Abstract
The sorption of both classic and emerging organic contaminants onto aquatic solids is a critical process that controls their fate in natural waters. Sorption is affected by numerous factors, including coexisting heavy metals. The mechanisms of the influence of heavy metals, especially those occurring in acid radical anions, are still unclear. Here, the effects of Pb, Cd, Cr, and As on the sorption of lindane and norfloxacin (NOR) onto natural biofilms, suspended particles, and sediments from one river were investigated following batch equilibration methods. In addition, changes in representative components that have important roles in sorption from these solids in the presence and absence of metals were characterized by spectrum analyses. The results indicated that sorption of lindane and NOR on the three solids in the absence of heavy metals was highly linear and nonlinear, respectively. Pb and Cd promoted and Cr and As suppressed hydrophobic lindane sorption on the three solids. This was because Pb and Cd enhanced but Cr and As weakened the hydrophobicity of these solids. Pb, Cd, Cr, and As decreased NOR sorption on sediments and suspended particles at pH 5.7~6.3. This was due to electrostatic competition between cationic Pb/Cd and NORH2 +, and the combination of Cr/As acid radicals with NORH2 +, which suppressed its ion-exchange adsorption. Pb, Cd, Cr, and As generally increased the sorption of NOR onto the biofilms at pH 5.7~6.3. Pb and Cd strengthened the flocculation of dissolved organic matter combined with NORH2 + onto the biofilms. Cr and As enhanced the hydrophilicity of biofilms, and then increased their sorption of NOR with active hydrophilic groups. The mechanisms of how different heavy metals affect NOR sorption by biofilms were more complicated than the mechanisms affecting lindane sorption, as well as by sediments and particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs), a large group of persistent organic pollutants (POPs), have received great concern as they are produced and used in large quantities, they have deleterious effects on non-target organisms, they are ubiquitous, and they bioaccumulate and persist in the environment (Ali et al. 2014). Gamma-hexachlorocyclohexane (γ-HCH) is a classic OCP, also known as lindane. Most countries have prohibited the use of the persistent compounds, but some still use lindane for economic reasons. Historical use, coupled with its extreme persistence, has caused a global legacy environmental issue (Abhilash et al. 2008). Antibiotics, which are regarded as emerging contaminants of concern, have been identified in many natural environmental compartments, such as river water, sediments, soils, and groundwater (Dong et al. 2016). Residual antibiotics in the environment could have adverse effects on nontarget organisms and cause increased bacterial resistance (Zhang et al. 2015). Norfloxacin (NOR) with a fluoroquinolone backbone is a common and useful antibiotic. Similar to other antibiotics, the presence of NOR in effluent and sludge from domestic wastewater treatment plants, hospitals, and livestock farms results in their release to recipient environments, such as surface waters (Pei et al. 2011).
The aquatic environment is a complex system in which inorganic heavy metals and organic contaminants commonly coexist (Pei et al. 2014; Guo et al. 2015). The sorption of both classic and emerging contaminants onto aquatic solids, such as sediments, suspended particles, sludge, and biosolids is of great importance from an environmental viewpoint (Wei et al. 2015, 2016b, c; You et al. 2017). The sorption of organic pollutants is affected by many factors, including coexisting heavy metals (Guo et al. 2015). It was previously stated that metal ions could influence the sorption of POPs and antibiotics onto various sorbents (Pei et al. 2014; Luo et al. 2008, 2010; Dong et al. 2011; Guo et al. 2015). These studies indicated that coexisting metal ions can facilitate or suppress the sorption of these organic contaminants, and thereby affect their fate and risk levels. Metal ions cannot usually directly interact with most nonpolar hydrophobic organic compounds (HOCs) and affect their sorption. Metal ions can affect the hydrophobicity and hydrophilicity of solid sorbents by changing their structure and surface charges, and then enhance or weaken the hydrophobic partition of HOCs. For example, it was found that the presence of metal cations could increase the capacity and nonlinearity of HOCs sorption to soils through decreasing the negatively charged density of soil surfaces and causing rubbery organic carbons, such as humic acids and biopolymers, to become more condensed and rigid (Luo et al. 2008, 2010). The mechanisms of mutual effects between metal ions and antibiotics with various reactive functional groups are more complex. In general, metal cations could increase the sorption of antibiotics through electrostatic attraction, salting-out effects, and cation bridging. However, metal cations could decrease their sorption through competition or outer-sphere complexation (Wu et al. 2014). For example, NOR is a zwitterionic molecule and it was found that the presence of Cu(II) suppressed the sorption of NORH2 + onto soil minerals at pH 4.5 due to competition. In contrast, Cu cations increased the sorption of NOR at pH 7.0 and 9.0 because Cu(II)-NOR complexes were more positively charged than NORH± and NOR− (Pei et al. 2011).
However, to the best of our knowledge, the mechanisms causing the effects of different heavy metal ions on antibiotics, as well as OCPs, are complicated. Controversial results have been found in literature, and the relevant effect mechanisms have not been clearly addressed. Providing more information in the co-sorption of metal ions and these organic compounds onto various solids will facilitate further discussion about effect mechanisms. It should also be noted that previous studies generally focused on effects by cation metals under specific water chemistry conditions. There are many heavy metals or metalloids that do not exist as cations in the environment. For example, the majority of Cr(VI) and As(V) exist in water and soil environments as acid radical anions (Babel and Kurniawan 2004; Mohan and Pittman 2007). Our previous study found they differed from metal cations, as Cr(VI) and As(V) could significantly decrease the sorption of HOCs to natural solids (Guo et al. 2015). The mechanisms by which heavy metals affect the sorption of organic compounds on solid sorbents could reasonably be attributed to the combination of water chemistry conditions and organic compound, heavy metal, and solid sorbent properties. However, to the best of our knowledge, the underlying mechanisms have not been clearly addressed.
The specific aims of this study were to investigate how heavy metal cations and acid radical anions affect the sorption of lindane and NOR on three river solids, including biofilms, suspended particles, and sediments. Different organic compounds, heavy metals, and solid sorbents with different properties were selected. Lindane and NOR were selected as the model POPs and antibiotics, respectively. Four hazardous priority metal and metalloid pollutants, Pb, Cd, Cr, and As, listed by the US Environmental Protection Agency were selected to study (Dai and Hu 2015; Dai et al. 2016). Pb and Cd commonly occur as divalent cations, but Cr and As commonly occur as acid radical anions in the aquatic environment (Babel and Kurniawan 2004; Mohan and Pittman 2007). Organic components from natural solids also play an important role in the sorption of trace contaminants. Among the organic components in natural solids, dissolved organic matters (DOMs) have been regarded as one of the most active organic components that play a role in the sorption of inorganic heavy metals and organic pollutants onto solids (Polubesova et al. 2007). It could, therefore, be speculated that DOM also play an important role in the co-sorption of organic and inorganic pollutants. In addition, the compositions of DOM samples can be rapidly and sensitively characterized by spectrum analysis, such as ultraviolet-visible (UV-vis) spectra and three-dimensional excitation-emission matrix (3D-EEM) fluorescence spectroscopy (Wei et al. 2016a; Dong et al. 2017). In this study, these methods were employed to analyze changes in DOM in sorption systems with the presence and absence of heavy metals to elucidate related mechanisms.
Materials and methods
Materials
Natural biofilms, suspended particles, and surface sediments were collected from Liao River in Jilin Province, northeastern China. Surface sediments (0–5 cm) were collected, biofilms were scraped from rocks in shallow waters, and suspended particles were collected from river water that passed through a 0.45-μm filter membrane. Biofilms and sediments were passed through a 0.18-mm sieve to remove large plant residues and stones. A portion of the three solid samples were freeze-dried and ground so that they could pass through a 0.15-mm sieve to determine their pH values, cation exchange capacities (CEC), and dissolved organic carbon (DOC) content that constituted their DOM content. The methods of measuring these physicochemical parameters are listed in Table S1 in Supplementary material. The remainder (5 mg freeze-dried weight mL−1) of the three solid samples were suspended for the sorption experiment.
Lindane (99% purity) and NOR (98% purity) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany) and Bomei Biotechnology Co. Ltd. (HeFei, China), respectively. Some of the physicochemical properties of lindane and NOR are presented in Table S2. Reagent-grade Pb(NO3)2, Cd(NO3)2, K2CrO4, and Na2HAsO4 were used to prepare Pb, Cd, Cr, and As solutions, respectively. The organic reagents used in the chromatographic analysis were of HPLC grade.
Sorption experiments
All sorption experiments were conducted in duplicate following a batch equilibration technique at 25 ± 1 °C. Sorption of lindane (0.2, 0.5, 1.0, 1.5, and 2.0 μmol L−1) and NOR (40, 100, 150, 200, and 400 μmol L−1) were conducted by mixing 20 mg dry weight of the solid sample in 40-mL glass centrifuge tubes with 0.003 mol L−1 NaN3 to suppress bacterial activity and 0.1 mol L−1 KNO3 to maintain ionic strength. These tubes were rotated continuously for 24 h at 100 r min−1 in the dark. Our previous experiments indicated that the apparent equilibrium was reached within 24 h. The pH values of the initial and equilibrium sorption solutions were measured. The equilibrium sorption solutions were centrifuged at 1788×g for 5 min. Aliquots of the supernatant (5 mL) were further filtered through a 0.45-μm glass fiber filter membrane, and the lindane content was measured by a gas chromatograph (GC) and NOR by high-performance liquid chromatography (HPLC). Two controls containing sorbents without solutes and solutes without sorbents were simultaneously studied to assess solute dissolution from sorbents and the loss of solutes, respectively.
Effects of heavy metals on the sorption
Two types of sorption experiments were conducted in the presence of Pb, Cd, Cr, and As: (I) lindane and NOR with different concentrations to determine how they are affected by heavy metals with a constant concentration, and (II) lindane and NOR at a constant concentration to determine how they are affected by heavy metals with different concentrations. For the type I experiments, batch sorption experiments were conducted, adding 10 μmol L−1 Pb(NO3)2, Cd(NO3)2, K2CrO4, and Na2HAsO4 to the initial sorption solution. For the type II experiments, lindane and NOR concentrations were initially 1 and 150 μmol L−1, respectively, and 5, 10, 25, and 50 μmol L−1 of heavy metal salts were added to the initial sorption solution.
Spectral analysis
The UV-vis absorption spectra and 3D-EEM fluorescence spectroscopy of DOM from biofilms were recorded. Biofilms (20 mg dry weight) were added to 40-mL glass centrifuge tubes with 10 μmol L−1 Pb(NO3)2, Cd(NO3)2, K2CrO4, Na2HAsO4, and Milli-Q water, and were then rotated continuously for 24 h at 100 r min−1. Following this, aliquots of supernatant were filtered through 0.45-μm glass fiber membranes were characterized by spectrum analysis. The UV-vis absorption spectra (UV-1800, Shimadzu, Japan) were recorded at 1-nm intervals over a range of 200–600 nm, with a scan speed of 120 nm min−1. The 3D-EEM fluorescence spectra were determined using a fluorescence spectrophotometer (F-2700, Hitachi, Japan) equipped with a 150-W xenon arc lamp. The spectra were recorded at 5-nm intervals over an excitation range of 220–500 nm, with an emission range of 220–550- and 2-nm intervals. The excitation and emission slits were both set to 5 nm of the band-pass. The voltage of the photomultiplier tube (PMT) was 700 V, and the scan speed was set to 1200 nm min−1 with an auto response time. Milli-Q water was used as the blank for spectral analysis. The entire procedure was conducted in triplicate, and the mean values were utilized for discussion.
Determination of lindane and norfloxacin
The lindane in the equilibrium solution was analyzed using a gas chromatograph equipped with an electron capture detector (GC-ECD, GC-2014C, Shimadzu, Japan) after extraction using C18 solid-phase extraction columns. The GC was equipped with a capillary column (Rtx-1, 30 m × 0.25 mm × 0.25 μm). During analysis, the column was initially maintained at a temperature of 100 °C, which was then increased at a rate of 20 °C min−1 to 210 °C, and increased further at a rate of 3 °C min−1 to 230 °C, which was held for 5 min. The temperature of the injector and detector were maintained at 250 and 300 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 1.6 mL min−1. A 1 μL sample was injected in the splitless mode. The NOR in the equilibrium solution was analyzed using a HPLC (LC-20AT, Shimadzu, Japan) equipped with a C18 column (5 μm, 4.6 mm × 150 mm). The mobile phase consisted of a mixed solution of 0.025 mol L−1 phosphoric acid-acetonitrile (80/20, V/V), for which the pH was adjusted to 3.0 with triethylamine, and the flow rate was 1.0 mL min−1. The UV detection wavelength was set at 278 nm, and the concentrations of lindane and NOR were determined following the working curve method. The control tests containing the three solids without lindane or NOR, and containing lindane or NOR without solids, indicated that the loss and increase of lindane and NOR by other reactions or dissolution from solids were negligible.
Data analysis
The equilibrium sorption quantities q e (μmol g−1) of lindane and NOR on solids were calculated by Eq. (1):
where W(g) and V(L) are the mass of the sorbent and volume of the aqueous solution, respectively, and C 0 (μmol L−1) and C e (μmol L−1) are the initial and equilibrium concentrations in the sorption solution, respectively.
The single-point distribution coefficient K d (L g−1) was used to directly compare the sorption capacities of sorbents at a certain equilibrium concentration (Xiao et al. 2007). It was calculated by Eq. (2):
Linear, Langmuir, and Freundlich models were used to fit the equilibrium sorption data. These models are expressed as follows:
where K p (L g−1), K L (L g−1), and K F (μmol1-n Ln g−1) are the linear, Langmuir, and Freundlich model sorption coefficients, respectively. q m (μmol g−1) is the maximum sorption quantity in the Langmuir model. n is the Freundlich model’s site energy heterogeneity factor and is an indicator of isotherm nonlinearity.
The adjusted coefficient of determination (R 2 adj ) was used to compare the performance of the three models and was calculated as follows:
where N and M are the numbers of experimental data points and parameters in the model, respectively (Pan et al. 2012).
Results and discussion
Physicochemical properties of the three solid materials
Four physicochemical properties of the natural biofilms, suspended particles, and surface sediments are listed in Table S1. The biofilms had the lowest pH value, indicating that they contained more acidic functional groups, such as carboxyl or sulfur functional groups (Chen et al. 2013). The CEC of the three solid materials decreased in the following order: sediments > particles > biofilms. Sediments could have had the highest CEC as they had a higher proportion of clay components (Pils and Laird 2007). Both the contents of TOC and DOC in the three solids decreased in the following order: biofilms > particles > sediments. The proportion of DOC in the TOC of sediments, particles, and biofilms were 7, 8, and 9%, respectively. The biofilms had the highest TOC and DOC contents as they contained a higher proportion of fresh organic matter, including algae, microbes, and extracellular polymeric substances (Flemming et al. 2016; Xu et al. 2016; Hou et al. 2016). Based on the four properties of the three solids, the compositions of the biofilms and sediments were vastly different from the suspended particles. This pattern was consistent with the results of our previous studies, in which more physicochemical and biological properties of different batches of the three solid samples were determined (Guo et al. 2012; Dong et al. 2011).
Sorption isotherms of lindane and NOR on the three solids
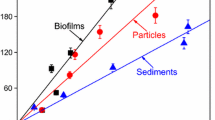
The parameters of the lindane and NOR sorption isotherms for the biofilms, suspended particles, and sediments fitted by the linear, Langmuir, and Freundlich models are presented in Tables S3 and S4. The lindane sorption isotherms for the three solids fitted well with the Freundlich model (R adj 2 > 0.94), and the NOR sorption isotherms for the three solids fitted well with the Langmuir model (R adj 2 > 0.81), which was determined by comparing the R adj 2 values. Figure 1 shows the sorption isotherms of lindane fitted by the Freundlich model and NOR fitted by the Langmuir model on the three solid materials in the absence of heavy metals. The sorption of lindane on the three solids appeared to be highly linear. The Freundlich linearity index n values for biofilms, particles, and sediments were 0.87, 0.80, and 0.95, respectively. The quantities of lindane sorbed on suspended particles and biofilms were significantly higher than those sorbed on sediments (p < 0.05). The sorption of NOR on the three solids appeared to be highly nonlinear. The quantities of NOR sorbed on suspended particles and sediments were significantly higher than those sorbed on biofilms (p < 0.05).
The distribution coefficient K d, calculated by Eq. (2) and based on five sorption data, was used to quantitatively compare the sorption of lindane and NOR on the three solids. The K d values are listed in Table S5. The K d values for lindane sorption on the three solids decreased in the following order: suspended particles (0.82 L g−1) > biofilms (0.70 L g−1) > sediments (0.49 L g−1). The K d values for lindane sorption on the three solids were slightly positively correlated with the DOC (r = 0.780) of the three solids. The K d values for NOR sorption on the three solid materials decreased in the following order: suspended particles (6.39 L g−1) > sediments (5.03 L g−1) > biofilms (2.04 L g−1). The K d values for NOR sorption on the three solid materials were slightly positively correlated with the CEC (r = 0.793) of the three solid materials. This indicated that the DOC and CEC content of the solids had significant effects on the sorption of lindane and NOR, respectively.
The sorption of organic pollutants mainly occurs by (1) absorption, in which hydrophobic interactions occur between the aliphatic and aromatic groups of an organic compound and organic components, such as the fat fraction of natural solids and the extracellular polymeric substances of biofilms, and (2) adsorption, involving electrostatic interactions between the positively charged groups with the negatively charged surfaces of natural solids (Luo et al. 2010). For lindane, its hydrophobicity should be the most important mechanism causing its sorption on the three solids, whereas for the dissociable NOR, ion exchange should be one of the main mechanisms causing its sorption on the three solids (Wu et al. 2014).
Effects of four heavy metals with a single concentration on the sorption
The sorption data of lindane and NOR in the presence of Pb, Cd, Cr, and As were also well fitted by the Freundlich and Langmuir models, respectively (listed in Tables S3 and S4). As shown in Fig. 2a–c, Pb and Cd significantly promoted the sorption of lindane on the three solids (p < 0.05). In contrast, the presence of Cr and As significantly inhibited the sorption of higher concentrations of lindane (> 1 μM) on the three solids (p < 0.05). The K d values for lindane sorption on the biofilms increased by 13.6 and 14.5%, sorption on the suspended particles increased by 7.8 and 9.3%, and sorption on the sediments increased by 29.3 and 45.6% in the presence of Pb and Cd, respectively. In contrast, the K d values for lindane sorption on biofilms decreased by 16.3 and 18.9%, sorption on the suspended particles decreased by 12.3 and 30.3%, and sorption on the sediments decreased by 3.2 and 5.1% in the presence of Cr and As, respectively. As shown in Fig. 2d–f, Pb, Cd, Cr, and As significantly suppressed the sorption of NOR on suspended particles and sediments (p < 0.05), whereas Pb, Cd, Cr, and As significantly promoted the sorption of higher concentrations of NOR (> 150 μM) on the biofilms (p < 0.05). The K d values for NOR sorption on the suspended particles decreased by 38.9, 29.3, 26.7, and 32.2%, and sorption on the sediments decreased by 39.5, 36.4, 27.8, and 29.1% in the presence of Pb, Cd, Cr, and As, respectively. However, the K d values for NOR sorption at concentrations greater than 150 μM on the biofilms increased by 50.1, 67.8, 83.3, and 98.9% in the presence of Pb, Cd, Cr, and As, respectively.
Effects of four heavy metals with different concentrations on the sorption
The effects of four different concentrations of Pb, Cd, Cr, and As on the sorption quantities of lindane and NOR on the three solids are shown in Fig. 3. The molar concentration of the four heavy metals to lindane and NOR ratios ranged from 5 to 50 and from 0.03 to 0.3, respectively. All the quantities of lindane sorbed onto the three solids increased with increasing ratios of Pb and Cd to lindane. The quantities sorbed increased by 3.3–21.5, 21.3–32.6, and 4.3–25.1% on the biofilms, suspended particles, and sediments, respectively. When the ratio of Pb or Cd to lindane was reached the maximum, the sorption quantities of lindane on the three solids correspondingly reached the maximums. In contrast, the sorption quantities of lindane on the three solids decreased with increasing ratios of Cr and As to lindane. With the increasing Cr and As to lindane ratios, the sorption quantities decreased by 2.3–6.7, 3.2–12.3, and 1.4–14.5% on the biofilms, suspended particles, and sediments, respectively. When the ratio of Cr or As reached the maximum, the quantities of lindane sorbed on the three solids correspondingly reached the minimum.
All quantities of NOR sorbed on the three solids decreased with increasing ratios of Pb and Cd to NOR. The sorption quantities decreased by 3.3–16.7, 4.2–12.3, and 10.1–20.3% on the biofilms, suspended particles, and sediments, respectively. The sorption quantities of NOR on the three solids also decreased with increasing ratios of Cr and As to NOR. With increasing Cr and As to NOR ratios, the sorption quantities decreased by 6.7–16.1, 4.2–15.3, and 10.2–17.8% on the biofilms, suspended particles, and sediments, respectively.
The effects of four different concentrations of the heavy metals on the sorption of NOR and lindane at fixed concentrations were also compared through the ratio of K d to K d0, where K d was the distribution coefficient in the presence of different heavy metals at different concentrations and K d0 was the distribution coefficient in the absence of heavy metals. Both K d and K d0 were calculated by Eq. (2). The K d/K d0 values as functions of the four heavy metals’ concentrations are shown in Fig. 4. Most K d/K d0 values for lindane sorption onto the three solids in the presence of Pb and Cd exceeded 1, whereas most K d/K d0 values were below 1 in the presence of Cr and As, and decreased with increasing Cr and As concentrations. This also indicated that Pb and Cd promoted lindane sorption onto the three solids, but Cr and As inhibited sorption. From the degrees of K d/K d0 value discretization for the sorption of lindane onto the three solids, the degrees of the effects of the four heavy metals on lindane sorption onto biofilms and suspended particles were higher than that onto sediments. All the K d/K d0 values for NOR sorption onto suspended particles and sediments in the presence of four heavy metals with four concentrations were below 1. This indicated that Pb, Cd, Cr, and As all inhibited NOR sorption onto suspended particles and sediments. However, at different concentrations, most heavy metals promoted NOR sorption onto biofilms, with the exclusion of Cr at higher concentrations.
The radio of sorption distribution coefficients for the sorption of lindane and norfloxacin (NOR) and on the three solids in the presence of four concentrations of Pb, Cd, Cr, and As. K d0 is the sorption distribution coefficient for lindane or NOR in the absence of metals; K d is the sorption distribution coefficient for lindane or NOR in the presence of metals with different concentrations
Analysis of mechanisms of the sorption affected by heavy metals
In this study, water chemistry conditions were almost constant, so the speciation of heavy metals, properties of organic compounds, and changes in key solid components were analyzed to elucidate the effect mechanisms of heavy metals on the sorption. First, we aimed to explain the effects on sorption by the speciation of lindane, NOR, and heavy metals. Due to the complexity of natural solids, the discussion was based on the calculated speciation under sorption solution chemistry conditions. The calculated speciations of heavy metals by Visual MINTEQ (version 3.0) are listed in Tables S6 and S7. The Pb and Cd present in the sorption solution were primarily Pb2+ (proportion of > 62%) and Cd2+ (> 89%), respectively, and Cr and As were primarily HCrO4 − (> 60%) and H2AsO4 − (> 81%), respectively. Therefore, cationic Pb(II), Cd(II), and anionic Cr(VI) and As(V) acid radicals were the main species of the four heavy metals present in the sorption solution. At slightly acidic pH values (5.7~6.3), cationic NORH2 + and zwitterionic NORH± were the main NOR species (Zhang and Dong 2008). The distributions of cationic (NORH2 +), zwitterionic (NORH±), and anionic (NOR−) norfloxacin in an aqueous solution as a function of pH is shown in Fig. S1.
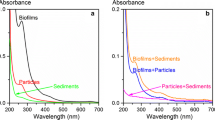
Second, we aimed to explain the effects caused by changes in the solid sorbents in the sorption systems. DOMs from natural solid materials are one of the most active organic components and have significant effects on both the sorption of inorganic heavy metals and organic pollutants onto the solids, although they exist as a small proportion of the total organic components. Therefore, it could be speculated that DOM had an important effect on the co-sorption of the two pollutants. EEM spectra could provide qualitative and quantitative information about the chemical compositions of DOM in solution. To evaluate the effects of interactions between heavy metals and a DOM solution during the sorption process, the highest DOC content of the biofilms was measured after the addition of 10 μM of Pb, Cd, Cr, and As. The EEM spectra of DOM from biofilms in the presence and absence of heavy metals are presented in Fig. 5. As shown in Fig. 5a, four regions indicating fluorescence peaks A, B, C, and D had been detected in the DOM solutions of the biofilms without heavy metals. The corresponding fluorescent parameters, including peak locations and intensities, are summarized in Table S8. Among the four peaks, peak B was located at the excitation/emission wavelength (EX/EM = 250–340/380–430 nm), which corresponds with the presence of humic acid-like substances. Peak D (EX/EM = 200–250/350–405 nm) is indicative of fulvic acid-like substances. The fluorescence peaks of humic and fulvic acid-like substances are due to the structure of carboxyl and carbonyl groups in the DOM (Chen et al. 2003). Peak A (EX/EM = 250–285/280–330 nm) and peak C (EX/EM = 200–235/280–330 nm) are both indicative of protein-like substances, which were associated with aromatic amino acid structures in DOM (He et al. 2011). From Fig. 5b–e, the intensities of all fluorescence peaks changed in the presence of heavy metals, suggesting interactions between DOM and heavy metals. Compared to peaks A and C, the intensities of peaks B and D were noticeably reduced by 36.4–69.8 and 55.6–81.9%, respectively, suggesting that humic and fulvic acid-like substances in DOM could interact with metals (Zhang et al. 2010).
The corresponding UV-vis scanning spectra for DOM from biofilms are also presented in Fig. 5f. The interval ranging from 250 to 280 nm represents aromatic organics owing to π–π* electronic transitions (Dilling and Kaiser 2002). E203, E250, E253, and E365 represent absorbances at 203, 250, 253, and 365 nm, respectively. The related absorbance ratios were E250/E365 and E253/E203 (Uyguner and Bekbolet 2005). Quotient E250/E365 has been found to negatively correlate with the molecular size and aromaticity of DOM, and when the E250/E365 ratio increases, the molecular size of DOM solutes decreases (Peuravuori and Pihlaja 2004). The E253/E203 ratio can indicate the type of substituents and degree of substitution. When the E253/E203 ratio increases, it indicates that aromatic ring substituents in DOM contain more carbonyl, carboxyl, and hydroxyl groups; otherwise, DOM primarily contains irreplaceable aromatic rings (Korshin et al. 1997). In this study, the E250/E365 values (listed in Table S9) of DOM increased by 12.3 and 30.3% in the presence of Pb and Cd, respectively, and decreased by 70.1 and 6.3% in the presence of Cr and As, respectively, indicating that the molecular size of DOM decreased in the presence of Pb and Cd, while it increased in the presence of Cr and As. This could be due to the flocculation of DOM macromolecules on solid surfaces through cation bridges between the hydrophilic anionic DOM functional groups and cationic Pb and Cd (Luo et al. 2008). In contrast, due to anionic electrostatic repulsion, the structure of organic components in the biofilms became looser, and the organic matter in the biofilms further dissolved in the presence of anionic Cr(VI) and As(V) acid radicals. The E253/E203 values of DOM all decreased in the presence of all four heavy metals, suggesting that these heavy metals could interact with the carbonyl, carboxyl, and hydroxyl groups in DOM.
A schematic diagram of the sorption mechanisms of lindane and NOR onto the biofilms, particles, and sediments as affected by Pb, Cd, Cr, and As is shown in Fig. 6. When Pb and Cd existed in the divalent cation form, the loose organic compounds could first flocculate on the solid surfaces through cationic bridges (Luo et al. 2008). This caused the structure of organic components on the solid surface to become more condensed, and some hydrophilic groups could simultaneously be wrapped by the flocculates. It has been found that the sorption capacity of fresh organic matters flocculated on solid surfaces through ion bridging for hydrophobic organic contaminants was stronger than that of inherent insoluble organic matters onto solid surfaces (Gao et al. 2006). Second, metal cations in solution commonly exist as hydrated ions with thick hydration shells. When these hydrated ions contact with solids, the blocking effects caused by the hydration shells could also enhance the solids’ hydrophobicity (Volkov et al. 1997). Cd(II) had much thicker hydration shells than Pb(II), which could be one of the reasons causing Cd to increase the sorption of hydrophobic lindane to a greater extent than Pb. In addition, compared with sediments and suspended particles, the sorption of lindane onto biofilms that had the highest DOC content of the three solids increased more significantly with the presence of Pb and Cd (p < 0.05). Cr and As existed mainly as acid radical anions and had opposing effects on the sorption of lindane onto the three solids, compared with Pb and Cd. Anionic Cr(VI) and As(V) acid radicals caused the solid surface to become more loose through electrostatic repulsion. This decreased the hydrophobicity of the solids, and subsequently suppressed the sorption of hydrophobic lindane.
For the sediments and suspended particles with higher CEC, the sorption of NOR was noticeably decreased by the presence of Pb and Cd. One of the primary reasons for this could be that cationic Pb(II) and Cd(II) directly competed with NORH2 + for the cation sorption sites. Conversely, metal cations could also increase NOR sorption through ion bridging between solid surfaces and NORH± (Gu et al. 2015). Therefore, the sorption of NOR onto biofilms in the presence of Pb and Cd appeared to decrease with lower NOR concentrations (< 150 μM) and increase with higher NOR concentrations (> 150 μM). Competition with sorption and ion bridging should be a dominant mechanism causing these two sorption effects, respectively. Cr and As decreased NOR sorption onto suspended particles and sediments, decreased the sorption of lower concentrations (< 150 μM) and increased the sorption of higher concentrations of NOR (> 150 μM) onto biofilms. Similar effects were caused by Cr, As, Pb, and Cd on the sorption of NOR onto the three solids. This could be relative to the properties of dissociable zwitterionic NOR in the solution. Similar phenomena could result from different mechanisms. For example, anionic Cr(VI) and As(V) salts caused the solid surface to become more loose through electrostatic repulsion, and subsequently more solid components with adsorbed NOR dissolved in the solution. The mechanism of this effect on sorption was different from those of Pb(II) and Cd(II), which were due to directly competing sorption sites.
Conclusions
Pb and Cd promoted lindane sorption, but Cr and As suppressed lindane sorption onto biofilms, suspended particles, and surface sediments from the same river. The enhanced and weakened hydrophobicity of these solids, affected by the four heavy metals, were the main causes of these apparent increased and decreased sorptions. The presence of Pb, Cd, Cr, and As decreased the sorption of NOR onto sediments and suspended particles at pH 5.7~6.3. The mechanism of the effects of Pb and Cd could be due to competition between divalent metal cations and NORH2 + for the same positively charged sites on sediments and suspended particles with higher CEC values. The mechanism of the effects of Cr and As could due to the initial combination of NORH2 + with chromate and arsenate, weakening ion-exchange adsorption. Pb, Cd, Cr, and As increased the sorption of NOR onto biofilms at pH 5.7~6.3. Biofilms had the highest organic matter content of the three solids. The presence of Pb and Cd enhanced the flocculation of DOM combined with NORH2 + onto biofilms. In addition, Cr and As enhanced the hydrophilicity of biofilms, and then increased their sorption capacity for NOR with hydrophilic groups. Similar sorption phenomena could originate from different inherent mechanisms. The mechanisms of NOR sorption onto biofilms were more complicatedly affected by heavy metals.
References
Abhilash PC, Jamila S, Singh V, Singh A, Singh N, Srivastava SC (2008) Occurrence and distribution of hexachlorocyclohexane isomers in vegetation samples from a contaminated area. Chemosphere 72(1):79–86. https://doi.org/10.1016/j.chemosphere.2008.01.056
Ali U, Syed JH, Malik RN, Katsoyiannis A, Li J, Zhang G, Jones KC (2014) Organochlorine pesticides (OCPs) in South Asian region: a review. Sci Total Environ 476:705–717. https://doi.org/10.1016/j.scitotenv.2013.12.107
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54(7):951–967. https://doi.org/10.1016/j.chemosphere.2003.10.001
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation - emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37(24):5701–5710. https://doi.org/10.1021/es034354c
Chen YP, Zhang P, Guo JS, Fang F, Gao X, Li C (2013) Functional groups characteristics of EPS in biofilm growing on different carriers. Chemosphere 92(6):633–638. https://doi.org/10.1016/j.chemosphere.2013.01.059
Dai C, Hu YD (2015) Fe(III) hydroxide nucleation and growth on quartz in the presence of Cu(II), Pb(II), and Cr(III): metal hydrolysis and adsorption. Environ Sci Technol 49(1):292–300. https://doi.org/10.1021/es504140k
Dai C, Zuo XB, Cao B, Hu YD (2016) Homogeneous and heterogeneous (Fe-x, Cr1-x)(OH)(3) precipitation: implications for Cr sequestration. Environ Sci Technol 50(4):1741–1749. https://doi.org/10.1021/acs.est.5b04319
Dong DM, Guo ZY, Hua XY, Lan Y, Zhou JT, Ding XO, Qiao QQ (2011) Sorption of DDTs on biofilms, suspended particles and river sediments: effects of heavy metals. Environ Chem Lett 9(3):361–367. https://doi.org/10.1007/s10311-010-0287-x
Dong DM, Zhang LW, Guo ZY, Hua XY (2017) The role of extracellular polymeric substances on the sorption of pentachlorophenol onto natural biofilms in different incubation times: a fluorescence study. Chem Ecol 33(2):131–142. https://doi.org/10.1080/02757540.2017.1281253
Dong DM, Zhang LW, Liu S, Guo ZY, Hua XY (2016) Antibiotics in water and sediments from Liao River in Jilin Province, China: occurrence, distribution, and risk assessment. Environ Earth Sci 75(16):1202. https://doi.org/10.1007/s12665-016-6008-4
Dilling J, Kaiser K (2002) Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry. Water Res 36(20):5037–5044. https://doi.org/10.1016/S0043-1354(02)00365-2
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Gao YZ, Xiong W, Ling WT, Xu JM (2006) Sorption of phenanthrene by soils contaminated with heavy metals. Chemosphere 65(8):1355–1361. https://doi.org/10.1016/j.chemosphere.2006.04.030
Guo ZY, Hua XY, Lan XH, Sun YY, Dong DM (2012) Evidence for a mutual effect of biofilms, suspended particles and sediments on DDT sorption. Environ Chem Lett 10(4):407–411. https://doi.org/10.1007/s10311-012-0369-z
Guo ZY, Dong DM, Hua XY, Zhang LW, Zhu SJ, Lan XH, Liang DP (2015) Cr and As decrease lindane sorption on river solids. Environ Chem Lett 13(1):111–116. https://doi.org/10.1007/s10311-014-0489-8
Gu XY, Tan YY, Tong F, Gu C (2015) Surface complexation modeling of coadsorption of antibiotic ciprofloxacin and Cu(II) and onto goethite surfaces. Chem Eng J 269:113–120
He XS, Xi BD, Wei ZM, Jiang YH, Yang Y, An D, Cao JL, Liu HL (2011) Fluorescence excitation-emission matrix spectroscopy with regional integration analysis for characterizing composition and transformation of dissolved organic matter in landfill leachates. J Hazard Mater 190(1-3):293–299. https://doi.org/10.1016/j.jhazmat.2011.03.047
Hou J, You GX, Xu Y, Wang C, Wang PF, Miao LZ, Ao YH, Li Y, Lv BW, Yang YY (2016) Impacts of CuO nanoparticles on nitrogen removal in sequencing batch biofilm reactors after short-term and long-term exposure and the functions of natural organic matter. Environ Sci Pollut Res 23(21):22116–22125. https://doi.org/10.1007/s11356-016-7281-1
Korshin GV, Benjamin MM, Sletten RS (1997) Adsorption of natural organic matter (NOM) on iron oxide: effects on NOM composition and formation of organo-halide compounds during chlorination. Water Res 31(7):1643–1650. https://doi.org/10.1016/S0043-1354(97)00007-9
Luo L, Zhang SZ, Ma YB, Christie P, Huang HL (2008) Facilitating effects of metal cations on phenanthrene sorption in soils. Environ Sci Technol 42(7):2414–2419. https://doi.org/10.1021/es702843m
Luo L, Zhang SZ, Christie P (2010) New insights into the influence of heavy metals on phenanthrene sorption in soils. Environ Sci Technol 44(20):7846–7851. https://doi.org/10.1021/es1024433
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142(1-2):1–53. https://doi.org/10.1016/j.jhazmat.2007.01.006
Pan B, Wang P, Wu M, Li J, Zhang D, Xiao D (2012) Sorption kinetics of ofloxacin in soils and mineral particles. Environ Pollut 171:185–190. https://doi.org/10.1016/j.envpol.2012.07.037
Pei ZG, Yang S, Li LY, Li CM, Zhang SZ, Shan XQ, Wen B, Guo BY (2014) Effects of copper and aluminum on the adsorption of sulfathiazole and tylosin on peat and soil. Environ Pollut 184:579–585. https://doi.org/10.1016/j.envpol.2013.09.038
Pei ZG, Shan XQ, Zhang SZ, Kong JJ, Wen B, Zhang J, Zheng LR, Xie YN, Janssens K (2011) Insight to ternary complexes of co-adsorption of norfloxacin and Cu(II) onto montmorillonite at different pH using EXAFS. J Hazard Mater 186(1):842–848. https://doi.org/10.1016/j.jhazmat.2010.11.076
Peuravuori J, Pihlaja K (2004) Preliminary study of lake dissolved organic matter in light of nanoscale supramolecular assembly. Environ Sci Technol 38(22):5958–5967. https://doi.org/10.1021/es040041l
Pils JRV, Laird DA (2007) Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay-humic complexes. Environ Sci Technol 41(6):1928–1933. https://doi.org/10.1021/es062316y
Polubesova T, Sherman-Nakache M, Chefetz B (2007) Binding of pyrene to hydrophobic fractions of dissolved organic matter: effect of polyvalent metal complexation. Environ Sci Technol 41(15):5389–5394. https://doi.org/10.1021/es070722r
Uyguner CS, Bekbolet M (2005) Evaluation of humic acid photocatalytic degradation by UV-vis and fluorescence spectroscopy. Catal Today 101(3-4):267–274. https://doi.org/10.1016/j.cattod.2005.03.011
Volkov AG, Paula S, Deamer DW (1997) Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem Bioenerg 42(2):153–160. https://doi.org/10.1016/S0302-4598(96)05097-0
Wei D, Dong H, Wu N, Ngo HH, Guo WS, Du B, Wei Q (2016a) A fluorescence approach to assess the production of soluble microbial products from aerobic granular sludge under the stress of 2,4-dichlorophenol. Sci Rep-UK 6(1):24444. https://doi.org/10.1038/srep24444
Wei D, Li MT, Wang XD, Han F, Li LS, Guo J, Ai LJ, Fang LL, Liu L, Du B, Wei Q (2016b) Extracellular polymeric substances for Zn (II) binding during its sorption process onto aerobic granular sludge. J Hazard Mater 301:407–415. https://doi.org/10.1016/j.jhazmat.2015.09.018
Wei D, Ngo HH, Guo WS, Xu WY, Zhang YF, Du B, Wei Q (2016c) Biosorption of effluent organic matter onto magnetic biochar composite: behavior of fluorescent components and their binding properties. Bioresour Technol 214:259–265. https://doi.org/10.1016/j.biortech.2016.04.109
Wei D, Wang BF, Ngo HH, Guo WS, Han F, Wang XD, Du B, Wei Q (2015) Role of extracellular polymeric substances in biosorption of dye wastewater using aerobic granular sludge. Bioresour Technol 185:14–20. https://doi.org/10.1016/j.biortech.2015.02.084
Wu D, Li H, Liao SH, Sun XL, Peng HB, Zhang D, Pan B (2014) Co-sorption of ofloxacin and Cu(II) in soils before and after organic matter removal. Sci Total Environ 481:209–216. https://doi.org/10.1016/j.scitotenv.2014.02.041
Xiao L, Qu XL, Zhu DQ (2007) Biosorption of nonpolar hydrophobic organic compounds to Escherichia coli facilitated by metal and proton surface binding. Environ Sci Technol 41(8):2750–2755. https://doi.org/10.1021/es062343o
Xu Y, Wang C, Hou J, Dai SS, Wang PF, Miao LZ, Lv BW, Yang YY, You GX (2016) Effects of ZnO nanoparticles and Zn2+ on fluvial biofilms and the related toxicity mechanisms. Sci Total Environ 544:230–237. https://doi.org/10.1016/j.scitotenv.2015.11.130
You GX, Wang PF, Hou J, Wang C, Xu Y, Miao LZ, Lv YY, Liu ZL, Zhang F (2017) Insights into the short-term effects of CeO2 nanoparticles on sludge dewatering and related mechanism. Water Res 118:93–103. https://doi.org/10.1016/j.watres.2017.04.011
Zhang DY, Pan XL, Mostofa KMG, Chen X, Mu GJ, Wu FC, Liu J, Song WJ, Yang JY, Liu YL, Fu QL (2010) Complexation between Hg(II) and biofilm extracellular polymeric substances: an application of fluorescence spectroscopy. J Hazard Mater 175(1-3):359–365. https://doi.org/10.1016/j.jhazmat.2009.10.011
Zhang JQ, Dong YH (2008) Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J Hazard Mater 151:833–839. https://doi.org/10.1016/j.jhazmat.2007.11.046
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of china: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49(11):6772–6782. https://doi.org/10.1021/acs.est.5b00729
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 21577047, 21307041, and 21277056), the Science and Technology Development Program of Jilin Province (No. 20150520078JH), and the 111 Project (No. B16020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 101 kb)
Rights and permissions
About this article
Cite this article
Dong, D., Li, L., Zhang, L. et al. Effects of lead, cadmium, chromium, and arsenic on the sorption of lindane and norfloxacin by river biofilms, particles, and sediments. Environ Sci Pollut Res 25, 4632–4642 (2018). https://doi.org/10.1007/s11356-017-0840-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0840-2