Abstract
Vitamin deficiency arises when the dietary intake of essential vitamins is too low. Insufficient levels of vitamin weaken the body and induce diseases. Although the dietary intake of essential vitamins has increased through food fortification, there are still major issues of vitamin deficiency. Moreover, consumption of vitamins in classical food supplements presents drawbacks such as poor bioavailability and low stability, notably in the gastrointestinal tract conditions. To overcome such issues, vitamin nanoemulsions have been recently developed. Here, we review the design of vitamin nanoemulsions with various oils to meet specific needs; actual research and markets for vitamin nanoemulsions; and techniques for nanoemulsion characterization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has been explored extensively as the next revolution, notably in the field of agriculture and food industry (Donsì 2018). Nanotechnology can also enhance water solubility, thermal stability, gastrointestinal stability and oral bioavailability of bioactive compounds and vitamins like A, D, E and K (Ezhilarasi et al. 2013). This is achieved by encapsulating essential vitamins, using various types of wall materials. In food and pharmaceutical industries, encapsulation allows to protect bioactive compounds and vitamins within a wall material in the form of capsules (Bartusik et al. 2016; Borthakur et al. 2016). Emulsification, coacervation, inclusion complexation, emulsification-solvent evaporation, nanoprecipitation, and supercritical fluid techniques are used to produce nanocapsules in the range of 10–1000 nm (Ezhilarasi et al. 2013). Figure 1 shows the different types of emulsion forms. This review majorly focuses on the use of oils as a carrier material to protect vitamins from temperature, light, and controlled release in the human body. Various researchers have extensively studied the production of nanoemulsions using different vitamins and carrier materials (oils). Table 1 presents the studies conducted by various researchers pertaining to vitamin nanoemulsions. This article is an abridged version of the chapter published by Dasgupta and Ranjan (2018) in the series Environmental Chemistry for a Sustainable World (http://www.springer.com/series/11480).
Nanoemulsion-based delivery systems and functional properties
Vitamin E is one of the nutraceutical compounds that have been a major research area for the researchers. Vitamin E is one of the nutraceutical compounds that have been one of the potent attention seekers of research. The most nutritionally imperative form of vitamin E is α-tocopherol, so there has been more interest of incorporating it into various food products. However, while incorporating the α-tocopherol into functional food products, there are various challenges associated with incorporation: poor water solubility, chemical instability (to oxygen, light, and heat), and variable oral bioavailability (Ozturk et al. 2015a). Researchers have studied the natural emulsifiers to develop the vitamin E acetate emulsion-based delivery system. Natural emulsifiers like protein (whey protein isolate) and polysaccharides (gum Arabic) have been used to formulate vitamin E acetate emulsions, and they observed that the protein emulsifier was more effective in producing smaller size droplets in the range of 110 nm, as compared to polysaccharide emulsifier (380 nm). On the other hand, emulsion prepared with gum Arabic has been found to be more stable, especially at elevated temperatures compared to whey protein isolate. This may be due to the protein surfactants that get denatured in protein-based emulsions (Ozturk et al. 2015a).
Ozturk et al. (2015b) studied the influence of different carrier oils on the bioaccessibility of vitamin D3-encapsulated nanoemulsion in simulated gastrointestinal conditions. Stable nanoemulsions in the range of 140–190 nm were synthesized using natural surfactants by high-pressure homogenization technique. Researchers have observed that the medium-chain triglycerides (MCT), carrier oil enabled better free fatty acid release during lipid digestion of vitamin D3, compared to other carrier oils like corn oil, fish oil, orange oil and mineral oil, whereas long-chain triglycerides (LCT) effectively increased bioaccessibility of vitamin D3. This can be attributed to the fact that long-chain free fatty acids protectively surround lipid droplets and thus prevent them from lipase action (Ozturk et al. 2015b).
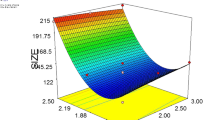
A study has been conducted by Mehmood (2015) where response surface methodology was used to optimize the formulation of nanoemulsion (Mehmood 2015). In his work, he used canola oil, as a carrier oil to encapsulate the vitamin E acetate using high-energy approach to understand the changes in nanoemulsion with edible mustard oil as carrier oil. Researchers have used low-energy approach to formulate vitamin E nanoemulsion with edible mustard oil as carrier oil. Analysis such as stability test, antioxidant analysis, and HPLC is done/performed for measuring encapsulation efficiency, and antimicrobial test in order to synthesize an effective emulsion system for vitamin E. The prepared nanoemulsion was found to be much stable, for nearly more than 15 days with significantly higher antioxidant activity of 62.55%. It has higher antibacterial property, making it a suitable additive for enhancing the shelf life of health drinks. High encapsulating efficiency (99.65%) proves that the synthesized nanoemulsion is highly effective in encapsulating and protecting vitamin E from unfavourable conditions (Dasgupta et al. 2016b). This study is a supportive evidence for the advantages of using nanoemulsion system as a health supplement in beverage industries (Figs. 2, 3).
Characterization of nanoemulsions
Separation techniques
Chromatography technique
In colloidal food science, separation technology is mainly used for identification and quantification of the different bioactive compounds. Many researchers have established the use of these separation techniques in nanocolloidal food science, i.e., food-grade nanoemulsions or nanoencapsulated products. The main challenge for food-grade nanoemulsion is the in situ characterization of them; nevertheless, in most cases it is not possible to detect them in the food matrixes. Therefore, separation techniques are mandatory/utmost important to isolate the nanoemulsion from food prior to their characterization.
Size exclusion chromatography
It is one of the chromatographic methods which accounts for size exclusion wherein molecules are separated on the basis of their size and molecular weight. It is usually applied to larger molecules or macromolecular complexes such as proteins and industrial polymers. It can be noted that size is the main characteristic of nanoemulsions, so it is the most suitable type of liquid chromatography for the separation of nanoemulsions from food matrix (Saifullah et al. 2016). It has been used in nanoemulsion-based drug delivery analysis.
Development of a nanoemulsion should focus on droplets with a well-defined size, i.e., with a narrow size distribution. Otherwise, contaminations with droplets of large size might be taken up more efficiently and/or carry a higher which leads to carrying of higher perfluorocarbon load, affecting both the specificity and quantity of the perfluorocarbon label. Grapentin and co-workers generated perfluorocarbon nanoemulsion by using phospholipids that usually show a broad size distribution—a challenge which cannot be overcome by the conventional manufacturing process. However, the formation of well-defined perfluorocarbon nanoemulsion is feasible by combining centrifugation with size exclusion chromatography. Size exclusion chromatographic technique was able to separate effectively well-defined fractions of a perfluorodecalin emulsion via a Toyopearl HW-75S column (Grapentin et al. 2014). Similarly, this technique has been used for characterization of nanoemulsion-based drug delivery by ketene-based polyester synthesized using electron rich carbon/silica composite surface (Swarnalatha et al. 2008). The use of size exclusion chromatographic technique in nanoemulsion characterization is quite rare nowadays and can be employed in drug delivery system (Bae and Chung 2014; Vezocnik et al. 2015).
Ion exchange chromatography
Ion exchange chromatography separates ions and polar molecules based on their affinity to the ion exchanger. It works on wide variety of charged molecule—including large proteins, small nucleotides, and amino acids. Simultaneously, as charge size is also one among the main characteristics of nanoemulsions, so it is also the most suitable type of liquid chromatography for the separation of nanoemulsions from food matrix (Saifullah et al. 2016). Ion exchange chromatography has been reported in a purifier for nanoemulsion which includes sucrose fatty acid ester (Bromley 2011).
High-performance liquid chromatography (HPLC)
HPLC method has also been highly explored to identify the presence of some unidentified compounds in natural/real extracts, e.g., leaf, fruit, or stem samples including environmental samples such as soil, water, and other samples. It mainly works on the principle of separation-based technique.
Recently, Dasgupta and co-workers have employed HPLC to quantify non-encapsulated vitamin E and have calculated the encapsulation efficiency for food-grade mustard oil nanoemulsion. It was for the first of its kind, an article in the field of food-grade nanoemulsions (Dasgupta et al. 2016b). During the similar time period, many other researchers have also reported/employed/practiced/explored/used HPLC for characterization of different nanoemulsions, calculating encapsulation efficiency and in analyzing the stability of selective/target molecule in nanoemulsion (Li et al. 2015; Panatieri et al. 2016; Youssof et al. 2016). Overall, similar to other chromatographic techniques, HPLC was also used to identify and quantify the active compounds and was found more precise, easier and efficient for nanoemulsions characterization.
Physical characterization
UV–Vis spectrophotometer
UV–visible spectrophotometer analysis can be performed to identify and quantify the concentration of the compounds based on their optical density and the amount of light transmitted or refracted. The UV–visible spectrophotometer cannot give absolute structural confirmation, and the quantification and identification of the particular sample result should also be calibrated with the controls. Earlier researchers have used this method for various estimations, e.g., detection of oil content in the final nanoemulsion (Costa et al. 2013), to estimate the encapsulated curcumin (Rachmawati et al. 2014) and to measure the transmittance percentage of nanoemulsions (Jaiswal et al. 2015).
Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC)
FTIR and DSC are the commonly used instrumental techniques for analyzing the structural identity of the selective molecule or mixture of compounds. These techniques were performed to identify the structural identity of active compound present in food materials. FTIR and DSC are normally used for assessing structure, encapsulating active compound, analyzing their integrity, and further ensuring their compatibility among other ingredients (Pathan and Mallikarjuna Setty 2011, 2012; Ahmad et al. 2014).
X-ray diffraction (XRD)
XRD is used to determine the crystal structure of any compound. Researchers have analyzed the crystalline structure of electrospinned nanofibre from nanoemulsion (Gordon et al. 2015; Sugumar et al. 2015). Other researchers also analyzed the crystal structure of specifically designed nanoparticles from nanoemulsions (Shams and Ahi 2013; Filipović et al. 2015; Soltani et al. 2016). Further extending the applications of XRD in core-nanoemulsion characterization, few researchers have used it on derived forms of nanoemulsions by using several food engineering steps (Ahmad et al. 2014; Zhou et al. 2014). Additionally, the protocol has been established to analyze the presence of ions in nanoemulsions (Mahendran and Philip 2013). Other X-ray-based technologies are also being used to identify/study lipid-based nanoparticles or nanoemulsion-based solid nanomaterials—small-angle X-ray scattering (Jenning et al. 2000; Alaimo et al. 2015; Truong et al. 2015; Uzun et al. 2016).
The other physical perspective of the nanoemulsion characterization techniques has been described in the later section—e.g., size distribution, zeta potential, crystallinity, viscosity, stability, etc., of the nanoemulsions.
Dynamic light scattering (DLS)
DLS is commonly used technique for determining the size distribution profile of small particles in suspension or polymeric solutions. In this technique, the temporal fluctuations are usually analyzed/examined/studied by means of the intensity or photon autocorrelation function; hence, DLS is also termed as photon correlation spectroscopy or quasi-elastic light scattering spectroscopy. Recently, DLS analysis has become a basic but compulsory methodology to measure the hydrodynamic size of nanoemulsion droplets. This is so because of one-step process of DLS size measurement and precise estimation of hydrodynamic size range of nanoemulsion droplets. Many food-grade nanoemulsions have been fabricated recently; almost all of them have used DLS size measurement in their research (Dasgupta et al. 2014, 2016b; Guttoff et al. 2015; Komaiko and McClements 2015; Ma et al. 2016). It has been reviewed and summarized in many recent scientific articles (Azeem et al. 2009; Rajpoot et al. 2011; Shakeel et al. 2012; Ranjan et al. 2014, 2016a, b, Dasgupta et al. 2015, 2016a; Jain et al. 2017). Nowadays, DLS is getting integrated with the zeta potential analyzer which is changing the normal DLS cuvette to the double-electrode integrated cuvette, which further helps to analyze the zeta potential of the nanoemulsion (Dasgupta et al. 2016b; Jain et al. 2017).
Viscometer
Viscometer is an instrument used to measure the viscosity of a fluid. Nanoemulsions are mainly characterized by using rotational viscometer. Rotational viscometers use the idea of torque required to turn an object into a fluid as a function of its viscosity. They measure the torque required to rotate a disk or bob in a fluid at a known speed. It is very important to know the viscosity of the food-grade nanoemulsions if ingested or passed through gastrointestinal tract or applied transdermally. Positively charged nanoemulsion-based steroidal drug for transdermal application has been analyzed using rotary viscometer (Da Costa et al. 2014). Similarly, many researchers have used rotary viscometer for analyzing the food- and drug-grade nanoemulsions (Tsai et al. 2014). Recently, few reviews have also highlighted the recent characterization technologies for nanoemulsions (Jaiswal et al. 2015; Jain et al. 2017).
Stability and pH analysis of nanoemulsion
Stability of the nanoemulsion can be analyzed only visually, and the main forms of instability of nanoemulsions are due to gravitational separation, flocculation, coalescence and phase separation. It can be noted that one has to be very precise while analyzing stability through naked eyes or the magnifying glasses. Similarly, stability and pH analysis are a need/requirement to be determined for food- and drug-grade nanoemulsions, because these emulsions will interact with the biological matrices which ultimately depend upon the pH (Jain et al. 2017).
Imaging techniques
Atomic force microscopy (AFM)
Atomic force microscopy technique is used for the nanoemulsions which is present in the colloidal film on slides. AFM images will provide a clear idea about the shape and size of the nanoemulsions droplets.
Recently, many researchers have focused on AFM imaging technique confirmed using dynamic light scattering (Makidon et al. 2008; Ghosh et al. 2013; Dasgupta et al. 2016b; Ma et al. 2016), whereas others researchers have confirmed that AFM results with other imaging techniques (Salvia-Trujillo et al. 2013; Neeru et al. 2014; Singh et al. 2015b; Song et al. 2016).
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
TEM is a microscopy technique in which a beam of electrons is transmitted through an ultra-thin specimen interacting with the specimen as it passes through it. An image is formed from the interaction of the electrons transmitted through the specimen; the image is magnified and focused onto an imaging device. In the case of SEM, the scanned electrons are responsible for image formation. TEM and SEM are efficiently used for nanoparticles from early decade of the twenty-first century, but recently after several instrumental advancements now researchers are performing TEM and SEM images for nanoemulsions too (Klang et al. 2012; Jaiswal et al. 2015). In the current decade, several protocols have been established for SEM and TEM analyses of nanoemulsions and many food-grade and drug nanoemulsions have been characterized by SEM and TEM (Da Costa et al. 2014; Singh et al. 2015a, b; Lee et al. 2016).
References
Ahmad J, Mir SR, Kohli K et al (2014) Solid-nanoemulsion preconcentrate for oral delivery of paclitaxel: formulation design, biodistribution, and γ scintigraphy imaging. Biomed Res Int. https://doi.org/10.1155/2014/984756
Alaimo D, Hermida Merino D, Grignard B et al (2015) Small-angle X-ray scattering insights into the architecture-dependent emulsifying properties of amphiphilic copolymers in supercritical carbon dioxide. J Phys Chem B 119:1706–1716. https://doi.org/10.1021/jp5086558
Asmawi AA, Salim N, Ngan CL, Ahmad H, Abdulmalek E, Masarudin MJ, Abdul Rahman MB (2018) Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-018-0526-4
Azeem A, Rizwan M, Ahmad FJ et al (2009) Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech 10:69–76. https://doi.org/10.1208/s12249-008-9178-x
Bae P, Chung B (2014) Multiplexed detection of various breast cancer cells by perfluorocarbon/quantum dot nanoemulsions conjugated with antibodies. Nano Converg 1:23. https://doi.org/10.1186/s40580-014-0023-5
Bartusik D, Aebisher D, Tomanek B (2016) The synthesis and application of vitamins in nanoemulsion delivery systems. Elsevier, Amsterdam
Borthakur P, Boruah PK, Sharma B, Das MR (2016) Nanoemulsion: preparation and its application in food industry A2 - Grumezescu, Alexandru Mihai BT - Emulsions. Elsevier, Amsterdam
Bromley PJ (2011) Nanoemulsion including sucrose fatty acid ester
Costa JA, Farias NC, Queirós YGC, Queirós YGC, Mansur CRE (2013) Determination of oil-in-water using nanoemulsions as solvents and UV visible and total organic carbon detection methods. Talanta 107:304–311. https://doi.org/10.1016/j.talanta.2013.01.040
Da Costa S, Basri M, Shamsudin N, Basri H (2014) Stability of positively charged nanoemulsion formulation containing steroidal drug for effective transdermal application. J Chem. https://doi.org/10.1155/2014/748680
Dasgupta N, Ranjan S (2018) Research updates on different vitamins based nanoemulsions and characterization of nanoemulsions. In: Dasgupta N, Ranjan S (eds) An introduction to food grade nanoemulsions, 1st edn. Springer, Singapore, pp 105–122
Dasgupta S, Ghosh SK, Ray S et al (2014) In vitro and in vivo studies on lornoxicam loaded nanoemulsion gels for topical application. Curr Drug Deliv 11:132–138. https://doi.org/10.2174/15672018113106660063
Dasgupta N, Ranjan S, Mundekkad D et al (2015) Nanotechnology in agro-food: from field to plate. Food Res Int 69:381–400
Dasgupta N, Ranjan S, Chakraborty AR et al (2016a) Nanoagriculture and water quality management. Nanosci Food Agric 1(1):1–18. https://doi.org/10.1007/978-3-319-39303-2_1
Dasgupta N, Ranjan S, Mundra S et al (2016b) Fabrication of food grade vitamin E nanoemulsion by low energy approach, characterization and its application. Int J Food Prop 19:700–708. https://doi.org/10.1080/10942912.2015.1042587
Donsì F (2018) Chapter 11: applications of nanoemulsions in foods. Appl Nanoemulsions Fooions 2:2. https://doi.org/10.1016/B978-0-12-811838-2.00011-4
Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C (2013) Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol 6:628–647. https://doi.org/10.1007/s11947-012-0944-0
Filipović J, Pezo L, Filipović V et al (2015) The effects of ω-3 fatty acids and inulin addition to spelt pasta quality. LWT-Food Sci Technol 63:43–51
Ghosh V, Mukherjee A, Chandrasekaran N (2013) Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason Sonochem 20:338–344. https://doi.org/10.1016/j.ultsonch.2012.08.010
Gordon V, Marom G, Magdassi S (2015) Formation of hydrophilic nanofibers from nanoemulsions through electrospinning. Int J Pharm 478:172–179. https://doi.org/10.1016/j.ijpharm.2014.11.038
Grapentin C, Temme S, Mayenfels F, et al (2014) Optimization of perfluorocarbon nanoemulsions for molecular imaging by 19F MRI
Guttoff M, Saberi AH, Mcclements DJ (2015) Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem 171:117–122. https://doi.org/10.1016/j.foodchem.2014.08.087
Hategekimana J, Chamba MVM, Shoemaker CF, Majeed H, Zhong F (2015) Vitamin E nanoemulsions by emulsion phase inversion: effect of environmental stress and long-term storage on stability and degradation in different carrier oil types. Colloids Surf A Physicochem Eng Asp 483:70–80. https://doi.org/10.1016/j.colsurfa.2015.03.020
Jain A, Ranjan S, Dasgupta N, Ramalingam C (2017) Nanomaterials in food and agriculture: an overview on their safety concerns and regulatory issues. Crit Rev Food Sci Nutr 58(2):297–317
Jaiswal M, Dudhe R, Sharma PK (2015) Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 5:123–127
Jenning V, Thünemann AF, Gohla SH (2000) Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm 199:167–177
Kadappan AS, Guo C, Gumus CE, Bessey A, Wood RJ, McClements DJ, Liu Z (2018) The efficacy of nanoemulsion-based delivery to improve vitamin D absorption: comparison of in vitro and in vivo studies. Mol Nutr Food Res 62(4):1–24. https://doi.org/10.1002/mnfr.201700836
Klang V, Matsko NB, Valenta C, Hofer F (2012) Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron 43:85–103
Komaiko J, McClements DJ (2014) Low-energy formation of edible nanoemulsions by spontaneous emulsification: factors influencing particle size. J Food Eng 146:122–128. https://doi.org/10.1016/j.jfoodeng.2014.09.003
Komaiko J, McClements DJ (2015) Food-grade nanoemulsion filled hydrogels formed by spontaneous emulsification and gelation: optical properties, rheology, and stability. Food Hydrocoll 46:67–75. https://doi.org/10.1016/j.foodhyd.2014.12.031
Laouini A, Fessi H, Charcosset C (2012) Membrane emulsification: a promising alternative for vitamin E encapsulation within nano-emulsion. J Membr Sci 423–424:85–96. https://doi.org/10.1016/j.memsci.2012.07.031
Lee HS, Morrison ED, Zhang Q, McCormick AV (2016) Cryogenic transmission electron microscopy study: preparation of vesicular dispersions by quenching microemulsions. J Microsc 263(3):293–299
Li Y, Teng Z, Chen P et al (2015) Enhancement of aqueous stability of allyl isothiocyanate using nanoemulsions prepared by an emulsion inversion point method. J Colloid Interface Sci 438:130–137. https://doi.org/10.1016/j.jcis.2014.09.055
Ma Q, Davidson PM, Zhong Q (2016) Nanoemulsions of thymol and eugenol co-emulsified by lauric arginate and lecithin. Food Chem 206:167–173. https://doi.org/10.1016/j.foodchem.2016.03.065
Mahendran V, Philip J (2013) Sensing of biologically important cations such as Na+, K +, Ca2+, Cu2+, and Fe3+ using magnetic nanoemulsions. Langmuir 29:4252–4258. https://doi.org/10.1021/la400502b
Makidon PE, Bielinska AU, Nigavekar SS et al (2008) Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS ONE. https://doi.org/10.1371/journal.pone.0002954
Meghani N, Patel P, Kansara K, Ranjan S, Dasgupta N, Ramalingam C, Kumar A (2018) Formulation of vitamin D encapsulated cinnamon oil nanoemulsion: its potential anti-cancerous activity in human alveolar carcinoma cells. Colloids Surf B Biointerfaces. https://doi.org/10.1016/j.colsurfb.2018.03.041
Mehmood T (2015) Optimization of the canola oil based vitamin E nanoemulsions stabilized by food grade mixed surfactants using response surface methodology. Food Chem 183:1–7. https://doi.org/10.1016/j.foodchem.2015.03.021
Mehmood T, Ahmed A, Ahmad A, Ahmad MS, Sandhu MA (2018) Optimization of mixed surfactants-based β-carotene nanoemulsions using response surface methodology: an ultrasonic homogenization approach. Food Chem 253(October 2017):179–184. https://doi.org/10.1016/j.foodchem.2018.01.136
Miyake M, Kakizawa Y, Tobori N, Kurioka M, Tabuchi N, Kon R, Shimokawa N, Tsujino Y, Takagi M (2018) Membrane permeation of giant unilamellar vesicles and corneal epithelial cells with lipophilic vitamin nanoemulsions. Colloids Surf B Biointerfaces. 169(December 2017):444–452. https://doi.org/10.1016/j.colsurfb.2018.05.052
Morais DJM, Burgess J (2014a) Vitamin E nanoemulsions characterization and analysis. Int J Pharm 465(1–2):455–463. https://doi.org/10.1016/j.ijpharm.2014.02.034
Morais JM, Burgess DJ (2014b) In vitro release testing methods for vitamin E nanoemulsions. Int J Pharm 475(1):393–400
Neeru S, Saurabh Manaswita V, Sandeep Kumar S, Priya Ranjan Prasad V (2014) Consequences of lipidic nanoemulsions on membrane integrity and ultrastructural morphology of Staphylococcus aureus. Mater Res Express 1:25401. https://doi.org/10.1088/2053-1591/1/2/025401
Ozturk B, Argin S, Ozilgen M, McClements DJ (2014) Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural surfactants: quillaja saponin and lecithin. J Food Eng 142:57–63
Ozturk B, Argin S, Ozilgen M, McClements DJ (2015a) Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: whey protein isolate and gum arabic. Food Chem. https://doi.org/10.1016/j.foodchem.2015.05.005
Ozturk B, Argin S, Ozilgen M, McClements DJ (2015b) Nanoemulsion delivery systems for oil-soluble vitamins: influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem 187:499–506. https://doi.org/10.1016/j.foodchem.2015.04.065
Panatieri LF, Brazil NT, Faber K et al (2016) Nanoemulsions containing a coumarin-rich extract from Pterocaulon balansae (Asteraceae) for the treatment of ocular acanthamoeba keratitis. AAPS PharmSciTech 18(3):721–728
Pathan IB, Mallikarjuna Setty C (2011) Enhancement of transdermal delivery of tamoxifen citrate using nanoemulsion vehicle. Int J PharmTech Res 3:287–297
Pathan IB, Mallikarjuna Setty C (2012) Nanoemulsion system for transdermal delivery of tamoxifen citrate: design, characterization, effect of penetration enhancers and in vivo studies. Dig J Nanomater Biostruct 7:1373–1387
Pawar VK, Panchal SB, Singh Y, Meher JG, Sharma K, Singh P, Bora HK, Singh A, Datta D, Chourasia MK (2014) Immunotherapeutic vitamin e nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. J Control Release 196:295–306. https://doi.org/10.1016/j.jconrel.2014.10.010
Rachmawati H, Budiputra DK, Mauludin R (2014) Curcumin nanoemulsion for transdermal application: formulation and evaluation. Drug Dev Ind Pharm 9045:560–566. https://doi.org/10.3109/03639045.2014.884127
Rachmawati H, Arvin YA, Asyarie S, Anggadiredja K (2018) Local sustained delivery of bupivacaine HCl from a new castor oil-based nanoemulsion system. Drug Deliv Transl Res 8(3):515–524
Rajpoot P, Pathak K, Bali V (2011) Therapeutic applications of nanoemulsion based drug delivery systems: a review of patents in last two decades. Recent Pat Drug Deliv Formul 5:163–172. https://doi.org/10.2174/187221111795471427
Ramli S, Norhman N, Zainuddin N, Ja’afar SM, Rahman IA (2017) Nanoemulsion based palm olein as vitamin E carrier. Malays J Anal Sci 21(6):1399–1408. https://doi.org/10.17576/mjas-2017-2106-22
Ranjan S, Dasgupta N, Chakraborty AR et al (2014) Nanoscience and nanotechnologies in food industries: opportunities and research trends. J Nanoparticle Res 16(6):2464
Ranjan S, Nandita D, Lichtfouse E (2016a) Nanoscience in food and agriculture 1, 1st edn. Springer, Switzerland
Ranjan S, Nandita D, Lichtfouse E (2016b) Nanoscience in food and agriculture 2, 1st edn. Springer, Switzerland
Saberi AH, Fang Y, McClements DJ (2013) Effect of glycerol on formation, stability, and properties of vitamin-E enriched nanoemulsions produced using spontaneous emulsification. J Colloid Interface Sci 411:105–113. https://doi.org/10.1016/j.jcis.2013.08.041
Saifullah M, Ahsan A, Shishir MRI (2016) Production stability and applications of micro- and nano-emulsion in food processing industry. In: Grumezescu A (ed) emulsions, 1st edn. Academic Press, London, pp 405–433
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2013) Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll 30:401–407. https://doi.org/10.1016/j.foodhyd.2012.07.004
Shakeel F, Shafiq S, Haq N et al (2012) Nanoemulsions as potential vehicles for transdermal and dermal delivery of hydrophobic compounds: an overview. Expert Opin Drug Deliv 9:953–974
Shams K, Ahi H (2013) Synthesis of 5A zeolite nanocrystals using kaolin via nanoemulsion- ultrasonic technique and study of its sorption using a known kerosene cut. Microporous Mesoporous Mater 180:61–71. https://doi.org/10.1016/j.micromeso.2013.06.019
Sherbiny EM, Eldosoky M, El-Shafey M, Othman G, Elkattawy HA, Bedir T, Elsherbiny NM (2018) Vitamin D nanoemulsion enhances hepatoprotective effect of conventional vitamin D in rats fed with a high-fat diet. Chem Biol Interact 288(February):65–75. https://doi.org/10.1016/j.cbi.2018.04.010
Singh N, Verma SM, Singh SK et al (2015a) Antibacterial activity of cationised and non-cationised placebo lipidic nanoemulsion using transmission electron microscopy. J Exp Nanosci 10:299–309. https://doi.org/10.1080/17458080.2013.830199
Singh N, Verma SM, Singh SK, Verma PRP (2015b) Antibacterial action of lipidic nanoemulsions using atomic force microscopy and scanning electron microscopy on Escherichia coli. J Exp Nanosci 10:381–391
Soltani S, Zakeri-Milani P, Barzegar-Jalali M, Jelvehgari M (2016) Design of eudragit RL nanoparticles by nanoemulsion method as carriers for ophthalmic drug delivery of ketotifen fumarate. Iran J Basic Med Sci 19:850–860
Song Z, Sun H, Yang Y et al (2016) Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant MRSA infections. Nanomed Nanotechnol Biol Med 12:1543–1555
Sugumar S, Mukherjee A, Chandrasekaran N (2015) Eucalyptus oil nanoemulsion-impregnated chitosan film: antibacterial effects against a clinical pathogen, Staphylococcus aureus, in vitro. Int J Nanomed 10:67–75. https://doi.org/10.2147/IJN.S79982
Swarnalatha S, Selvi PK, Ganesh Kumar A, Sekaran G (2008) Nanoemulsion drug delivery by ketene based polyester synthesized using electron rich carbon/silica composite surface. Colloids Surf B Biointerfaces 65:292–299. https://doi.org/10.1016/j.colsurfb.2008.04.012
Truong T, Morgan GP, Bansal N et al (2015) Crystal structures and morphologies of fractionated milk fat in nanoemulsions. Food Chem 171:157–167. https://doi.org/10.1016/j.foodchem.2014.08.113
Tsai M-J, Fu Y-S, Lin Y-H et al (2014) The effect of nanoemulsion as a carrier of hydrophilic compound for transdermal delivery. PLoS ONE 9:e102850
Uzun S, Kim H, Leal C, Padua GW (2016) Ethanol-induced whey protein gels as carriers for lutein droplets. Food Hydrocoll 61:426–432
Vezocnik V, Rebolj K, Sitar S et al (2015) Size fractionation and size characterization of nanoemulsions of lipid droplets and large unilamellar lipid vesicles by asymmetric-flow field-flow fractionation/multi-angle light scattering and dynamic light scattering. J Chromatogr A 1418:185–191. https://doi.org/10.1016/j.chroma.2015.09.048
Walia N, Dasgupta N, Ranjan S, Chen L, Ramalingam C (2017) Fish oil based vitamin D nanoencapsulation by ultrasonication and bioaccessibility analysis in simulated gastro-intestinal tract. Ultrason Sonochem 39:623–635
Yoshida K, Sekine T, Matsuzaki F, Yanaki T, Yamaguchi M (1999) Stability of vitamin A in oil-in-water-in-oil-type multiple emulsions. J Am Oil Chem Soc 76(2):1–6. https://doi.org/10.1007/s11746-999-0212-2
Youssof AME, Salem-Bekhit MM, Shakeel F et al (2016) Analysis of anti-neoplastic drug in bacterial ghost matrix, w/o/w double nanoemulsion and w/o nanoemulsion by a validated ‘green’ liquid chromatographic method. Talanta 154:292–298
Zhou K, Zhang QG, Li HM et al (2014) Ultrathin cellulose nanosheet membranes for superfast separation of oil-in-water nanoemulsions. Nanoscale 6:10363–10369. https://doi.org/10.1039/c4nr03227f
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Walia, N., Dasgupta, N., Ranjan, S. et al. Food-grade nanoencapsulation of vitamins. Environ Chem Lett 17, 991–1002 (2019). https://doi.org/10.1007/s10311-018-00855-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-00855-9