Abstract
The photoconversion of 2-chloronaphthalene (CN-2) in water in a simulated sunlight system was investigated. The photoconversion efficiency, photoproducts and mechanisms were inspected, and the effects of inorganic ions (NO3 −, NO2 −) and fulvic acid (FA) were discussed. The results showed that CN-2 could be transformed in water under the irradiation. NO3 − and NO2 − promoted the photoconversion of CN-2 owing to ·OH generated by the photolysis of NO3 − and NO2 −; FA at a lower concentration promoted the photoconversion, but it had an inhibition effect at a higher concentration. It was demonstrated that the acidic conditions promoted the photoconversion of CN-2 by the active groups such as superoxide radical anion, hydrogen peroxide and hydroxyl radical produced in the system. Eight photoproducts of CN-2 were characterized by the GC-MS method and the possible photoconversion mechanisms were proposed accordingly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polychlorinated naphthalenes (PCNs), which have similar structure and toxicity to those of polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), are ubiquitous persistent organic pollutants (POPs) in the environment. Owing to their toxic equivalents (TEQs) comparable to or even higher than those of PCDDs and PCDFs (Li et al. 2016), PCNs have been nominated by the European Union (EU) in 2011 to be listed in the Stockholm Convention on Persistent Organic Pollutants (POPs) for the global control (Huang et al. 2015). Although the production and use of PCNs have been banned since 1970s, the historical residue is still the main source of PCNs in many countries because of their high physicochemical stability (Xu et al. 2015a, b; Barc and Gregoraszczuk 2014). Additionally, other important sources of PCNs are impurities in commercial polychlorinated biphenyl mixtures and other chemicals, and unintentional formation and emission as byproducts from industrial thermal and incomplete combustion processes such as waste incineration and metal refining (Jiang et al. 2015; Die et al. 2016; Liu et al. 2015). PCNs have been detected in various environmental media worldwide, even including remote polar areas because of their long range atmospheric transport potential (Li et al. 2016; Moukas et al. 2016). PCNs pose a great threat to human health and ecosystems due to the stronger toxicity and the higher bioaccumulation and biomagnification (Fromme et al. 2015; Zhang et al. 2014).
Although PCNs are easily adsorbed onto the sediments because of the hydrophobicity and are thus found in many lake surface deposits (Cetin 2016), they are also detected in many natural waters (Ali et al. 2016), therefore, the transformation of PCNs in waters should be taken seriously. Because PCNs can absorb the sunlight with wavelengths of more than 300 nm, the photoconversion is an important way for the transformation of PCNs in water (Ruzo et al. 1975). However, the existing studies on the photoconversion of PCNs are all performed in organic solutions. Jarnberhg et al. (1999) deduced that the photolysis mechanisms of PCNs in methyl alcohol involved dechlorination and dimerization. Keum and Li (2004) found that 8-chloronaphthalene was completely degraded into several low-chlorinated PCNs in hexane solution after irradiation for 9 h. Up to the present, the research on the photoconversion of PCNs in water has not been reported yet.
As one of the components of PCNs, 2-Chloronaphthalene (CN-2) is mainly used as a chemical-resistant gauge fluid, a heat-exchange fluid, engine crank-case additives to dissolve sludge and gum, and an ingredient in motor tune-up compounds (Sinha and Bose 2006). Improper disposal of wastes containing CN-2 can lead to the contamination of ground and surface waters (Xu et al. 2015a, b). CN-2 was selected as a typical component of PCNs because of the better solubility in water and the simplicity of the structure, and its photoconversion in water was studied in this paper. The results should contribute to an in-depth understanding of the environmental behaviors of PCNs.
Materials and Methods
The photochemistry experiments were carried out in a self-made photoconversion device. An aqueous solution (0.1 mg L−1) of CN-2 was prepared and distributed into a series of glass tubes and then irradiated by an 80 W high-pressure mercury lamp. The tubes were set around the lamp to get the same quantity of photos. The wavelength of the light through the glass is more than 280 nm, therefore, the irradiation the samples obtained can be almost considered as the sunlight. The reaction temperature was kept at 25 ± 1°C. Two parallel samples were punctually taken for analysis during the irradiation at regular intervals. The mean values of the duplicate samples are reported.
The aqueous solution (5 mL) after the irradiation was transferred into an extraction flask containing 2 mL hexane. The mixture was shaken for 5 min, and then stand for 30 min. The concentration of CN-2 in the upper hexane phase was analyzed on a Shimadzu GC-2010 Plus (Shimadzu Corp., Japan) with an electron capture detector, equipped with a Rtx-5 capillary column (30 m × 0.25 mm × 0.25 μm). The initial column temperature was set at 180°C for 1 min and then increased to 200°C at a rate of 5°C min−1. The carrier gas was high-purity nitrogen (99.999%) with a flow rate of 1.0 mL min−1. The samples were analyzed by injecting 1 μLof each sample with the split ratio of 10:1. External standard and calibration curve method were used for the quantification. The detection limit of the analytical method was 0.0021 mg L−1, and the percent recoveries were in the range of 83%–95%.
A chemical probe method was used to determine the concentration of ·OH produced in the photoconversion process. The mixed solution containing 0.64 mmol L−1 benzoic acid (BA) and 5.76 mmol L−1 sodium benzoate (BA–Na) was added into the initial reaction solution to capture ·OH in the system. The concentration of ·OH in the sample was indirectly determined by analyzing the concentration of p-hydroxybenzoic acid (p-HBA) in it (Chu and Anastasio 2003; Lindsey and Tarr 2000). The concentration of p-HBA was analyzed on a LC-20A (Shimadzu Corp, Japan) equipped with a UV detector and a C18 chromatographic column(150 mm × 3.9 mm, 5 µm).The mobile phase was methanol and water (30:70, v/v) with a flow rate of 1.0 mL·min−1. The samples were analyzed by injecting 20 μL of each sample and the detection wavelength was 256 nm.
The photoproducts were analyzed on an Agilent 6890 N GC with a 5975 mass selective detector (Agilent Technologies Inc., US), equipped with a HP-1701 capillary column (60 m × 0.25 mm × 0.25 μm); the carrier gas was high-purity helium; the initial column temperature was set at 50°C for 3 min, and then increased to 260°C at a rate of 10°C min−1 and held for 10 min. An electron bombing ion source was used and the electron energy was 70 eV, the emission current was 0.35 mA, the electron multiplier voltage was 1000 V, the resolution rate was 2 M, and the mass spectra were collected in the scan range m/z 20–850.
In the experiment, all reagents were used without any purification and all solutions were prepared with deionized water.
Results and Discussions
The comparison of the light and dark reactions of CN-2 in water is presented in Fig. 1, and it shows that the concentration of CN-2 remained unchanged under the dark condition. Therefore, the decrease in the concentration of CN-2 was completely attributed to the direct photolysis. The photoconversion efficiency of CN-2 reached 45.4% after 12 h of irradiation. This is because the spectral range of the irradiation filtered through the glass tubes is λ > 280 nm, which overlapped largely with the UV absorption spectrum of CN-2; therefore, CN-2 was directly photolyzed under the experimental conditions. The photoconversion of CN-2 in water fits the first-order kinetic model, and it can be described by the kinetic equation: ln(c t /c 0 ) = −0.0557 × −0.0719; the apparent rate constant was 0.0557 h−1, the half-life was 12.44 h, and the correlation coefficient (R 2) was 0.9741.
The natural water contains numerous photo-active materials, such as NO3 −, NO2 − and humic acid. NO3 − and NO2 − can produce active groups such as ·OH and are considered as the main sources of ·OH in natural water (Jacobi et al. 2006). ·OH can easily react with organic pollutants to promote the degradation. Humic acid, as an important natural photosensitizer, can absorb visible light to generate the triplet excited state and a variety of reactive oxygen radicals, and thus induce indirect photochemical degradation of organic pollutants. Hence, the effects of NO3 −, NO2 −, as well as fulvic acid (FA), an important component of humic acid, were investigated here.
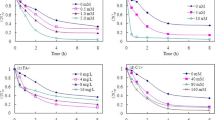
NO3 − can absorb sunlight with wavelengths of more than 290 nm, and its characteristic absorption peak occurs at 305 nm. In general, the content of NO3 − in natural water is between 5 and 50 mg L−1 (0.081–0.81 mmol L−1) (Russi et al. 1982). The effects of different concentrations of NO3 − on the photoconversion of CN-2 in water are summarized in Fig. 2. It clearly shows that NO3 − can significantly promote the photoconversion of CN-2, and with the increasing of the concentration of NO3 −, the promotion was strengthened. It is proposed that NO3 − could produce ·OH in the aqueous solution after irradiated as Eq. (1) (Jacobi et al. 2006).
To attest the production of ·OH and its effect on the conversion of CN-2, the concentration of ·OH in the reaction solution was determined by means of the chemical probe method; and the promoting effect of ·OH on the conversion of CN-2 was proved by using isopropanol to quench ·OH in the solution (Kormali et al. 2007). The results (Fig. 3) show that no ·OH was detected in the solution without NO3 −presence during the irradiation process, and the photoconversion of CN-2 was induced by direct photolysis under this condition. With the presence of NO3 −(0.2 mmol L−1), the content of ·OH gradually increased with the increasing of the irradiation time, indicating that NO3 − produced ·OH via irradiation, while the photoconversion rate of CN-2 also increased significantly. When 0.2 mmol L−1 isopropanol was added into the solution containing 0.2 mmol L−1 NO3 −, ·OH was completely quenched, the photoconversion efficiency of CN-2 decreased seriously and it was almost the same with that of the direct photolysis. This further proved that ·OH produced by NO3 − promoted the photoconversion of CN-2.
NO2 − can absorb the sunlight with wavelengths of more than 290 nm; the characteristic absorption peak occurs at 355 nm. Similar to NO3 −, NO2 − also had a promoting effect on the photoconversion of CN-2 (Fig. 4), which should be attributed to the ·OH generated by NO2 − as Eqs. (2 and 3) (Boxe et al. 2005).
Because the presence of NO2 − interfered with the determination of p-hydroxybenzoic acid, ·OH was failed to be accurately quantified in the solution. However, the photoconversion of CN-2 decreased after isopropanol was added, which also demonstrated the generation of ·OH by NO2 − as well as the promotion effect of ·OH on the photoconversion of CN-2 (Fig. 5).
FA is widely present in natural water, and its content is generally about 10 mg L−1 (Lou et al. 2017). It can be seen from Fig. 6 that the photoconversion of CN-2 was promoted both by 5 and 8 mg L−1 FA, while inhibited by 10 mg L−1 FA. The effects of FA on the phototransformation of organic pollutants are usually divided into two aspects. One is as a photosensitizer to promote the photolysis of the organic pollutants; the other is as an inhibitor to inhibit the photolysis owing to its light shielding effect (Zhan et al. 2006). In this experiment, the shielding effect of 5 mg L−1 FA was negligible and it only showed a promotion effect. As for 8 and 10 mg L−1 FA, the shielding effect couldn’t be neglected. If the promotion effect of FA (such as 8 mg L−1) was stronger than the shielding effect, it showed a positive effect. On the contrary, 10 mg L−1 FA showed its negative effect for its stronger shielding effect.
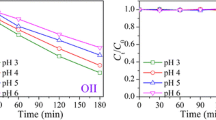
The pH value is an important factor influencing the photoconversion. The initial pH of 0.1 mg L −1 CN-2 is about 5.9. By adjusting the initial pH of the solution with 1.0 mol L−1 H2SO4 or NaOH aqueous solution, the effects of 5 pH levels were tested. Figure 7 clearly shows that the pH value greatly influenced the photoconversion of CN-2 in water. With the decreasing of the pH value from 5.9 to 2.0, the photoconversion of CN-2 increased significantly and the photoconversion efficiency of CN-2 reached 90% at pH 2.0. Nevertheless, alkaline conditions (pH 8.1 and 10.1) showed little effect on the photoconversion.
The results mean that the photoconversion of CN-2 under acidic and alkaline conditions proceeded by two different kinds of reaction mechanisms. It is reported that under irradiation, the aqueous solution is able to generate hydrated electrons which rapidly react with oxygen and generate superoxide radical anion (O2·−). Under acidic conditions, O2 −· can react with H+ to form hydrogen peroxide, the photolysis of which can produce strong oxidative intermediates such as ·OH as Eqs. (4, 5, 6) (Deng and Wu 2003).
To prove the roles of the active groups under the acidic conditions, benzoquinone, catalase and isopropanol were respectively selected as the scavengers of superoxide radical anion, hydrogen peroxide and hydroxyl radical. After scavengers added, the photoconversion efficiency of CN-2, which was 61.5% in water without the presence of the scavengers, was seriously inhibited under the condition of pH 4 (Fig. 8). Hence, such a conclusion can be drawn that superoxide radical, hydrogen peroxide and hydroxyl radical were produced in the photoconversion and showed positive effects on the photoconversion process.
Eight photoproducts were identified after CN-2 degraded in water by the GC-MS method. They are naphthalene (1), 1-chloronaphthalene (2), 1-naphthol (3), styrene (4), benzyl alcohol (5), benzaldehyde (6), 1-chlorobenzene (7) and 2-chlorophenol (8). The chromatogram of the photoproducts is given in Fig. 9. On the basis of characterizing the photoproducts, as well as analyzing the structure and properties of CN-2, the pathway of the photoconversion of CN-2 in water was proposed as Fig. 10. The photoconversion of CN-2 in water underwent the following steps.
The GC-MS chromatogram of the photoproducts of CN-2 after illuminated 12 h. The peaks in the chromatogram from left to right correspond to 1-chlorobenzene(4.020 min), styrene(4.572 min), benzaldehyde(6.539 min), 2-chlorophenol(7.197 min), benzyl alcohol(7.653 min), naphthalene(10.061 min), 1-naphthol(11.468 min), 1-chloronaphthalene(14.694 min)
-
1.
CN-2 generated naphthalene (1) and ·Cl through reductive dechlorination, and ·Cl shifted to the number 1 position of Naphthalene to form 1-chloronaphthalene (2). The chlorine group of 1-chloronaphthalene (2) was substituted by hydroxyl to produce 1-naphthol (3).
-
2.
1-naphthol (3) transformed to styrene (4) through a series of reactions such as oxidation reaction, ring-opening reaction and decarboxylic reaction.
-
3.
Styrene (4) changed to benzyl alcohol (5), and then to benzaldehyde (6) by oxidation. Benzaldehyde (6) changed into 1-chlorobenzene (7) and 2-chlorophenol (8) by further oxidation and the substitution of ·Cl and ·OH.
References
Ali U, Sanchez-Garcia L, Rehman MYA, Syed JH, Mahmood A, Li J, Zhang G, Jones KC, Malik RN (2016) Tracking the fingerprints and combined TOC-black carbon mediated soil-air partitioning of polychlorinated naphthalenes (PCNs) in the Indus River Basin of Pakistan. Environ Pollut 208:850–858
Barc J, Gregoraszczuk EL (2014) Effects of individual polychlorinated naphthalene(PCN) components of Halowax 1051 and two defined, artificial PCN mixtures on AHR and CYP1A1 protein expression, steroid secretion and expression of enzymes involved in steroidogenesis (CYP17, 17β-HSD and CYp19) in porcine ovarian follicles. Toxicology 322:14–22
Boxe CS, Colussi AJ, Hoffmann MR, Murphy JG, Wooldridge PJ, Bertram TH, Cohen RC (2005) Photochemical production and release of gaseous NO2 from nitrate-doped water ice. J Phys Chem A 109(38):8520–8525
Cetin B (2016) Investigation of PAHs, PCBs and PCNs in soils around a heavily industrialized area in Kocaeli, Turkey: concentarions, distributions, sources and toxicological effects. Sci Total Environ 560–561:160–169
Chu L, Anastasio C (2003) Quantum yields of hydroxyl radical and nitrogen dioxide from the photolysis of nitrate on ice. J Phys Chem A 107:9594–9602
Deng NS, Wu F (2003) Environmental photochemistry. Chemical Industry, Beijing
Die QQ, Nie ZQ, Fang YY, Yang YF, Gao XB, Tian YJ, He J, Liu F, Huang QF, Tian SL (2016) Seasonal and spatial distributions of atmospheric polychlorinated naphthalenes in Shanghai, China. Chemosphere 144:2134–2141
Fromme H, Cequier E, Kim JT, Hanssen L, Hilger B, Thomsen C, Chang YS, Volkel W (2015) Persistent and emerging pollutants in the blood of German adults: occurrence of deshloranes, polychlorinated naphthalenes, and siloxanes. Environ Int 85:292–298
Huang J, Yu G, Yamauchi M, Matsumura T, Yamazaki N, Weber R (2015) Congener-specific analysis of polychlorinated naphthalenes (PCNs) in the major Chinese technical PCB formulation from a stored Chinese electrical capacitor. Environ Sci Pollut Res 22:14471–14477
Jacobi HW, Annor T, Quansah E (2006) Investigation of the photochemical decomposition of nitrate, hydrogen peroxide, and formaldehyde in artificial snow. J Photochem Photobiol A 179:330–338
Jarnberg UG, Asplund LT, Egeback AL, Jansson B, Unger M, Wideqvist U (1999) Polychlorinated naphthalene congener profiles in background sediments compared to a degraded Halowax 1041 technical mixture. Environ Sci Technol 33(1):1–6
Jiang XX, Liu GR, Wang M, Liu WB, Tang C, Li L, Zheng MH (2015) Case study of polychlorinated naphthalenes emissions and factors influencing emission variations in secondary aluminum production. J Hazard Mater 286:545–552
Keum YS, Li QX (2004) Photolysis of octachloronaphthalene in hexane. Bull Environ ContamToxicol 72:999–1005
Kormali P, Troupis A, Triantis T, Hiskia A, Papaconstantinou E (2007) Photocatalysis by polyoxometallates and TiO2. Catal Today 124:149–155
Li F, Jin J, Gao Y, Geng NB, Tao DQ, Zhang HJ (2016) Occurrence distribution and source apportionment of polychlorinated naphthalenes(PCNs) in sediments and soils from the Liaohe River Basin, China. Environ Pollut 211:226–232
Lindsey ME, Tarr MA (2000) Quantitaion of hydroxyl radical during Fenton oxidation following a single addition of iron and peroxide. Chemosphere 41:409–417
Liu GR, Lv P, Jiang XX, Nie ZQ, Liu WB, Zheng MH (2015) Identification and preliminary evaluation of polychlorinated naphthalenes emissions from hot dip galvanizing plants. Chemosphere 118:112–116
Lou T, Wang XJ, Song GJ, Gui GP (2017) Synthesis and flocculation performance of a chitosan–acrylamide–fulvic acid ternary copolymer. Carbohydr Polym 170:182–189
Moukas AI, Thomaidis NS, Calokerinos AC (2016) Novel determination of polychlorinated naphthalence in water by liquid chromatography-mass spectrometry with atmospheric pressure photoionization. Res Paper 408:191–201
Russi H, Kotzias D, Korte F (1982) Photoinduzierte hydrox-ylierungsreaktionen organischer chemikalien in naturlichen gewassern: nitrate als potentielle OH-radikalquellen. Chemosphere 10:1041–1048
Ruzo LO, Bunce NJ, Safe S (1975) Photodegradation of polychloronaphthalenes in methanol solution. Bull Environ ContamToxicol 14 (3):341–345
Sinha A, Bose P (2006) Dehalogenation of 2-chloronapthalene by cast iron. Water Air Soil Pollut 172:375–390
Xu F, Zhang RM, Li YF, Zhang QZ (2015a) Homogeneous gas-phase formation of polychlorinated naphthalene from dimerization of 4-chlorophenoxy radicals and cross-condensation of phenoxy radical with 4-chlorophenoxy radical: mechanism and kinetics study. Chem Phys Lett 638:153–160
Xu F, Zhang RM, Li YF, Zhang QZ, Wang WX (2015b) Theoretical Mechanistic and Kinetic Studies on homogeneous gas-phase formation of polychlorinated naphthalenes from 2-chlorophenol as forerunner. Mol Sci 16:25641–25656
Zhan M, Yang X, Xian Q, Kong L (2006) Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances. Chemosphere 63:378–386
Zhang HY, Xiao K, Liu JY, Wang T, Liu GR, Wang YW, Jiang GB (2014) Polychlorinated naphthalenes in sewage sludge from wastewater treatment plants in China. Sci Total Environ 490:555–560
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 41073063 and No. 41403101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, C., Bao, S., Chen, B. et al. Photoconversion of 2-Chloronaphthalene in Water. Bull Environ Contam Toxicol 99, 415–421 (2017). https://doi.org/10.1007/s00128-017-2146-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2146-y