org/10.1007/s10311-010-0287-x
Dong DM, Li LF, Zhang LW et al (2017a) Effects of lead, cadmium, chromium, and arsenic on the sorption of lindane and norfloxacin by river biofilms, particles, and sediments. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0840-2
Dong DM, Zhang LW, Guo ZY et al (2017b) The role of extracellular polymeric substances on the sorption of pentachlorophenol onto natural biofilms in different incubation times: a fluorescence study. Chem Ecol 33:131–142. https://doi.org/10.1080/02757540.2017.1281253
Dorado J, González-Vila FJ, Zancada MC et al (2003) Pyrolytic descriptors responsive to changes in humic acid characteristics after long-term sustainable management of dryland farming systems in Central Spain. J Anal Appl Pyrol 68:299–314. https://doi.org/10.1016/S0165-2370(03)00057-3
Du CF, Shang SL, Dong QA et al (2010) Characteristics of chromophoric dissolved organic matter in the nearshore waters of the western Taiwan Strait. Estuar Coast Shelf Sci 88:350–356. https://doi.org/10.1016/j.ecss.2010.04.014
Fan Y, Zhao Z, Yu F et al (2017) A 21-year record of methoxylated and hydroxylated polybrominated diphenyl ethers in sediments from the East China Sea. Environ Chem Lett 15:679–687. https://doi.org/10.1007/s10311-017-0637-z
Guo ZY, Hua XY, Lan XH et al (2012) Evidence for a mutual effect of biofilms, suspended particles and sediments on DDT sorption. Environ Chem Lett 10:407–411. https://doi.org/10.1007/s10311-012-0369-z
Guo ZY, Dong DM, Hua XY et al (2015) Cr and As decrease lindane sorption on river solids. Environ Chem Lett 13:111–116. https://doi.org/10.1007/s10311-014-0489-8
Kalathoor R, Zeiner M, Schmidt B et al (2015) First evidence for covalent linkage of acidic metabolites of metalaxyl and DDT as non-extractable pesticide residues in soil and sediment. Environ Chem Lett 13:431–437. https://doi.org/10.1007/s10311-015-0514-6
Kucher S, Schwarzbauer J (2017) DDT-related compounds as non-extractable residues in submarine sediments of the Palos Verdes Shelf, California, USA. Chemosphere 185:529–538. https://doi.org/10.1016/j.chemosphere.2017.07.041
Matott LS, Singh A, Rabideau AJ (2017) Parameterizing sorption isotherms using a hybrid global–local fitting procedure. J Contam Hydrol 200:35–48. https://doi.org/10.1016/j.jconhyd.2017.03.006
Miao LZ, Wang C, Hou J et al (2014) Kinetics and equilibrium biosorption of nano-ZnO particles on periphytic biofilm under different environmental conditions. J Environ Inform 23:1–9. https://doi.org/10.3808/jei.201400267
Moura L, Moufawad T, Ferreira M et al (2017) Deep eutectic solvents as green absorbents of volatile organic pollutants. Environ Chem Lett 15:747–753. https://doi.org/10.1007/s10311-017-0654-y
Poerschmann J, Kopinke FD (2001) Sorption of very hydrophobic organic compounds (VHOCs) on dissolved humic organic matter (DOM). 2. Measurement of sorption and application of a Flory–Huggins concept to interpret the data. Environ Sci Technol 35:1142–1148. https://doi.org/10.1021/es0017615
Polubesova T, Sherman-Nakache M, Chefetz B (2007) Binding of pyrene to hydrophobic fractions of dissolved organic matter: effect of polyvalent metal complexation. Environ Sci Technol 41:5389–5394. https://doi.org/10.1021/es070722r
Tan YY, Guo Y, Gu XY et al (2015) Effects of metal cations and fulvic acid on the adsorption of ciprofloxacin onto goethite. Environ Sci Pollut Res 22:609–617. https://doi.org/10.1007/s11356-014-3351-4
Tijani JO, Fatoba OO, Babajide OO et al (2016) Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: a review. Environ Chem Lett 14:27–49. https://doi.org/10.1007/s10311-015-0537-z
Zhang JQ, Dong YH (2008) Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J Hazard Mater 151:833–839. https://doi.org/10.1016/j.jhazmat.2007.11.046
Zhang LW, Dong DM, Hua XY et al (2018) Inhibitory effects of extracellular polymeric substances on ofloxacin sorption by natural biofilms. Sci Total Environ 625:178–184. https://doi.org/10.1016/j.scitotenv.2017.12.271
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 21577047 and 21307041) and the 111 Project (No. B16020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Dong, D., Xie, Y. et al. Coexisting sediments and suspended particles change the sorption of lindane and ciprofloxacin in waters. Environ Chem Lett 16, 1043–1048 (2018). https://doi.org/10.1007/s10311-018-0715-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0715-x