Abstract
We report the first data for atrazine removal in low-turbidity freshwaters. Atrazine is a globally applied herbicide, contamination by which may lead to direct and indirect ecotoxicological impacts. Although a common contaminant of surface waters, microbial biodegradation of atrazine in this environment has been little studied, with most work focused on soils by means of selected, atrazine-degrading bacteria-enriched cultures. Here, we measured atrazine removal from river water using a batch incubation system designed to represent environmental conditions, with water from two contrasting UK rivers, the Tamar and Mersey. Atrazine and bacterial inocula prepared from the source water were added to cleaned river water for 21-day incubations that were analysed directly by electrospray ionisation-mass spectrometry. The experimental approach was validated using peptides of different molecular mass. Results show that atrazine concentrations decreased by 11% over 21 days in Tamar samples, a rural catchment with low population density, when atrazine was the only substrate added. In contrast no removal was evident in Mersey samples, an urban catchment with high population density. When a tripeptide was added as a co-substrate, atrazine removal in the Tamar water remained at 11% while that for the Mersey water increased from 0 to 37%. Although degradation of atrazine in aerobic freshwaters is predicted according to its chemical structure, our data suggest that the composition of the bacterial population determines whether removal occurs under these conditions and at these environmentally realistic concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrazine is the most widely used herbicide of the s-triazine group across the globe. It was finally banned for use in the European Union in 2007 (Graymore et al. 2001; Ackerman 2007) and is included in the EU Directive on Priority Substances (Directive 2008/105/EC, Annex II), while 2-hydroxyatrazine is cited as a high priority compound for detailed environmental risk assessment (von der Ohe et al. 2011). Atrazine contamination may lead to direct and indirect eco-toxicological impacts (Solomon et al. 1996; Graymore et al. 2001), although its role as an endocrine disrupter remains controversial (Solomon 2009).
Most studies on atrazine biotransformation have been on soils, using bacterial enrichment cultures (Shapir et al. 2007). Reports on atrazine biotransformation in other environmental compartments are much rarer, particularly those employing naturally occurring (i.e. non-selected) microbial populations. However, atrazine is a common contaminant of surface waters, in which it is reported to be relatively persistent (Ingerslev and Nyholm 2000; Smalling and Aelion 2006), with a half-life of up to 120 days (Graymore et al. 2001).
A number of procedures have been devised for measuring the microbial degradation of organic molecules in surface waters (Jaworska et al. 2003). Elements of these approaches have been formalised in guidelines for the determination of biodegradation rate constants derived from experiments where natural waters (with or without suspended particles) are spiked with appropriate 14C-labelled molecules at concentrations <100 μg L−1 (OECD 2004; USEPA 2008). This approach assumes that, in general, susceptibility to biodegradation is related to compound structure (Jaworska et al. 2003). The identification and quantification of biotransformation products is viewed as a secondary goal in the guidelines (OECD 2004; USEPA 2008), primarily because relatively high concentrations of non-labelled compounds and alternative analytical methods would be required. Nevertheless, the identification of biotransformation products from anthropogenic molecules is important if the ecological impact of a compound is to be properly assessed (Kosjek et al. 2007). For example, the phototransformation products of the pharmaceutical naproxen were found to be significantly more toxic than the parent compound (Isidori et al. 2005).
The objective of this study was to measure atrazine removal in freshwaters, under environmentally realistic conditions, through a systematic laboratory batch incubation procedure simulating low-turbidity riverine systems. Samples were analysed directly by electrospray ionisation-mass spectrometry (ESI-MS), a powerful technique for studying polar, non-volatile compounds in surface waters. This approach enables detailed analyses of intact molecular ions and their fragments (Pérez and Barceló 2007) and so can be applied to studies where both the fate of parent molecules and transformation products are of interest. As such, ESI-MS is widely employed for analysis of pharmaceuticals and their degradation products in wastewater effluent (Kosjek and Heath 2008).
The incubation system was first validated using two peptides of contrasting molecular mass as recommended by OECD (2004) and then used for the atrazine experiments, with both the chemical and the bacterial inoculum added at representative environmental concentrations. Water from two contrasting rivers (Tamar and Mersey) with their corresponding bacterial inocula was used, and the effect of adding a potential co-substrate was also investigated.
Materials and methods
Chemicals
Atrazine (Pestanel® analytical standard grade, Sigma-Aldrich, UK), a tripeptide (≥98% purity, Sigma-Aldrich, UK; termed peptide A) and an octa-peptide (99.02% purity, Biomatik Corp., USA; termed peptide B) were used for the study (see Table 1 for composition of peptides).
Incubation setup
All plasticware and glassware were cleaned prior to use (2% v/v Decon, ≥24 h; 10% v/v HCl, ≥24 h; final rinse with 18 MΩ cm−1 water). Glassware was combusted (450°C, 6 h) as a final cleaning step. Pipette tips and glassware were also autoclaved when required, and all critical handling steps were undertaken in a Class 100 laminar flow hood using clean techniques.
Requirements of the incubation medium were that, as far as possible, it should be low in alternative carbon- and nitrogen-containing substrates (i.e. dissolved organic matter, nitrate) and give a low background signal for the ESI-MS determinations without further treatment of the incubated samples. A bulk freshwater sample was collected from the tidal limit of the Tamar (southwest England) and Mersey (northwest England) rivers, which are well-aerated, relatively low-turbidity rivers draining predominantly agricultural and urban catchments, respectively. In order to remove matrix interferences and competitive substrates from the collected river water, it was filtered (GF/F; 0.7 μm nominal pore size) to remove suspended particles, passed through a strong anion-exchange resin (Dowex® X-100, 200 mesh; water flow rate 80 mL h−1) to remove nitrate, UV-irradiated (400 W medium pressure Hg lamp, 6 h) to remove dissolved organic carbon (DOC) and re-filtered through a 0.2-μm filter membrane (Whatman Anodisc 47, aluminium oxide) to remove any remaining particulate matter. Finally, the water was sterilised by autoclaving (115°C, 15 min). All incubation samples and standards for the experiment were then prepared using this water. The bacterial inoculum was prepared using additional water samples collected from the same sampling site less than 24 h before the incubations were started. Each of these samples was filtered through a 1.6-μm pore size membrane (combusted GF/A) to remove zooplankton and particle-bound bacteria (Ainsworth and Goulder 1998) and then re-filtered through a 0.2-μm pore diameter membrane filter (Whatman Anodisc 47). The bacteria retained on the membrane were resuspended in a small volume of the 0.2-μm filtered water to provide the inoculum, which was then added to produce a final bacterial concentration similar to that measured in situ.
Concentrated stock solutions of atrazine were made up in methanol (LC–MS grade, Fisher Scientific, UK) while the peptides were dissolved in the river water used for the incubations. Sterilised river water (60 mL) was transferred to a 125-mL screw-capped amber glass bottle to which was added 30 μL of the stock solution and 1 mL of the bacterial inoculum. Control incubations, with bacteria only and compound only, were run in parallel. Orthophosphate was added to a final concentration of ca. 1–2 μM P, typical for the waters studied. The bottles were loosely capped, placed in a re-sealable plastic bag and transferred to an orbital shaker. Incubations were performed at ambient temperature. At the beginning and end of the incubation, duplicate incubated samples were filtered (combusted GF/F) and filtrates retained for measurement of analytes and potential degradation products by ESI-MS, while total dissolved nitrogen (TDN), DOC, ammonium, nitrate + nitrite and orthophosphate were also measured. The samples for TDN/DOC analysis were acidified to pH 2–3 using 6 M HCl. All samples were stored at −20°C until analysis. Separate duplicate incubated samples were used for viable counts of chemoorganotrophic bacteria.
An important consideration in the experimental design was to relate the timescales of the incubations to the timescale(s) of river water advection through a catchment and its exit at the tidal limit, as this will constrain the extent of biogeochemical processing. However, the accurate and precise determination of water transit time (i.e. the age of the water as it exits the catchment) in river basins is challenging. Published mean transit times for river basins of similar area to the Tamar and Mersey are in the region of 60–720 days (McGuire and McDonnell 2006). However, transit times of river water will be shorter as these estimates typically exclude faster surface flows. Our incubation time of 21 days was chosen as a reasonable approximation of the river water transit time in the Tamar and Mersey catchments.
Analytical
Analyses by ESI-MS were performed using a Finnegan MAT LCQ MS, a quadrupole ion trap mass spectrometer with an external source atmospheric pressure interface capable of electro-spray ionisation. Solutions were introduced by direct injection from a 250-μL Hamilton syringe at a rate of 3 μL min−1. The sample matrix was 50:50 methanol/water amended with 0.1% (v/v) formic acid. The signal sensitivity for each compound, in positive-ion mode, was optimised by adjustment of instrumental parameters using in-built tuning procedures. Ion counts were integrated for 2 min, and 5 × 2 min replicates were recorded per sample. Ion count stability was recorded in real-time via single ion monitoring. Analyte quantification was performed by external standard calibration. Specifically, a calibration curve was generated prior to sample analysis on each analytical day, and as analysis of samples proceeded, these were bracketed with a drift calibration standard to account for variations in detector response. Changes in sensitivity were accounted for using an algorithm developed for automated nutrient analysis (Skalar Analytical BV 2004); in this implementation, baseline drift was assumed unimportant as shown in the following equation:
where U″(n) = drift corrected ion count for sample n, U′(n) = ion count for sample n, D1 = ion count of the first drift standard, D(a) = ion count of the preceding drift standard, D(b) = ion count of the following drift standard, n = sample number, a = sample number of preceding drift, and b = sample number of following drift. Correlation coefficients (r 2) for the calibration lines were 0.98–0.99, while detection limits (mean blank + 3 standard deviations) were 70–110 nM for atrazine, 100–740 nM for peptide A and 50–450 nM for peptide B. The selection of m/z values for monitoring potential biotransformation products was informed by earlier studies on atrazine biodegradation (Graymore et al. 2001; Strong et al. 2002).
The TDN and DOC concentrations were determined simultaneously by high-temperature catalytic combustion (Badr et al. 2003). Concentrations measured in a certified reference material (US National Science Foundation/University of Miami, Batch 9, 2009) were in good agreement with the consensus values: DOC, 51.3 ± 6.9 and 41–44 μM, respectively; TDN, 31.9 ± 3.8 and 32.3–33.8 μM, respectively. Analytical precisions were ±2% and ±5% for DOC and TDN, respectively. Nitrate + nitrite and orthophosphate were determined by colorimetry with a precision of <1% (Skalar Analytical BV 2004) and ammonium by ο-phthaldialdehyde fluorescence (Holmes et al. 1999) with a precision of 4%. Viable counts of chemoorganotrophic bacteria were obtained in 100-μL aliquots which were serially diluted in phosphate-buffered saline solution (PBS, Sigma Aldrich, UK) and 100 μL of each diluted solution spread on half-strength Luria–Bertani agar (Merck, Germany) and incubated at 30°C for 2 days. Colonies were enumerated as colony-forming units (cfu) mL−1 of the incubated sample.
Results and discussion
Evaluation of the incubation protocol
The viable chemoorganotrophic bacterial count determinations at days 0 and 21 (0 cfu mL−1) confirmed that the substrate-only abiotic controls remained sterile during the incubations. Incubations with bacterial inocula added gave counts in the range 103–105 cfu mL−1 at the beginning and end of the incubations, except for one experiment (Table 1). These counts are within the ranges reported for free-living bacteria in UK rivers (Ainsworth and Goulder 1998) and an inter-calibration exercise of OECD 309 involving 7 laboratories (OECD 2004).
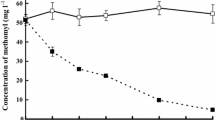
Nitrate was efficiently removed by the anion-exchange column (to <15 μM, Fig. 1), restricting it as an alternative source of N for the bacteria. The low concentrations of ammonium in the modified river water (≤4 μM, Fig. 1) confirmed that little was generated during the UV-irradiation step. The modified river water used in the incubation experiments gave a background signal that enabled direct analyses of samples by ESI-MS. Ion counts were generally low relative to those from atrazine and the peptides. The background ion count of the prepared river water was consistent with a low DOC concentration (<60 μM) following UV-irradiation, which is below the maximum background DOC concentration (83 μM) recommended for studies of this type (OECD 2004).
Concentrations (μM) of total dissolved nitrogen, the substrate added (i.e. atrazine or peptides A or B), ammonium, nitrate + nitrite and orthophosphate in solution at days 0 and 21. (a) peptide A, (b) peptide B, (c) atrazine (Tamar), (d) atrazine (Mersey). The relatively high background concentration of ammonium for day 0 in (b) was due to the compound preparation and not the river water used. Error bars represent ±1σ of the results from the duplicate incubations with each sample analysed 3–5 times (i.e. n = 6–10)
Incubations with peptides
Peptide A, an easily utilised substrate used to validate the experimental protocol, was added at a concentration of ca. 30 μM on day 0. By day 21, 95% had been removed in the biotic incubation (Fig. 1a, Table 2), as indicated by a marked decrease in the counts of the parent ion (m/z 352.1) in the ESI-MS chromatograms. The absence of transformation product ions in these chromatograms suggests that the tripeptide was assimilated, consistent with the ability of bacteria to directly absorb peptides containing up to six amino acid residues (Bronk 2002). The concentration of ammonium increased by two orders of magnitude in the biotic incubation, reflecting ammonification and release of ca. 30% of peptide N, while the mean TDN concentration decreased by ca. 30% (Fig. 1a). Viable bacterial counts increased from 1.5 × 104 to 4.8 × 105 cfu mL−1 (Table 1), indicating that peptide A had been utilised for the growth of a bacterial community. Peptide A was also completely removed from solution in bacterial incubations using water and bacteria from the River Mersey. The removal of peptide A confirmed that the bacterial inoculum was able to utilise substrates introduced into the water sample.
Peptide B, which was too large to be directly taken up by bacteria, was added to assess the ability of the bacterial population to externally hydrolyse substrates. Its concentration decreased from 13.0 ± 1.6 μM to 1.0 ± 0.2 μM between days 0 and 21 in the biotic incubation, a 92% decrease (Fig. 1b, Table 2); this was accompanied by an increase in the ammonium concentration (from 31.6 ± 0.3 μM to 140 ± 3 μM). In contrast to peptide A, there was a marked increase in TDN, from 138 ± 6 μM at day 0 to 305 ± 6 μM at day 21, as shown in Fig. 1b. This suggests that the bacteria released cellular organic nitrogen, as well as ammonium, in response to the peptide B addition.
Incubations with atrazine
In the River Tamar water, atrazine concentrations in the abiotic control at day 21 (25.1 ± 2.1 μM, t-test, P = 0.21, n = 10) were not significantly different from those measured at day 0 as shown in Fig. 1c and Table 2). In the biotic incubation, the concentration of atrazine had decreased by 11% at day 21 (Table 2). This change was statistically significant (P = 0.032, n = 10). When peptide A was added as a co-substrate, the total removal value remained at 11%. Cell counts were low at the start of the experiment, relative to the other incubations, but were of a similar order after 21 days (Table 1). In the River Mersey water, no removal was measured in the abiotic or biotic incubations. (Fig. 1d, Table 2). However, with peptide A present as a co-substrate, the concentration of atrazine had decreased by 37% after 21 days. These are the first reported observations of atrazine removal from low-turbidity freshwaters.
Atrazine contains two secondary amine groups. Microbial alteration of these functional groups in aerobic systems has been shown to occur via dealkylation of the N groups to form primary amines, as has dechlorination to yield 2-hydroxy derivatives (Graymore et al. 2001). Atrazine was removed from the biotic incubations in our study but not systematically, as changing the water source with its associated bacterial community altered the extent of removal. The addition of peptide A to the Mersey samples appears to have had a ‘priming effect’, resulting in 37% removal of atrazine. The priming effect occurs when inputs of labile organic matter tend to modify, and more generally increase, the mineralisation rate of the more recalcitrant organic matter (Guenet et al. 2010). It was first identified in soils but has since been reported for aquatic systems. For example, the biodegradation of phenols by a natural microbial lacustrine community was shown to be stimulated by addition of amino acids (Shimp and Pfaender 1985). A priming effect was not observed for the Tamar incubations of atrazine, so it would appear that stimulation of xenobiotic degradation by the addition of a readily utilisable source of carbon and nitrogen is dependent on the composition of the resident bacterial community and, particularly, the presence of species that can respond in this way. Interestingly, although addition of co-substrates is not compatible with some databases for predicting microbial catabolism of organic compounds (e.g. University of Minnesota Biocatalysis/Biodegradation Database, Gao et al. 2010), it creates experimental conditions that are more representative of low-turbidity aquatic systems (i.e. presence of natural dissolved organic matter). Furthermore, we were able to incorporate this approach into our method, while retaining the ability to identify xenobiotic biotransformation products by mass spectrometry.
The loss of atrazine did not result in any identifiable and specific biotransformation products, including those reported previously, when analysed by ESI-MS (Fig. 2). Strong et al. (2002) detected the atrazine metabolites hydroxyatrazine, N-isopropylammelide and cyanuric acid, via ESI-MS, following incubation of atrazine with the bacterium Arthrobacter aurescens TC1.). Elsewhere, in a microcosm containing river water and sediment, atrazine was degraded by sediment-bound bacteria to cyanuric acid, presumably via de-chlorination and subsequent hydrolysis to give 2-hydroxyatrazine, followed by removal of the alkyl substituents on the triazine ring (Satsuma et al. 2002; Satsuma 2006). The cyanuric acid was then mineralised to CO2. Degradation occurred within a few weeks, although the rate and extent depended upon the type of sediment and the season of collection. In contrast, Smalling and Aelion (2006) reported limited microbial production of deethylatrazine from atrazine via de-alkylation in slurries containing either pond or tidal creek sediments. Microbial de-alkylation of atrazine has also been reported for Mississippi river waters (Pereira and Rostad 1990). In addition, atrazine can sorb to several marine fungi and was shown to be bio-accumulated by the marine fungus Periconia prolifica (Schocken and Speedie 1982a,b; 1984). P. prolifica was also able to transform the parent compound to deisopropylatrazine and hydroxyatrazine (Schocken and Speedie 1984).
Ion chromatograms of atrazine in samples inoculated with bacteria and containing peptide A. (a) Tamar and (b) Mersey at days 0 and 21. Each m/z ion is a singly charged adduct ([M + H]+). The m/z 218.2 peak reflects the heavier 37Cl isotope. An ESI-MS calibration standard is included for comparison. Biotransformation products reported in the literature (Graymore et al. 2001; Strong et al. 2002) would have m/z values of 130.2, 146.2, 156.2, 170.2, 171.2, 174.2 and 188.2 (single charge adduct, 35Cl isotope)
In our experiments, the abiotic controls confirmed that atrazine did not hydrolyse or sorb to the walls of the incubation bottle, at least on these timescales (Table 2). Sorption of atrazine to microbes, namely marine fungi, has been reported, however (Schocken and Speedie 1982a, b), and may also be a plausible removal mechanism for bacteria (Geller 1979; 1980). Alternatively, the absence of detectable transformation products in our samples may be because this relatively low concentration of atrazine is being attacked by an aerobic community of microorganisms, such that intermediates are quickly further metabolised and thus do not accumulate to detectable levels. This may be analogous to observations on the composting of pentachlorophenol by heterogenous aerobic microbial communities in which mineralisation occurs without the accumulation of detectable levels of well-known intermediates (Jaspers et al. 2002).
Conclusions
In this study, we have made the first measurements of atrazine removal in low-turbidity freshwater systems at realistic concentrations of both substrate and bacteria. Its removal, as the sole substrate added, was higher in the River Tamar incubation, from a low-population, rural catchment, compared with the River Mersey, a high population, urban catchment. However, addition of a peptide co-substrate increased atrazine removal in the River Mersey incubation from 0 to 37%, but did not increase the removal rate in the River Tamar incubation. This suggests that, under these conditions, atrazine removal is more dependent on the characteristics of the indigenous bacterial population than on the structure of atrazine, contradicting previous assertions that removal is primarily dependent on chemical structure. As we did not observe any previously reported or predicted biotransformation products, it was not possible to deduce the specific removal mechanisms.
The incubation protocol developed for this study provides a controlled system in which to study the aquatic biotransformation of atrazine, or indeed many other anthropogenic dissolved organic nitrogen compounds, including pharmaceuticals. The major variable is the bacterial inoculum chosen, which in itself allows the significance of bacterial communities from different environments (e.g. pristine vs. impacted) to be investigated. Further advantages are that ESI-MS analyses can be undertaken with minimum sample manipulation and that the data show good precision for a range of chemical and biological measurements. The protocol was easily manipulated to include incubations with a co-substrate, and time-step sampling for rate constant determinations can be incorporated. In addition, the concentrations of atrazine to be tested could be reduced, commensurate with variations in measured in situ concentrations, analytical capabilities and the potential identification and quantification of transformation products.
References
Ackerman F (2007) The economics of atrazine. Int J Occup Env Heal 13:441–449
Ainsworth AM, Goulder R (1998) Microbial organic-nitrogen transformations along the Swale-Ouse river system, Northern England. Sci Total Environ 210(211):329–355
Badr ESA, Achterberg EP, Tappin AD, Hill SJ, Braungardt CB (2003) Determination of dissolved organic nitrogen in natural waters using high-temperature catalytic oxidation. TRAC Trends Anal Chem 22:819–827
Bronk DA (2002) Dynamics of DON. In: Hansell DA, Carlson CA (eds) Biogeochemistry of marine dissolved organic matter. Academic Press, London, pp 153–247
Gao J, Ellis LBM, Wackett LP (2010) The University of Minnesota biocatalysis/biodegradation database: improving public access. Nucleic Acids Res 38:D488–D491
Geller A (1979) Sorption and desorption of atrazine by three bacterial species isolated from aquatic systems. Arch Environ Contam Toxicol 8:713–720
Geller A (1980) Studies on the degradation of atrazine by bacterial communities enriched from various biotopes. Arch Environ Contam Toxicol 9:289–305
Graymore M, Stagnitti F, Allinson G (2001) Impacts of atrazine in aquatic systems. Environ Intern 26:483–495
Guenet B, Danger M, Abbadie L, Lacroix G (2010) Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91:2850–2861
Holmes RM, Aminot A, Kérouel R, Hooker BA, Peterson BJ (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56:1801–1808
Ingerslev F, Nyholm N (2000) Shake-flask test for determination of biodegradation rates of 14C-labelled chemicals at low concentrations in surface water systems. Ecotoxicol Environ Safety 45:274–283
Isidori M, Lavorgna M, Nardelli A, Parrella A, Previtera L, Rubino M (2005) Ecotoxicity of naproxen and its phototransformation products. Sci Total Environ 348:93–101
Jaspers CJ, Ewbank G, McCarthy AJ, Penninckx MJ (2002) Successive rapid reductive dehalogenation and mineralization of pentachlorophenol by the indigenous microflora of farmyard manure compost. J Appl Microbiol 92:127–133
Jaworska JS, Boethling RS, Howard PH (2003) Recent developments in broadly applicable structure-biodegradability relationships. Environ Toxicol Chem 22:1710–1723
Kosjek T, Heath E (2008) Applications of mass spectrometry to identifying pharmaceutical transformation products in water treatment. TRAC Trends Anal Chem 27:807–820
Kosjek T, Heath E, Petrovic M, Barceló D (2007) Mass spectrometry for identifying pharmaceutical biotransformation products in the environment. TRAC Trends Anal Chem 26:1076–1085
McGuire KJ, McDonnell JJ (2006) A review of catchment transit time modeling. J Hydrol 330:543–563
OECD (2004) OECD Guideline for the testing of chemicals. Aerobic mineralisation in surface water—simulation biodegradation test
Pereira WE, Rostad CE (1990) Occurrence, distribution and transport of herbicides and their degradation products in the lower Mississippi River and its tributaries. Environ Sci Technol 24:1400–1406
Pérez S, Barceló D (2007) Application of advanced MS techniques to analysis and identification of human and microbial metabolites of pharmaceuticals in the aquatic environment. TRAC Trends Anal Chem 26:494–514
Satsuma K (2006) Characterisation of new strains of atrazine degrading Nocardioides sp. isolated from Japanese riverbed sediment using naturally derived river ecosystem. Pest Manag Sci 62:340–349
Satsuma K, Tanaka H, Sato K, Kato Y (2002) Role and behaviour of benthic microbes able to degrade herbicide atrazine in naturally derived water/sediment microcosm. Microbes and Environments 17:179–184
Schocken MJ, Speedie MK (1982a) Interaction of higher marine fungi with the herbicide atrazine. 2. Sorption of atrazine to 4 species of marine fungi. Bull Environ Contam Toxicol 29:101–106
Schocken MJ, Speedie MK (1982b) Interaction of higher marine fungi with the herbicide atrazine. 3. Adsorption of atrazine to the marine fungus Dendryphiella salina. Chemosphere 11:885–890
Schocken MJ, Speedie MK (1984) Physiological aspects of atrazine degradation by higher marine fungi. Arch Environ Contam Toxicol 13:707–714
Shapir N, Mongodin EF, Sadowsky MJ, Daugherty SC, Nelson KE, Wackett LP (2007) Evolution of catabolic pathways: genomic insights into microbial s-triazine metabolism. J Bacteriol 189:674–682
Shimp R, Pfaender FK (1985) Influence of naturally occurring humic acids on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol 49:402–407
Skalar Analytical BV (2004) Skalar SanPlus analyzer general information. PO Box 3237 4800 DE, Breda, The Netherlands
Smalling KL, Aelion MC (2006) Biological and chemical transformation of atrazine in coastal aquatic sediments. Chemosphere 62:188–196
Solomon KR (2009) Is atrazine a potent endocrine disrupting chemical? Environ Sci Technol 43:2993
Solomon KR, Baker DB, Richards P, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
Strong L, Rosendahl C, Johnson G, Sadowsky MJ, Wackett LP (2002) Arthrobacter aurescens TC1 metabilizes diverse s-triazine ring compounds. App Environ Microb 68:5973–5980
USEPA (2008) Fate, transport and transformation test guidelines. OPPTS 835.3190. Aerobic mineralization in surface water—simulation biodegradation test
von der Ohe PC, Dulio V, Slobodnick J, de Deckere E, Kühne R, Ebert RU, Ginebreda A, de Cooman W, Schüürmann G, Brack W (2011) A new risk assessment approach for the prioritization of 500 classical and emerging organic microcontaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci Total Environ 409:2064–2077
Acknowledgments
This work was funded by the UK Natural Environment Research Council (grant NE/E006302/1). We thank Dr Clare Redshaw for advice on the use of the Finnegan MAT LCQ mass spectrometer and Dr Claire Williams for the nitrate + nitrite and orthophosphate analyses. The incubations were undertaken in an ISO 9001:2001 accredited laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tappin, A.D., Loughnane, J.P., McCarthy, A.J. et al. Removal of atrazine from river waters by indigenous microorganisms. Environ Chem Lett 10, 89–96 (2012). https://doi.org/10.1007/s10311-011-0332-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-011-0332-4