Abstract
Functional properties of liposoluble vitamins have been extensively used in the food industry for the development of nutrition-based products. But their efficacy tends to decline because of oxidation reactions as they are highly sensitive to free radicals and other metallic ions. In addition, lipid-soluble vitamins also demonstrate limited water solubility. Thus, dissolution in aqueous-based foods is a challenge. Some techniques have been suggested for the protection of lipid-soluble vitamins. One such method is nanoencapsulation which has recently posed a novel platform for showing enhanced bioavailability, higher shelf life and controlled release of entrapped liposoluble vitamins. In this work, we focus on five model lipid-based nanodelivery systems: (1) conventional nanoemulsions, (2) nanoliposomes, (3) solid lipid nanoparticles, (4) nanostructured lipid carriers and (5) nanosuspensions. First, we focus on the specific liquid and electrochemical techniques available for the preparation of such nanostructures. In the next phase, the five different lipid-based nanostructures have been discussed with their formulation techniques (energy and pressure attributes) concerning their encapsulation strategy for lipid-soluble vitamins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing interest in the application of nanotechnology to food products has initiated an emerging field of research for distinct nanoencapsulation strategies. In particular, the last two decades have witnessed extensive research on nanoencapsulation of nutrients that has garnered much attention for enhanced delivery of biomolecules including vitamins. Vitamins are figured as one of the important class of nutrients that deliver diverse biochemical functions that include improving the immune system and neurological disorders. In addition, vitamins may help in mitigating cholesterol levels and prevent cardiovascular diseases, improve physical endurance along with maintenance of normal metabolic rate (Webb 2014; Abbasi et al. 2014). They are either supplied through food products or in the form of dietary supplements. Owing to their chemical characteristics, these are classified into water- and liposoluble vitamins. Water-soluble vitamins include vitamins B complex (B1, B2, B3, B5, B6 and B12) and C (ascorbic acid). Fat-soluble vitamins include vitamin A, D, E and K. A summary of the biological functions of fat-soluble vitamins is shown in Table 1. According to Jafari (2017a, b), nanoencapsulation techniques have been sub-divided into five elemental groups: nature inspired, lipid based, special equipment, biopolymer based and others (nanoscrystals and dendrimers). However, the present review aims to critically summarize and explore the state-of-the-art liquid and electrochemical-based techniques to formulate lipid-based nanodelivery systems. This review has also elucidated several low- and high-energy and pressure techniques, respectively, for developing the desired lipid-based nanovehicle. Various strategies have been developed for the protection of lipid-soluble vitamins within food systems. One such method is known as nanoencapsulation. In short, it is a coating or a shell around the desired substance at sizes of nanoscale. This technique acts as an ultimate platform to carry out the required protection and delivery of these bioactive molecules in the determined time and zone. Moreover, these coatings possess improved functionality in the form of better solubility, retaining stability and controlled release of the entrapped or encapsulated material (Oehlke et al. 2014). Formulation of these systems must involve the use of generally recognized as safe (GRAS) materials and be compatible with various processing systems to accomplish such functionality (McClements et al. 2009). On the other hand, the encapsulation efficiency of these active components is considered as a vital factor. It describes the concentration of active ingredient detected in the formulation over the initial concentration used to make the formulation (Piacentini 2016; Chan 2011; Sun-Waterhouse et al. 2014).
Nanoencapsulation provides a better platform over conventional encapsulation in preserving the native properties (due to the large surface distribution and effective biodegradability than microencapsulation) of the bioactive components ultimately performing the required functions. In addition, nanoencapsulation also promotes precision targeting, higher intracellular uptake, quick dissociation within the tissues, improved shelf life of the active molecule and increased surface to volume ratio which further accelerates the cellular reactions. Moreover, nanoencapsulation changes the optical, mechanical and electrical properties of the incorporated substance due to intense molecular interactions within the shell (Neethirajan and Jayas 2011). Hence, for maintenance of proper physicochemical properties of the entrapped vitamins, appropriate techniques for encapsulation should be procured depending on their required stability (Quintanilla-Carvajal et al. 2010). These nanosystems should be carefully formulated to exhibit the essential functional attributes in the final food product. Specifically, they possess the ability to permeate into the tissues and enter the epithelial cell to carry out the proposed objective (Abaee et al. 2017). An overview of different liquid and electrochemical-based nanoencapsulation techniques, biopolymers used and structured assemblies have been demonstrated in Fig. 1.
Techniques available for nanoencapsulation
Liquid-based techniques
Nanoprecipitation
Nanoprecipitation is also known as solvent displacement or interfacial deposition method, where spontaneous emulsification of the internal organic phase results in the diffusion of the left out solvent. The solvent phase must be a solution of either polysorbate 80, poloxamer 188, ethanol, methyl chloride or acetone (Mora-Huertas et al. 2010). The added polymer or the ingredient gets precipitated from the organic solution and the left out solvent gets diffused into the aqueous system. This leads to the formation of nanocapsules which transport the labile active ingredients to the oral or colon-targeted sites (Fig. 2).
Ribeiro et al. (2008) designed β-carotene—loaded nanodispersions containing poly (d, l-lactic acid) and poly (d, l-lactic-co-glycolic acid) using the solvent displacement technique. β-carotene (a component with pro-vitamin A molecule) was encapsulated in the polymer matrix without any lipid core material, and Tween 20 or gelatin was used as an emulsifier for stabilizing the hydrocolloid. The nanoparticle formed in the dispersion phase exhibited a concentration of 150 mg/L which lasted for at least 5 months. During the period, no significant change in particle size distribution was observed, and the dispersion was quite stable against coalescence and Ostwald ripening. The mean diameter of the nanoparticle formed was less than 80 nm. Khayata et al. (2012) formulated antioxidant (Vitamin E)-loaded nanocapsules at pilot and laboratory scale, respectively, using a similar technique. Later, it was scaled up using membrane contactor technique. The biopolymers used were similar as in the case of that by Ribeiro et al. (2008). However, Tween 80 was used as an emulsifier for stabilization purpose. During encapsulation, three different types of oils were introduced along with the antioxidant. The formulation showed low ζ-potential values which indicated low stability. Yet, high encapsulation efficiency was observed even at pilot scale (97.65 ± 2.06%) and at laboratory scale (98 ± 1.68%). The mean diameter of the nanocapsules formed was 172 and 165 nm, respectively.
Emulsification-solvent evaporation
Emulsification-solvent evaporation is an improved version of solvent evaporation technique. In general, in a nanoencapsulation technique using oil-in-water emulsion system, the bioactive nutrient is emulsified in an organic polymer solution, which is then emulsified in an aqueous phase. This process is followed by organic solvent evaporation, which precipitates the bioactive nutrient in the droplets forming nanocapsules. This coupled technique also proposes quite a few advantages involving preference over other preparation techniques such as homogenization and sonication, as it necessitates mild conditions such as constant stirring and ambient temperature. Thus, several materials and processing parameters need to be taken care of for attaining stable nanoparticles without compromising the incorporated nutrient. These may include surfactant concentration, aqueous and organic phase volume and concentration, respectively, bioactive nutrient and polymer concentration in the organic phase, power and duration of energy supplied and stirring rate. Using emulsification-coupled solvent evaporation technique with a high-energy input, Silva et al. (2011) designed a β-carotene-encapsulated oil-in-water nanoemulsion system. Hexane was used as a polymer–solvent phase and Tween 20 as an emulsifier for stabilizing the surfactants. The particle size of the nanoemulsion formed ranged from 9.24 ± 0.16 to 276.77 ± 17.70 nm, and there was no significant change observed for 21 days.
Coacervation
Coacervation is a unique nanoencapsulation technique, as it can achieve a higher payload (up to 99%) than any other technique such as emulsification, solvent evaporation and nanoprecipitation (Ezhilarasi et al. 2013). It allows the controlled release of various labile active components depending upon external parameters such as temperature and mechanical stress. The mechanism involves the deposition of coacervate (spherical colloidal particles held by hydrophobic interaction) phase around the labile active compound resulting from phase separation of single or mixed polyelectrolytes from the aqueous solution. The resulting aggregated colloidal particle can further be mixed with some enzymatic or chemical cross-linkers such as transglutaminase or glutaraldehyde to enhance the strength of the coacervate (Zuidam and Shimoni 2010). Factors such as the ratio of biopolymers used, pH, charge, concentration and ionic strength help in the interaction of biopolymers and the coacervate formed (Butstraen and Salaün 2014). This technique is further classified into simple and complex coacervation. Simple coacervation involves only one polymer, whereas the complex method involves the use of two or more types of polymers. Complex coacervation technique has found wide applications in encapsulating various liposoluble vitamins. The most common biopolymers used are gum arabic and gelatin, having opposite charges. However, in some cases, gelatin can be replaced with chitosan or its derivatives or milk proteins, while gum arabic can be replaced with non-ionic polyelectrolytes such as alginates or pectin (Dima et al. 2015).
Supercritical fluid extraction of (O/W) emulsion (SFEE)
Recently, supercritical fluid extraction technique has attracted increasing scientific attention of food scientists (Ziani et al. 2012). The fluids used can either be a gas or a liquid exhibiting property between the two states. These properties include low density, high diffusivities, low viscosity and low solvating power, showing values above its thermodynamic critical point of pressure and temperature. Among other widely available compounds such as water, CO2, N2 and propane, CO2 is mostly preferred as its supercritical state can be achieved at low pressure (7.38 MPa) and temperature (304.2 K) (Fathi et al. 2014). This provides an inert medium suitable for encapsulating thermosensitive and oxidizable bioactive components. In this method, solubilization of the biopolymer and bioactive component in the fluid takes place above its critical temperature and pressure. It is expanded through a nozzle, followed by evaporation of the fluid which precipitates the solute particles (Mattea et al. 2009). This method eases the separation of supercritical fluid from the bioactive nutraceutical by depressurization. Thus, it reduces the use of harmful organic solvents and produces particles with narrow size distribution (Cocero et al. 2009). A widely used supercritical fluid technique based upon constituent formulation is rapid expansion of supercritical solutions (RESS). This involves the mixing of bioactive ingredient and polymer (surfactants) with the expanded supercritical fluid. The subsequent mixture experiences pressure drops upon expanding through the capillary tube and leads to the formation of a nanosized particle (Fig. 3). The second method is the supercritical antisolvent (SAS) technique. Davies et al. (2008) firstly discovered its implications using polymer system in drug encapsulation. Unlike RESS, here CO2 acts as an antisolvent factor which is directly pumped into a high-pressure container at a definite pressure. Then the solution containing the organic solvent (mainly polystyrene or polymethyl methacrylate), bioactive nutrient and polymer is sprayed via a nozzle. The solvent is diffused into the supercritical fluid, precipitating the bioactive nutrient. The second technique has a demerit over the former due to the involvement of organic solvents (Hu et al. 2012). SFEE has been recently in use for the encapsulation of liposoluble vitamins to produce custom-made nanoparticles based on the use of supercritical fluid (CO2) for effectively removing the polymer dissolved in the solution. Prieto and Calvo (2017) studied the influence of supercritical fluid extraction on the initial formulation of the vitamin E-incorporated nanoparticles using polycaprolactone (encapsulating polymer). Acetone was used as the organic solvent due to its high non-toxicity potential. A low residual solvent concentration of 50 ppm was obtained when operated at a critical pressure and temperature value of 8 MPa and 313 K, respectively, with a CO2 flow rate of 7.2 kg h−1 kg. The residue was obtained in 240 min after consuming 101 kg CO2 per of kg acetone. The obtained particle had nanoscale size ranging between 8 and 276 nm with narrow particle size distribution (between 0.24 and 0.54) and high encapsulation efficiency of around 90%. Prieto et al. (2017) then used a high-pressure packing column (3.5 m) in concurrent mode to continuously produce vitamin E-incorporated polycaprolactone nanoparticles. The resulting formulation exhibited particle size at nanoscale ranging between 9 and 84 nm with encapsulation efficiency higher than 70%. Limited work has been done using this technique in developing food-grade delivery systems.
Ionotropic gelation
This technique is also known as internal gelation where drug-loaded polymeric solution interacts with the polyelectrolytes (polyvalent cations) to form hydrogels or gelispheres. These hydrogels are spherical hydrophilic units which exhibit general swelling and gelation in simulated biofluids. The gelispheres then form a three-dimensional lattice structure which is cross-linked with the encapsulated bioactive component. Polymeric relaxation controls the release of vitamins in the system. Belščak-Cvitanović et al. (2016) encapsulated β-carotene in pectin hydrogels using the internal gelation technique. Hydroxypropyl methylcellulose (HPMC) was used as a carrier for designing the delivery system. The hydrogel beads formed exhibited slightly irregular shape. It was observed that the loading capacity of carotene depended on the carrier material and not on the particle size. Further research is needed to explore the implication of the gelation technique to formulate nanoscale delivery systems.
Electrochemical-based techniques
Electrospinning
Electrospinning involves the use of high voltage to convert the suspended droplet of a polymer solution into a fiber. In this method, the liquid polymer is extruded from the tip of the needle through a syringe pump at a constant rate, resulting in droplet formation on the tip (Ghorani and Tucker 2015). When an electric field (103 V/cm) meets the droplet, it stretches in the direction of the lower potential area forming a Taylor cone (Katouzian and Jafari 2016). On reaching the critical field, the droplet loses its surface tension from the tip and consequently a polymer jet is ejected in the liquid phase. The liquid polymer continues to flow in the form of thin fibers until all the solvent gets evaporated. It is a unique as well as a cost-effective technique for producing fibers of ≤ 100 nm in size. With different polymer solution and processing parameters, a wide range of polymer fibers can be produced with a variety of size and cross-sectional shapes.
There has been a growing interest in the use of electrospun fibers in the food and nutraceuticals sector. They have been extensively used in encapsulating bioactive compounds for preservation and control release purpose (Alborzi et al. 2013). Encapsulation involves prior mixing of bioactive compounds in the polymer liquid phase, followed by electrospinning. Ethanol and water are the most widely used solvents for the preparation of the polymer phase (Ghorani and Tucker 2015). In most cases, a two-phase electrospinning technique is used for the encapsulation of bioactive compounds. This involves a biphasic suspension, prepared by mixing of polymer solution with the solution of the bioactive component (Anandharamakrishnan 2014). A proper polymer solution also decides the formation of the electrospun fiber. Viscosity and surface tension of the polymer solution act as the crucial parameters in determining the propensity of the bead formation and the diameter of the fiber (Spasova et al. 2006).
Fernandez et al. (2009) used light-sensitive β-carotene for encapsulation in ultrafine electrospun fibers of zein prolamine. Fiber capsules produced were in the range of 310–2910 nm and preserved the fluorescence of the encapsulated molecule. Characterization through scanning electron microscope showed that the bioactive nutrient was stable and evenly distributed in the zein fiber. Furthermore, when exposed to UV–Vis irradiation, a significant increase in the stability of the bioactive nutrient was observed. Carotene was not observed to be oxidized or isomerized when encapsulated in the zein prolamine fiber.
Electrospraying
Electrospraying technique works on the same principle as electrospinning. However, a low voltage is required during the initial processing stage. The liquid polymer is extruded from the tip of the needle through a syringe pump at a constant rate, resulting in the droplet formation at the tip. When the liquid flowing out of the capillary nozzle meets the electric field, it elongates toward the higher potential area forming a Taylor cone. On reaching the critical stage, the droplet loses its surface tension from the tip and consequently ejects out in the form of a jet which deforms or disrupts due to the electrical force acting upon it. The electrospraying method generates particle size ≤ 10 nm with high monodispersity index. It has few applications in encapsulating bioactive compounds and is on the verge of enhancing its effectiveness in the food industries.
López-Rubio and Lagaron (2012) used the electrospraying technique for producing whey protein concentrate (WPC) nanocapsule for encapsulating antioxidant β-carotene molecule. Water was used as a solvent for developing the encapsulating morphologies widely applicable for food applications. The concentration of WPC was optimized, and the effect of pH and addition of glycerol in the nanocapsule were studied. Glycerol emerged as a crucial component for the photostabilization of carotene in the hydrocolloid matrices. A high encapsulation efficiency of around 90% was observed in the initial sample.
Nanoformulations and structured assemblies for delivery systems
Functionality and physicochemical properties of delivery systems
Nanosized vehicles have attained greater attention in the development of potential delivery systems for lipophilic bioactive compounds, supplements and nutrients. For incorporating these substances in the food matrix, some valuable factors are needed to be considered. First, the rheological properties of the food matrix are an important factor. Second, whether the matrix can maintain its stability throughout the shelf life of the food product and, third, whether the encapsulated components will release at the target site are the other factors (Oehlke et al. 2014; Shin et al. 2015). Due to their nano size, some food-grade matrix has shown numerous functional benefits inside the body such as augmenting the bioaccessibility, stability, solubility or membrane permeability. These properties help in proper absorption in the gastrointestinal tract (GIT), lessen the variability in pH in the GIT, enhance the intestinal permeation, control release at the targeted site and promote transcellular and intracellular delivery (Fathi et al. 2014).
These nanoformulated structured assemblies are classified into three types depending on their wall composition: protein-based systems, polysaccharide-based system (comprising of nanovehicles and polysaccharide-based lipid nanoparticles) and lipid and surfactant-based systems including nanoemulsions, nanoliposomes, solid lipid nanoparticle (SLNs), nanostructured lipid carrier (NLCs) and nanosuspensions (Katouzian and Jafari 2016; Rodríguez et al. 2016; Davidov-Pardo et al. 2015). However, for formulating nanoscale encapsulation of liposoluble vitamins, only lipid and surfactant-based systems have been studied so far. These systems have been shown schematically in Fig. 4. These systems have two types of release mechanisms: sustained release in which constant concentration is maintained at the target site and delayed release in which the release is prolonged from a lag time up to the target site with no obstacles (Fathi et al. 2012). Since each structured assembly has its functional property such as oral absorption, stability, solubility, bioavailability and encapsulation efficiency, it is crucial to analyze the material to be encapsulated for creating efficient delivery systems for liposoluble components (Table 2).
Nanoformulations for lipid-soluble vitamins
Conventional nanoemulsion-based system
Nanoemulsions are regarded as the most effective means of delivery systems for various bioactive compounds including fat-soluble vitamins. Their formulation involves water phase, surfactant and oil phase and their proportion for mixing depends upon the type of component to be encapsulated (Fig. 5). Their droplet size ranges between 10 and 100 nm, which depends mainly upon the component ratios involved and shearing and mechanical forces applied during the processing time (Jaiswal et al. 2015; Shin et al. 2015). Emulsifiers (Tween 20, lecithin, caseinate, WPI) also affect the initial droplet size and concentration in the system (Teo et al. 2016). They control the environmental stresses such as enzyme activity, ionic strength and interfacial properties such as thickness, rheology and charge of the molecule (Akhavan et al. 2018). Smaller droplet size makes these emulsions slightly turbid or optically transparent in appearance due to poor scattering of light through the solution, as the size of the droplet is smaller than the wavelength of the light (Ahmed et al. 2012). They can, therefore, be used in liquid foods or beverages to deliver the incorporated liposoluble components such as vitamins inside the body. Also due to the nanosize of the droplets, nanoemulsions possess better stability to coalescence, gravitational separation and flocculation. Nanoemulsions have higher viscosity even at the same lipid concentration compared to conventional emulsions (McClements and Li 2010). In addition, they have higher surface area to volume ratio compared to conventional ones which accelerate the chemical reactions occurring at the oil–water interface. This makes liposoluble components a better bioactive nutrient in nanoemulsions rather than in conventional emulsions.
Nanoemulsions are formed by two approaches; high energy or shearing method, which involves the use of ultrasonicators or high-pressure homogenizers and low-energy method which relies on surrounding conditions for any change in the interfacial properties (Anton and Vandamme 2009). Due to the fast processing operations, high-energy methods are frequently used in the food industries. Emulsions are formulated by homogenizing an aqueous and an oil phase together in the presence of water-soluble emulsifier, thus preventing droplet coalescence and facilitating disruption within the sonicator or homogenizer (Wooster et al. 2008). Various experiments have been carried out for encapsulating different liposoluble components (vitamins) using oil-in-water nanoemulsion in recent years.
Ziani et al. (2012) encapsulated vitamin E, vitamin D3 and lemon oil in surfactant-based oil-in-water emulsions. Tween 20, 60 and 80 were used as surfactants. The influence of the bioactive nutrients and surfactant to bioactive nutrient ratio was determined using turbidity measurement and dynamic light scattering. Both the vitamins had broad monomodal distribution, with the majority of the droplets between 220 nm (vitamin D3) and 340 nm (vitamin E). Laouini et al. (2012) used Shirasu porous glass (SPG) membrane emulsification technique for encapsulating vitamin E within oil-in-water nanoemulsion. The goal was to create a delivery system which could efficiently deliver the vitamin to the lungs. Tween 80 and Brij 35 were used as surfactants. The average size of the emulsion droplets was found to be around 78 nm. Droplet coalescence did not occur due to the presence of high ζ-potential value (≈ − 22.9 mV). The formulated nanoemulsion-encapsulated vitamin E had an overall efficiency of 99.7%.
Nanoliposomes
Nanoliposome is a lipid-based nanocarrier with aqueous core vascular structure embedded in a phospholipid bilayer. These hydrophobic lipids are components of the cell membrane with more than one hydrophobic tail, but only one hydrophilic head (Bouarab et al. 2014; Fang and Bhandari 2010). This allows the accumulation of both liposoluble and water-soluble bioactive components in its encapsulating region. Fat-soluble components can be encapsulated between the phospholipid layers due to the presence of hydrophobic medium, whereas hydrophilic components are encapsulated inside the core shell. In addition, both water- and fat-soluble components can be entrapped simultaneously in the nanoliposome. The dual encapsulation property owing to good efficiency, biocompatibility and lower toxicity levels makes nanoliposomes more acceptable as a delivery system in food and pharmaceutical industries (Patel and Velikov 2011). They have been studied for encapsulating various unstable compounds such as vitamins, antimicrobials and flavors. The methods of production of liposomes for vitamin encapsulation are thin-film hydration technique, solvent injection technique, detergent removal method and reverse phase method (Fig. 6) (Demirci et al. 2017). In one study, nanoliposomes could enhance their bioavailability and efficacy at the target site itself and also improve their stability with the addition of cholesterol to phospholipid bilayers (Pezeshky et al. 2016; Shin et al. 2015). Ko and Lee (2010) prepared a retinol (a component of vitamin A)-loaded nanoliposome by the centrifugation method, so that the influence of the encapsulated efficiency could be easily examined. The average particle size of the formulated nanoliposome was 98 nm with an encapsulated efficiency of 99%. The increase in temperature degraded the encapsulated retinol inside the nanoliposome resulting in the decrease of its stability.
Mohammadi et al. (2014) used thin-film hydration sonication technique for formulating vitamin D3-loaded nanoliposome for beverage fortification. The span value and particle size of the formulated particles were 0.70–0.85 and 82–90 nm, respectively, with an overall encapsulation efficiency of 93%. Due to the high ζ-potential value (− 43 mV) on adding cholesterol to phospholipid, droplet coalescence did not occur which made it suitable for use in beverage products. β-Carotene-loaded nanoliposome was reported by de Freitas Zômpero et al. (2015) for incorporating in ultrathin polymeric fiber produced by the electrospinning technique. The incorporation was done to enhance the photostability of β-carotene. For producing the electrospun fibers of polyvinyl alcohol (PVOH) and polyethylene oxide (PEO), 24 °C and 60% relative humidity (RH) conditions were maintained. It was observed that on increasing the nanoliposome content, the diameter of the electrospun fiber increased. Between the two fibers, PVOH showed better encapsulation of carotene rather than PEO.
Bochicchio et al. (2016) used ultrasonic irradiation technique to formulate nanoliposome for encapsulating vitamin B12, vitamin E (tocopherol) and vitamin D (ergocalciferol), respectively. The particle size and encapsulation efficiency obtained for vitamin B12, vitamin D and vitamin E nanoemulsions were 51, 48 and 40 nm and 56.2, 57.5 and 76.3%, respectively. Pezeshky et al. (2016) optimized lecithin-cholesterol concentration for formulating vitamin A palmitate-loaded nanoliposome. The study indicated that the complex between the liposome and vitamin A was produced by physical interaction. The span value and the overall particle size were in the range of 0.60–0.88 nm and 76–115 nm, respectively. Results showed that higher concentration of cholesterol decreased the efficiency of vitamin E.
Solid lipid nanoparticle (SLNs)
SLNs are particles consisting of a crystallized lipid phase medium encapsulating the core material (Fathi et al. 2012). There are only two basic techniques for large-scale preparation of SLNs in food industries, namely hot and cold homogenization. Hot homogenization involves melting of the lipid constituent at around 10 °C higher than its melting point for proper mixing of bioactive nutrient in the lipid phase (Helgason et al. 2009). The solution is again dispersed and homogenized in a preheated surfactant medium at the same temperature. The formulated oil-in-water emulsion is cooled for lipid crystallization producing solid nanoparticles. In the case of a water-soluble bioactive component, there is loss due to mixing with the water medium and on applying this technique would lead to the formation of a solid matrix containing some of those components. Cold homogenization provides a better platform for proper encapsulation of water-soluble components. In this method, the lipid mixture phase is rather cooled and milled afterward. Then the ground particles are dispersed and homogenized in cold surfactant solution below room temperatures.
SLNs have been regarded as a novel delivery system and an alternative to nanoemulsion and nanoliposomes (Katouzian et al. 2017). This phenomenon can be attributed to higher encapsulation efficiency and slower degradation rate (due to the presence of crystallized lipid phase medium), allowing prolonged time for the release of the bioactive nutrient. In addition, it provides higher flexibility and protection against chemical degradation due to the formation of a solid matrix such as waxes, triacylglycerol and paraffin (Pardeshi et al. 2012; Katouzian et al. 2017).
Patel et al. (2012) prepared a vitamin D2 (ergocalciferol)-loaded solid lipid nanoparticle. In this study, the vitamin was encapsulated in tripalmitin SLNs stabilized by Tween 20. The vitamin concentration was optimized to determine the suitable concentration for formulating the nanoparticle. The result showed that the stability of the lipid crystal was affected by increasing the vitamin concentration. Thus, better clarity of the dispersion was achieved with an enhanced loading capacity of the bioactive nutrient. The particle size obtained was 65 nm in diameter. Qian et al. (2013) encapsulated β-carotene in SLNs. SLNs were prepared using Tween 20 as a surfactant and hydrogenated palm oil or cocoa butter as lipids. Equal concentrations of both the lipid were used for producing the nanoparticle at 30 °C temperature. The particle size obtained was less than 200 nm with a high loading capacity of the bioactive nutrient. The process flow diagram for encapsulation of bioactives by SLN and NLC as described by Pyo et al. (2017) is given below (Fig. 7).
Nanostructured lipid carrier (NLCs)
NLCs are particulate systems obtained from oil-in-water nanoemulsion. The overall composition remains the same as that of the oil-in-water nanoemulsion. Instead, they consist of liquid lipid (oil) in the space, and the majority of oil constituent is replaced by crystallized solid lipid (fats) particles at body temperature (Shin et al. 2015). NLCs are considered better assemblies than SLNs due to the higher loading capacity of the incorporated bioactive component (Katouzian et al. 2017). They can be formulated using different methods depending on polymeric nanoparticle manufacturing protocol. These include hot and cold homogenization, phase inversion, solvent injection/displacement method, solvent diffusion method, a solvent-emulsification evaporation method and membrane contractor (Iqbal et al. 2012). When the bioactive nutrient concentration is too high, recrystallization of solid lipid particles occurs which degrades the solubility of the encapsulated material, leading to expulsion from the particulate system. Hence, if any liposoluble component is encapsulated in the system, it gets dissolved with the liquid lipid phase further getting encapsulated into the solid lipid medium, thus, executing controlled release with a high loading capacity of the bioactive nutrient (Tamjidi et al. 2013; Varshosaz et al. 2010). Therefore, the nanostructured lipid carrier restrains the encapsulated lipophilic material inside the solid lipid matrix and protects it from degradation.
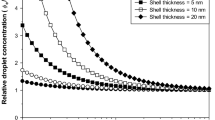
Hejri et al. (2013) used the solvent diffusion method for the formulation of β-carotene-loaded NLCs. The surfactant and the lipid phase concentration were optimized in this study. Furthermore, the temperature and the liquid to total (solid + liquid) lipid ratio during the carotene degradation were scrutinized carefully. The minimum particle size was found to be 8–15 nm with a degradation rate of 3%. Zhang et al. (2013) reported an anhydrous milk fat-based NLC using phase inversion technique to deliver β-carotene into the body. Transparent dispersions were observed at 0.8 M NaCl by heating at 90 °C with 10% (w/w) of anhydrous milk fat. The overall particle size obtained was smaller than 25 nm. After 16 days of storage, the net antioxidant potential decreased by 66.7%, while 47.3% decrement was observed in β-carotene concentration when loaded in NLCs.
Nanosuspensions
Nanocrystal suspensions consist of only solid nanoparticles dispersed in a liquid medium, which encapsulates a single pure bioactive component. The dispersed medium is totally crystallized and contains minimum surfactant molecules for attaining stabilization. The suspensions can be used in various aqueous formulations for subcutaneous, intravenous and pulmonary use as they tend to enhance the solubility and dissolution rate of the encapsulated bioactive component at the target site (Shin et al. 2015). These properties are associated with resistance to oxidation and hydrolysis, increased oral adsorption and bioavailability.
Campardelli and Reverchon (2015) used a supercritical-assisted process called supercritical-assisted injection in liquid antisolvent (SAILA) for formulating vitamin E (α-tocopherol)-loaded nanosuspension. The suspension produced by the application of high injection pressure and low solute concentration at a temperature of 60 °C contains particle size below 150 nm in diameter. The schematic of this process is shown in Fig. 3.
Controlled and targeted release of nanoencapsulated liposoluble vitamins
Controlled and targeted release are two of the many criteria responsible for proper bioavailability of liposoluble vitamins. The complex interaction of structured assemblies and the involved food matrix makes it a challenging aspect to study the release profile inside the gastrointestinal systems. However, the development of nanoencapsulation (nanocarriers) systems has posed better results, mitigating this trial (Katouzian and Jafari 2017). These systems provide controlled release upon degradation of the polymer matrix incorporating lipophilic bioactive components. This occurs in accordance with the equilibrium between the rate of mucous erosion and kinetics of ingredient adhesion. Therefore, it is essential to study the adhesion kinetics in relation to the release procedures for improvisation in controlled release processes (Jafari and McClements 2017). Functional molecules such as lecithin and chitosan have been introduced to improve the adhesive property of nanoformulations (Jafari 2017b).
Among various release procedures such as dissolution, swelling, osmosis, partitioning, erosion and diffusion, the last one has a major contribution in this stage (Jafari 2017a). The rate of diffusion is directly related to the hydrophilicity and particle size of the matrix polymer due to the presence of a lipophilic bioactive component in the core. Moreover, the nanoparticle size closely influences the release features of the nutrients at the targeted site. Therefore, an improved bioavailability is achieved in the case of nanocarriers when compared with their microcarrier counterpart (Katouzian and Jafari 2016). Initially, during the release, vitamins undergo a burst effect followed by its stationary release depending on the suiting environment of the system. Furthermore, this initiation is influenced by four major factors affecting the release profile and is depicted in Fig. 8.
Safety and regulatory issues of nanoencapsulated vitamins
Although the implementation of nanoscience in the encapsulation of bioactive components has risen to a new level, there are certain limitations in terms of its size as the nanoparticles have a tendency to pass through the cell barrier. In 2011, the European Food Safety Authority (EFSA) put forth some guidance for risk assessment of the incorporated nanoparticle with the food matrix involving analysis in five different stages: the time of preparation; type of food product to be utilized; maintaining the toxicity assay; complexity of the food network; interaction within the cell and biological fluid. In addition, in the same year, the US Food and Drug Administration (USFDA) announced a regulation guideline for specific manufacturers regarding the regulatory and safety issues for novel food processing industries (Food and Administration 2012). Other than Europe and the USA, there are other nations such as Africa (Cele et al. 2009), Australia (Bowman and Ludlow 2012) and some Asian countries (Delemarle and Throne-Holst 2013) which have recently developed their regulatory principles for their respective industries to maintain the quality of food production.
In 2014, it was observed that there was no carcinogenic or genotoxic effect from the food nanoparticles and thus these were considered safe for consumption (Bai and Liu 2015). Until now, in vivo and in vitro tests have been conducted in small animals to observe the effects of food nanostructures. However, clinical trials need to be investigated on humans to become commercialized globally (Katouzian and Jafari 2016).
Potential future works in the enhancement of nutritional attributes
There are various encapsulation techniques that have frequently been used for producing very small sized material for efficient delivery purposes. Liquid-based techniques like nanoprecipitation, emulsification-solvent evaporation, coacervation, supercritical fluid extraction and ionotropic gelation are widely known for encapsulating lipophilic vitamins. But due to unavailability of proper drying method, the desired nanosized powders for incorporating in nanocarriers are scarcely available, as they tend to form dust and micro-sized aggregates even after forming nanosized edible particles. Researchers are working to produce a suitable technique for drying of the material to the desired size for synthesizing novel biopolymers for formulating delivery systems. Considering the structured assemblies, apart from lipid-based systems, protein and polysaccharide-based delivery systems need more importance for carrying out the delivery of liposoluble vitamins due to better compatibility with food systems. Apart from these, research has been performed for evaluating various toxicokinetic properties of these fat-soluble vitamins during encapsulation in various nanoformulations. As most of these methodologies are adapted from non-food-grade industries, future research should focus on developing techniques imperative to only food-grade ingredients and related economic processes.
Conclusion
This review has provided an overview of the different functionalities of lipophilic vitamins, their encapsulation techniques and various nanoformulations, specifically for delivering fat-soluble vitamins. The development of structured assemblies for delivering lipophilic functional bioactive is a crucial area of research in food and nutritional sciences. For fabricating purposes, GRAS materials must be used if food ingredients are to be incorporated. They are known to enhance the bioavailability and dispersibility of these nutrients by in vivo or in vitro studies resulting in a reduction of the deficiency diseases. There is a challenge in establishing proper interaction between the encapsulating material and the bioactive nutrient. For delivery at the targeted site, these nanostructured assemblies must be designed considering a change in temperature and pH in the body, as they are the major parameters affecting the loss of vitamin activity. Hence, nanotechnology acts as a base for creating efficient systems for enhancing the nutritional attributes of food products.
References
Abaee A, Mohammadian M, Jafari SM (2017) Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci Technol 70:69–81. https://doi.org/10.1016/j.tifs.2017.10.011
Abbasi A, Emam-Djomeh Z, Mousavi MAE, Davoodi D (2014) Stability of vitamin D 3 encapsulated in nanoparticles of whey protein isolate. Food Chem 143:379–383. https://doi.org/10.1016/j.foodchem.2013.08.018
Ahmed K, Li Y, McClements DJ, Xiao H (2012) Nanoemulsion-and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem 132:799–807. https://doi.org/10.1016/j.foodchem.2011.11.039
Akhavan S, Assadpour E, Katouzian I, Jafari SM (2018) Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci Technol 74:132–146. https://doi.org/10.1016/j.tifs.2018.02.001
Alborzi S, Lim L-T, Kakuda Y (2013) Encapsulation of folic acid and its stability in sodium alginate-pectin-poly (ethylene oxide) electrospun fibres. J Microencapsul 30:64–71. https://doi.org/10.3109/02652048.2012.696153
Anandharamakrishnan C (2014) Techniques for formation of nanoemulsions. Techniques for nanoencapsulation of food ingredients. Springer, Berlin, pp 7–16
Anton N, Vandamme TF (2009) The universality of low-energy nano-emulsification. Int J Pharm 377:142–147. https://doi.org/10.1016/j.ijpharm.2009.05.014
Bai H, Liu X (2015) Food nanotechnology and nano food safety. In: Nanotechnology materials and devices conference (NMDC). IEEE, USA, pp 1–4
Belščak-Cvitanović A, Bušić A, Barišić L, Vrsaljko D, Karlović S, Špoljarić I, Vojvodić A, Mršić G, Komes D (2016) Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll 57:139–152. https://doi.org/10.1016/j.foodhyd.2016.01.020
Bochicchio S, Barba AA, Grassi G, Lamberti G (2016) Vitamin delivery: carriers based on nanoliposomes produced via ultrasonic irradiation LWT-Food. Sci Technol 69:9–16. https://doi.org/10.1016/j.lwt.2016.01.025
Bouarab L, Maherani B, Kheirolomoom A, Hasan M, Aliakbarian B, Linder M, Arab-Tehrany E (2014) Influence of lecithin–lipid composition on physico-chemical properties of nanoliposomes loaded with a hydrophobic molecule. Colloids Surf B Biointerfaces 115:197–204. https://doi.org/10.1016/j.colsurfb.2013.11.034
Bowman DM, Ludlow K (2012) Assessing the impact of a for government review on the nanotechnology regulatory landscape l. Monash UL Rev 38:168
Butstraen C, Salaün F (2014) Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr Polym 99:608–616. https://doi.org/10.1016/j.carbpol.2013.09.006
Campardelli R, Reverchon E (2015) α-Tocopherol nanosuspensions produced using a supercritical assisted process. J Food Eng 149:131–136. https://doi.org/10.1016/j.jfoodeng.2014.10.015
Cele LM, Ray SS, Coville NJ (2009) Guest editorial: nanoscience and nanotechnology in South Africa. S Afr J Sci 105:242
Chan E-S (2011) Preparation of Ca-alginate beads containing high oil content: influence of process variables on encapsulation efficiency and bead properties. Carbohydr Polym 84:1267–1275. https://doi.org/10.1016/j.carbpol.2011.01.015
Cocero MJ, Martín Á, Mattea F, Varona S (2009) Encapsulation and co-precipitation processes with supercritical fluids: fundamentals and applications. J Supercrit Fluids 47:546–555. https://doi.org/10.1016/j.supflu.2008.08.015
Davidov-Pardo G, Joye IJ, McClements DJ (2015) Chapter nine-food-grade protein-based nanoparticles and microparticles for bioactive delivery: fabrication characterization, and utilization. Adv Protein Chem Struct Biol 98:293–325
Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM (2008) Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Deliv Rev 60:373–387. https://doi.org/10.1016/j.addr.2006.12.001
de Freitas Zômpero RH, López-Rubio A, de Pinho SC, Lagaron JM, de la Torre LG (2015) Hybrid encapsulation structures based on β-carotene-loaded nanoliposomes within electrospun fibers. Colloids Surf B Biointerfaces 134:475–482. https://doi.org/10.1016/j.colsurfb.2015.03.015
Delemarle A, Throne-Holst H (2013) The role of standardization in the shaping of a vision for nanotechnology. Int J Innov Technol Manag 10:1340005
Demirci M, Caglar MY, Cakir B, Gülseren İ (2017) Encapsulation by nanoliposomes. In: Jafari SM (ed) Nanoencapsulation technologies for the food and nutraceutical industries. Academic Press, Cambridge, USA, pp 74–113. https://doi.org/10.1016/B978-0-12-809436-5.00003-3
Dima Ş, Dima C, Iordăchescu G (2015) Encapsulation of functional lipophilic food and drug biocomponents. Food Eng Rev 7:417–438. https://doi.org/10.1007/s12393-015-9115-1
Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C (2013) Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol 6:628–647. https://doi.org/10.1007/s11947-012-0944-0
Fang Z, Bhandari B (2010) Encapsulation of polyphenols–a review. Trends Food Sci Technol 21:510–523. https://doi.org/10.1016/j.tifs.2010.08.003
Fathi M, Mozafari M-R, Mohebbi M (2012) Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Technol 23:13–27. https://doi.org/10.1016/j.tifs.2011.08.003
Fathi M, Martín Á, McClements DJ (2014) Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci Technol 39:18–39. https://doi.org/10.1016/j.tifs.2014.06.007
Fernandez A, Torres-Giner S, Lagaron JM (2009) Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll 23:1427–1432. https://doi.org/10.1016/j.foodhyd.2008.10.011
Food U, Administration D (2012) Draft guidance for industry: assessing the effects of significant manufacturing process changes, including emerging technologies, on the safety and regulatory status of food ingredients and food contact substances, including food ingredients that are color additives Washington, DC: US Department of Health and Human Services
Ghorani B, Tucker N (2015) Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll 51:227–240. https://doi.org/10.1016/j.foodhyd.2015.05.024
Hejri A, Khosravi A, Gharanjig K, Hejazi M (2013) Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem 141:117–123. https://doi.org/10.1016/j.foodchem.2013.02.080
Helgason T, Awad T, Kristbergsson K, McClements DJ, Weiss J (2009) Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J Colloid Interface Sci 334:75–81. https://doi.org/10.1016/j.jcis.2009.03.012
Hu D, Lin C, Liu L, Li S, Zhao Y (2012) Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J Food Eng 109:545–552. https://doi.org/10.1016/j.jfoodeng.2011.10.025
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J (2012) Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target 20:813–830. https://doi.org/10.3109/1061186X.2012.716845
Jafari SM (2017a) An overview of nanoencapsulation techniques and their classification. Nanoencapsulation technologies for the food and nutraceutical industries. Academic Press, Cambridge, USA, pp 1–34. https://doi.org/10.1016/B978-0-12-809436-5.00001-X
Jafari SM (2017b) An Introduction to nanoencapsulation techniques for the food bioactive ingredients. Nanoencapsulation of food bioactive ingredients. Academic Press, Cambridge, USA, pp 1–62. https://doi.org/10.1016/B978-0-12-809740-3.00001-5
Jafari SM, McClements DJ (2017) Nanotechnology Approaches for Increasing Nutrient Bioavailability. In: Toldrá F (ed) Advances in Food and Nutrition Research, vol 81. Academic Press, Cambridge, USA, pp 1–30. https://doi.org/10.1016/bs.afnr.2016.12.008
Jaiswal M, Dudhe R, Sharma P (2015) Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 5:123–127. https://doi.org/10.1007/s13205-014-0214-0
Katouzian I, Jafari SM (2016) Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci Technol 53:34–48. https://doi.org/10.1016/j.tifs.2016.05.002
Katouzian I, Jafari SM (2017) Nanoencapsulation of vitamins. In: Nanoencapsulation of food bioactive ingredients. Academic Press, Cambridge, USA, pp 145–181. https://doi.org/10.1016/B978-0-12-809740-3.00004-0
Katouzian I, Esfanjani AF, Jafari SM, Akhavan S (2017) Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci Technol 68:14–25. https://doi.org/10.1016/j.tifs.2017.07.017
Khayata N, Abdelwahed W, Chehna M, Charcosset C, Fessi H (2012) Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: from laboratory scale to large scale using a membrane contactor. Int J Pharm 423:419–427. https://doi.org/10.1016/j.ijpharm.2011.12.016
Ko S, Lee S-C (2010) Effect of nanoliposomes on the stabilization of incorporated retinol. Afr J Biotech 9:6158–6161. https://doi.org/10.5897/AJB10.917
Laouini A, Fessi H, Charcosset C (2012) Membrane emulsification: a promising alternative for vitamin E encapsulation within nano-emulsion. J Membr Sci 423:85–96. https://doi.org/10.1016/j.memsci.2012.07.031
López-Rubio A, Lagaron JM (2012) Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov Food Sci Emerg Technol 13:200–206. https://doi.org/10.1016/j.ifset.2011.10.012
Mattea F, Martín Á, Cocero MJ (2009) Carotenoid processing with supercritical fluids. J Food Eng 93:255–265. https://doi.org/10.1016/j.jfoodeng.2009.01.030
Mayer S, Weiss J, McClements DJ (2013) Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: factors influencing droplet size and stability. J Colloid Interface Sci 402:122–130. https://doi.org/10.1016/j.jcis.2013.04.016
McClements DJ, Li Y (2010) Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv Colloid Interface Sci 159:213–228. https://doi.org/10.1016/j.cis.2010.06.010
McClements DJ, Decker EA, Park Y, Weiss J (2009) Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit Rev Food Sci Nutr 49:577–606. https://doi.org/10.1080/10408390902841529
Mohammadi M, Ghanbarzadeh B, Hamishehkar H (2014) Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv Pharm Bull 4:569. https://doi.org/10.5681/apb.2014.084
Mora-Huertas C, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385:113–142. https://doi.org/10.1016/j.ijpharm.2009.10.018
Neethirajan S, Jayas DS (2011) Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol 4:39–47. https://doi.org/10.1007/s11947-010-0328-2
Oehlke K, Adamiuk M, Behsnilian D, Gräf V, Mayer-Miebach E, Walz E, Greiner R (2014) Potential bioavailability enhancement of bioactive compounds using food-grade engineered nanomaterials: a review of the existing evidence. Food Funct 5:1341–1359. https://doi.org/10.1039/c3fo60067j
Pardeshi C, Rajput P, Belgamwar V, Tekade A, Patil G, Chaudhary K, Sonje A (2012) Solid lipid based nanocarriers: an overview/Nanonosači na bazi čvrstih lipida: Pregled. Acta Pharmaceutica 62:433–472
Patel A, Velikov KP (2011) Colloidal delivery systems in foods: a general comparison with oral drug delivery LWT-Food. Sci Technol 44:1958–1964. https://doi.org/10.1016/j.lwt.2011.04.005
Patel MR, Martin-Gonzalez S, Fernanda M (2012) Characterization of ergocalciferol loaded solid lipid nanoparticles. J Food Sci 77:N8–N13. https://doi.org/10.1111/j.1750-3841.2011.02517.x
Pezeshky A, Ghanbarzadeh B, Hamishehkar H, Moghadam M, Babazadeh A (2016) Vitamin A palmitate-bearing nanoliposomes: preparation and characterization Food. Bioscience 13:49–55. https://doi.org/10.1016/j.fbio.2015.12.002
Piacentini E (2016) In: Giorno L, Drioli E (eds) Encyclopedia of membranes. Springer, Berlin pp, pp 706–707
Prieto C, Calvo L (2017) Supercritical fluid extraction of emulsions to nanoencapsulate vitamin E in polycaprolactone. J Supercrit Fluids 119:274–282. https://doi.org/10.1016/j.supflu.2016.10.004
Prieto C, Calvo L, Duarte CM (2017) Continuous supercritical fluid extraction of emulsions to produce nanocapsules of vitamin E in polycaprolactone. J Supercrit Fluids 124:72–79. https://doi.org/10.1016/j.supflu.2017.01.014
Pyo S-M, Müller RH, Keck CM (2017) Encapsulation by nanostructured lipid carriers. In: Jafari SM (ed) Nanoencapsulation Technologies for the Food and Nutraceutical Industries. Academic Press, Cambridge, USA, pp 114–137. https://doi.org/10.1016/B978-0-12-809436-5.00004-5
Qian C, Decker EA, Xiao H, McClements DJ (2013) Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: potential limitations of solid lipid nanoparticles. Food Res Int 52:342–349. https://doi.org/10.1016/j.foodres.2013.03.035
Quintanilla-Carvajal MX, Camacho-Díaz BH, Meraz-Torres LS, Chanona-Pérez JJ, Alamilla-Beltrán L, Jimenéz-Aparicio A, Gutiérrez-López GF (2010) Nanoencapsulation: a new trend in food engineering processing. Food Eng Rev 2:39–50. https://doi.org/10.1007/s12393-009-9012-6
Ribeiro HS, Chu B-S, Ichikawa S, Nakajima M (2008) Preparation of nanodispersions containing β-carotene by solvent displacement method. Food Hydrocoll 22:12–17. https://doi.org/10.1016/j.foodhyd.2007.04.009
Rodríguez J, Martín MJ, Ruiz MA, Clares B (2016) Current encapsulation strategies for bioactive oils: from alimentary to pharmaceutical perspectives. Food Res Int 83:41–59. https://doi.org/10.1016/j.foodres.2016.01.032
Shin GH, Kim JT, Park HJ (2015) Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci Technol 46:144–157. https://doi.org/10.1016/j.tifs.2015.07.005
Silva HD et al (2011) Nanoemulsions of β-carotene using a high-energy emulsification–evaporation technique. J Food Eng 102:130–135. https://doi.org/10.1016/j.jfoodeng.2010.08.005
Spasova M, Mincheva R, Paneva D, Manolova N, Rashkov I (2006) Perspectives on: criteria for complex evaluation of the morphology and alignment of electrospun polymer nanofibers. J Bioact Compat Polym 21:465–479. https://doi.org/10.1177/0883911506068495
Sun-Waterhouse D, Wang W, Waterhouse GI (2014) Canola oil encapsulated by alginate and its combinations with starches of low and high amylose content: effect of quercetin on oil stability. Food Bioprocess Technol 7:2159–2177. https://doi.org/10.1007/s11947-013-1163-z
Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A (2013) Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol 19:29–43. https://doi.org/10.1016/j.ifset.2013.03.002
Teo A, Goh KK, Wen J, Oey I, Ko S, Kwak H-S, Lee SJ (2016) Physicochemical properties of whey protein, lactoferrin and Tween 20 stabilised nanoemulsions: effect of temperature, pH and salt. Food Chem 197:297–306. https://doi.org/10.1016/j.foodchem.2015.10.086
Varshosaz J, Eskandari S, Tabakhian M (2010) Production and optimization of valproic acid nanostructured lipid carriers by the Taguchi design. Pharm Dev Technol 15:89–96. https://doi.org/10.3109/10837450903013568
Webb G (2014) Vitamins/minerals as dietary supplements: a review of clinical studies. In: Berginc K, Kreft S (eds) Dietary Supplements: Safety, Efficacy and Quality. Woodhead Publishing, UK, pp 139–169. https://doi.org/10.1533/9781782420811.3.139
Wooster TJ, Golding M, Sanguansri P (2008) Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 24:12758–12765. https://doi.org/10.1021/la801685v
Zhang L, Hayes DG, Chen G, Zhong Q (2013) Transparent dispersions of milk-fat-based nanostructured lipid carriers for delivery of β-carotene. J Agric Food Chem 61:9435–9443. https://doi.org/10.1021/jf403512c
Ziani K, Fang Y, McClements DJ (2012) Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: vitamin E, vitamin D, and lemon oil. Food Chem 134:1106–1112. https://doi.org/10.1016/j.foodchem.2012.03.027
Zuidam NJ, Shimoni E (2010) Overview of microencapsulates for use in food products or processes and methods to make them. In: Encapsulation technologies for active food ingredients and food processing. Springer, Berlin, Germany, pp 3–29
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panigrahi, S.S., Syed, I., Sivabalan, S. et al. Nanoencapsulation strategies for lipid-soluble vitamins. Chem. Pap. 73, 1–16 (2019). https://doi.org/10.1007/s11696-018-0559-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0559-7