Abstract
Here we evidenced the photo-induced degradation of monolinuron, a phenylurea herbicide, through the 300–450 nm light excitation of nitrite and nitrate species. The degradation pathways were compared to those obtained under direct photolysis at 254 nm. When using NO3 − and NO2 − as photoinducers, hydroxyphenyl-substituted photodegradation products were found to be formed specifically through the involvement of OH° radicals. NO− and NO2-phenyl substituted compounds were also observed as a result of the production of NO° and NO2° radicals. Half-lives of monolinuron in aqueous solutions were measured in various conditions of concentrations of substrate and inducer, oxygen content and pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The massive and prolonged use of pesticides in agriculture explains their frequent occurrence in natural waters. Phenylureas are herbicides commonly applied for weed control. They inhibit photosynthesis. However, they may also damage non-target aquatic primary producers such as phytoplankton, periphyton and macrophytes, and, in turn, alter the ecosystem equilibrium. The consequences of this contamination depend on the sensitivity of the communities and on the potential eco-toxicity and bioavailability of the herbicide and its degradation products. In aquatic ecosystems, molecular transformations result from biotic or abiotic processes. Some of the phenylurea by-products are known to show phytotoxicity equivalent to or even higher than that of the initial pesticide (Tixier et al. 2000; Malato et al. 2003). Here we investigated the role of some environmental parameters involved in the photodegradation of monolinuron taken as a model.
Photodegradation can result from a direct process by which the pesticide absorbs photons and then undergoes degradation. A molecule can also be excited first by light energy and then transfer either energy (sensitization) or an electron (or a proton) to the pesticide. Photodegradation can also involve induced processes. A series of reactions may lead to the formation of active species such as singlet oxygen or a hydroxyl radical. In natural waters, after excitation by solar ultraviolet (UV) light, nitrate and nitrite ions are sources of hydroxyl radicals (Eqs. 1 and 2). Although less abundant than NO3 − ions, NO2 − ions are competitive reagents due to their more advantageous solar light absorption and higher quantum yields: NO2 −: λmax 352 nm, ε 22 L mol−1 cm−1, Φ 0.015–0.07 vs NO3 −: λmax 302 nm, ε 7 L mol−1 cm−1, Φ 0.009–0.017. By excitation of HNO2 form (pKa 3.37; Eq. 3) the quantum yield becomes even higher, about 0.45 (Meunier and Boule 2000).
Direct and induced photolyses of substituted phenylureas were reviewed by Boule et al. (2002) and Burrows et al. (2002) and reported to give as main degradation pathways: (i) photohydrolysis (ii) demethylation and/or demethoxylation of the substituted N -terminus, (iii) possible rearrangement and iv) formation of benzoquinone and analogues. Ring hydroxylation occurs in general under induced (radicalar) conditions and oligomerisation may be observed at high substrate concentration. The influence of nitrate and nitrite ions on the photodegradation of phenylureas is poorly documented. The specific contribution of NO3 − and NO2 − ions will thus be studied herein and compared to that of direct photolysis.
Experimental
Chemicals
Monolinuron (1, 3-(4-chlorophenyl)-1-methoxy,-1-methylurea, 97.1%) was purchased from Cluzeau-Info-Labo, France. Aqueous solutions for photolytic studies were prepared by sonication-activated dissolution in LC-grade water (LC: liquid chromatography), filtration through a 0.45-µm membrane, and concentrations were determined by LC, using methanol solutions as references.
Irradiation
For direct photolysis, 60 mL monolinuron 1 solutions in LC-grade water were irradiated at 254 nm in a 2-cm i.d. quartz tube parallel to a monochromatic low-pressure mercury lamp (germicide TUV 15 W, Philips, incident photon flow evaluated as 2.0×1014 photons cm−2 s−1), under magnetic stirring. Irradiations at 270, 290 and 300 nm were applied using a Schoeffel monochromator equipped with a xenon lamp (1,600 W). The incident light intensity was in the range of 1.1×1014–3.8x1014 photons cm−2 s−1. For polychromatic irradiations at 300–450 nm (induced photolysis), a pyrex tube (3 cm i.d., 295 mL solution) was used as reactor and located along the axis of a cylindrical device equipped with six actinic lamps (TLD 15 W, Philips). The incident light intensity was evaluated at about 1.0×1016 photons cm−2 s−1. Nitrogen, oxygen or air bubbling (150 mL min−1) was carried out, agitating the solution, and temperature was maintained below 30 °C by ventilation. Kinetics were measured by regular sampling and LC-UV direct analysis or on-line solid phase extraction-LC-UV. Standard concentrations of 1 were 10 mg L−1 (or 1 mg L−1, inducer effect studies). Quantum yields were determined using a low-pressure mercury or xenon lamp with monochromatic filter, the irradiation being evaluated by ferrioxalate actinometry.
Analytical determinations
Analysis was performed using an LC-UV-photodiode array equipment (Waters) by gradient reversed phase chromatography (Kromasil C18 250×4.6 mm column, water/acetonitrile mobile phase). For low initial concentrations, a large injection volume (2 mL) was directed to a C18 (10×4.6 mm) precolumn. Quantifications were carried out at 242 nm with external standards: monolinuron 1 and 6. The other compounds were evaluated on the basis of the monolinuron UV-response. Identifications were performed by LC-ESI-MS/MS with a triple quadrupole instrument (Quattro LC Micromass) in positive and negative modes (ESI: electrospray ionisation, MS: mass spectrometry). Collisional activated dissociation was obtained by argon (2.5×10−3 torr) with 15 eV collision energy.
Results and discussion
Direct photodegradation
Quantum yields and kinetics
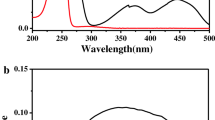
The ultraviolet (UV) spectrum of monolinuron exhibits a maximum at 242 nm (ε 19,000 L mol−1 cm−1), the absorption being also quite high at 254 nm (ε 10,950 L mol−1 cm−1). In contrast, UV absorption is weak at wavelengths above 300 nm (ε <200 L mol−1 cm−1). The quantum yield of the direct photodegradation of monolinuron was evaluated as 0.13±0.01 at 254 nm but was found to progressively decrease by increasing excitation wavelength (0.11, 0.06 and 0.03, all ±0.01) at 270, 290 and 300 nm, respectively. This excitation wavelength dependence has also been reported for chlorophenylureas like diuron (Jirkovsky et al. 1997) and linuron (Faure and Boule 1997), and tentatively explained as resulting from the “keto-enolic” equilibrium of the urea function.
Using irradiation at 254 nm, photodegradation of monolinuron (1) was fast, giving a total disappearance of the pesticide within 3–10 min. In a first approach, kinetics may be assumed to follow a first-order (with R2 >0.99). Pseudo-rate constants were respectively determined as 10.3×10−3, 32×10−3 and 34×10−3 s−1 at 10, 0.5 and 0.2 mg L−1 of substrate. The low rate constant observed for the higher concentration could be explained by an inner filter effect due to the by-products (Absorbance254 nm = 1). Inversely, the reaction was significantly accelerated when the solution was continuously purged with nitrogen which indicated that the photoreaction was inhibited by oxygen (14.2×10−3 s−1 at 10 mg L−1). The deactivation of the triplet excited state of monolinuron by oxygen was most likely the reason for this effect. When irradiated at 300–450 nm, monolinuron reacted very slowly as expected, with only 1–2% degradation after 48 h.
Reaction products
The direct photolysis of monolinuron proceeds via two main pathways (Scheme 1 and Fig. 1) yielding, respectively, 3-(4-chlorophenyl)-1-methylurea (2), which results from demethoxylation of the N-terminus substituted functional group, and 3-(4-hydroxyphenyl)-1-methoxy-1-methylurea (6) obtained by photohydrolysis of the C-Cl bond. Compound 5 formed by N-demethylation of 2 and compound 7 which combines N-demethoxylation and the Cl/OH substitution are second generation products. Most surprising is the occurrence of the carbinolamine 3. Such compound, probably unstable, has sometimes been suggested as an intermediate of N-dealkylation during photolysis of phenylureas, but has never been detected. Since it was not observed from monuron (3-(4-chlorophenyl)-1-dimethylurea) under the same conditions, the carbinolamine intermediate is assumed to be stabilized in a specific way when starting from a N-methoxy-methyl phenylurea. Interestingly, the same compound has been identified under oxidative reaction conditions (Amir Tahmasseb et al. 2002). Two rearrangement products 9 and 10 were also observed. Photo-Fries compounds like 9 have rarely been reported from phenylureas (Tixier et al. 2000; Aguer and Richard 1996). The formation of 10 presumably by radical cleavage of the N-OCH3 bond and recombination onto the phenyl ring is uncommon.
1,4-benzoquinone 8 appearing exclusively in the presence of oxygen (aerated solutions) is probably produced through a carbene intermediate as has been suggested previously (Boule et al. 2002). As shown by their evolution curves (Fig. 1), most of these photo-products are photodegraded by excitation at 254 nm in parallel with the parent pesticide, whereas 7 tends to accumulate.
Photodegradation induced by nitrites and nitrates
Effect of parameters
Under 300–450 nm irradiation in the pyrex reactor, the direct photodegradation of monolinuron was slow. Its transformation was enhanced, however, by adding nitrite or nitrate ions. This effect is due to the involvement of efficient radical species (Eqs. 1 and 2). The degradation efficiencies were compared by measuring monolinuron half-lives (Table 1).
Oxygen concentration (using N2, air or O2 bubbling) in water had only a small accelerating effect on the induced phototransformation both by nitrites and nitrates. Observed half-lives were of approximately 12–14 h for nitrites at 10−3 mol L−1 and ranged between 21 and 25 h when using nitrates at 5×10−3 mol L−1. However, the degradation rate changed more significantly with the initial concentration of the substrate. In the presence of 5×10−3 mol L−1 NO3 −, half-lives shifted from 22 to 3.2 h when the monolinuron concentration was decreased from 10 to 0.1 mg L−1. With NO2 − at 10−3 mol L−1 initial concentration, the effect was much less pronounced (t1/2 from 14.5 to 12.5 h).
As expected from the NO2 −/HNO2 relative quantum yields, lowering the pH accelerated the photodegradation induced by nitrites: half-lives of 14.5 and 2.7 h were obtained at pH 7 and pH 4, respectively. Finally, the influence of the inducer concentration on kinetics was examined. For nitrites, half-lives were not significantly different at “high” concentrations (t1/2 13.4 h and 12.4 h for 10−3 and 10−4 mol L−1 NO2 −, respectively), but increased when the concentration was lowered again (t1/2 18.3 h for 10−5 mol L−1 NO2 −). In contrast, the decrease in kinetics was progressive when the nitrate concentration was decreased in the studied range. This effect is consistent with the evolution of the OH°/ substrate ratio. Indeed, the stationary concentration of hydroxyl radicals increases with the increase in nitrate concentration, thus accelerating the degradation of the substrate.
Reaction products
In the presence of nitrates (and nitrites at pH 4), the main photoproducts (Scheme 1) include 11 and 12 (major) resulting from hydroxylation of the phenyl ring but absent in direct photolysis which clearly indicates the involvement of hydroxyl radicals. Substituted N-terminus degradation compounds (2-5) and the Cl/OH substituted product 6 are also observed. The photo-Fries rearranged compound 9 is detected in the presence of NO2 − ions at pH 4. All these photoproducts, assumed to originate from OH° radicalar attack, were previously observed under other OH° generating processes (AOP) such as O3/H2O2 or Fenton’s reaction (Amir Tahmasseb et. al 2002).
A number of new compounds resulting from the addition of NO° or NO2° onto the phenyl ring (13-15) are also produced. NO° is generated according to Eq. (2), and NO2° either through propagation of the radicalar reaction (Eq. 4) or via the further formation of nitrates in the presence of oxygen (Eq. 5 and then Eq. 1) (Halmann 1996).
Under the NO2 − photoinduced reaction and relatively diluted solutions (1 mg L−1 monolinuron, 10−4 mol L−1 NO2 −, pH 7), a 80% degradation was reached after 32 h giving a sum of by-products of 10% evaluated by LC-UV. The major detected compounds (comprising mainly 3,5,6 and 14) led to maximum yields after 8 h and remained thereafter at constant concentrations. With NO3 − (similar conditions) the photoproducts were found to accumulate constantly within 52 h (mostly 2, 3 and 12, reaching a total of ca. 4% at 35% degradation of 1).
Structural determinations
The photo-products were characterized by mass spectrometry and in some cases compared to standards by LC-UV (benzoquinone). Low collision energy LC-MS-MS was used extensively for full assignment of the original structures. Briefly, the positive mode (CAD of the MH+ ion) was effective in identifying the NHR-phenylurea type compounds 2 and 5 giving an anilinium ion at m/z 128 (Cl-C6H4NH3 +). Instead, a substituted phenyl cation of m/z 108 (analogous of m/z 126 for monolinuron) was obtained for dechlorinated 6.
Carbinolamine 3 was identified by studying the CAD of the [M – H]− anion at m/z 199 which eliminates primarily HCHO (m/z 169). The rearrangement products were also characterized in the negative mode starting from the deprotonated molecules at m/z 213 (yielding m/z 181, 152 and 126 for 9; m/z 156 and 141 for 10). The isomeric hydroxylated compounds are clearly differentiated using the CAD of the [M – H]− anion m/z 229 leading predominantly to the fragment ion m/z 141 by loss of the radical H3C(OCH3)NCO° (11) or to ion m/z 168 produced after anchimeric elimination of HN(OCH3)CH3 and thus specific to the ortho form (12). The major NO2-substituted photo-product 14 was assigned through the CAD of MH+ yielding fragments at m/z 60, 62, 68 also formed for monolinuron (showing the conservation of the urea functionality) and ions at m/z 214 (loss of NO2°) and m/z 156 (ClC6H3NO2 +).
Conclusion
Nitrite or nitrate induced-photolysis of monolinuron using the 300–450 nm light excitation gives rise to a variety of photo-products. The N-demethoxylated (NHCH3) and N-demethyldemethoxylated (NH2) phenylureas as well as the uncommon carbinolamine intermediate (NHCH2OH) are also produced under direct photolysis at 254 nm. However, the OH-phenyl substituted compounds (predominantly ortho) are specifically produced via OH° radicalar processes occurring at 300–450 nm in the presence of NO2 − and NO3 − ions. NO- and NO2-phenyl substituted ureas, reported for the first time from phenylureas, result similarly from NO° and NO2° attack on the phenyl ring. The inducer concentration, oxygen content and pH have significant effects on the photodegradation rates (cf. half-lives) indicating the importance of these parameters in the frame of risk assessment. The role of dissolved organic matter should not be excluded in certain circumstances (OH° scavenging effect).
References
Aguer JP, Richard C (1996) Transformation of fenuron induced by photochemical excitation of humic acids. Pestic Sci 46:151–155
Amir Tahmasseb L, Nélieu S, Kerhoas L, Einhorn J (2002) Ozonation of chlorophenylurea pesticides in water: reaction monitoring and degradation pathways. Sci Tot Environ 291:33–44
Boule P, Meunier L, Bonnemoy F, Boulkamh A, Zertal A, Lavedrine B (2002) Direct phototransformation of aromatic pesticides in aqueous solution. Int J Photoenergy 4:69–78
Burrows HD, Canle ML, Santabella JA, Steenken S (2002) Reaction pathways and mechanisms of photodegradation of pesticides. J Photochem Photobiol B: Biol 67:71–108
Faure V, Boule P (1997) Phototransformation of linuron and chlorbromuron in aqueous solution. Pestic Sci 51:413–418
Halmann MM (1996) Photodegradation of water pollutants, CRC Press, Boca Raton
Jirkovsky J, Faure V, Boule P (1997) Photolysis of diuron. Pestic Sci 50:42–52
Malato S, Caceres J, Fernandez-Alba AR, Piedra L, Hernando MD, Agüera A, Vial J (2003) Photocatalytic treatment of diuron by solar photocatalysis: evaluation of main intermediates and toxicity. Environ Sci Technol 37:2516–2524
Meunier L, Boule P (2000) Direct and induced degradation of mecoprop [2-(4-chloro-2-methylphenoxy)-propionic acid] in aqueous solution. Pest Manage Sci 56:1077–1085
Murov SL, Carmichael I, Hug GL (1993) Handbook of photochemistry, 2nd edn. Marcel Dekker, New York, 293 pp
Tixier C, Bogaerts P, Sancelme M, Bonnemoy F, Twagilimana L, Cuer A, Bohatier J, Veschambre H (2000) Fungal degradation of a phenylurea herbicide, diuron: structure and toxicity of metabolites. Pest Manage Sci 56:455–462
Acknowledgements
Dr. J.P. Aguer from the University Clermont-Ferrand, France, is acknowledged for fruitful discussions. The Région Ile de France is acknowledged for SESAME financial support CLC-MS equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nélieu, S., Kerhoas, L., Sarakha, M. et al. Nitrite and nitrate induced photodegradation of monolinuron in aqueous solution. Environ Chem Lett 2, 83–87 (2004). https://doi.org/10.1007/s10311-004-0066-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-004-0066-7