Abstract
Over the past four decades, many researchers have applied the theory of island biogeography (IBT) to predict and understand species loss and distribution in fragmented landscapes. Recent studies found that specialist species were more affected by fragment size and isolation than generalists. However, the mechanisms underlying different effects of area and isolation among specialists and generalists are unknown. We tested the predictions of IBT on butterfly assemblages in Tokyo, Japan, and hypothesized that the effects of fragment size and isolation would be stronger for specialists than for generalists. We classified butterfly species into specialists and generalists for each of two dimensions (food range and voltinism) and according to tolerance to the matrix. We recorded 26 feeding specialists and 27 generalists, 24 seasonal specialists and 29 generalists, 32 low matrix-tolerant species and 21 high matrix-tolerant species in 20 forest fragments. We used generalized linear models to relate the number of species in a fragment to fragment size and isolation (distance to the mainland). The averaged models based on AICc showed that fragment size had positive and significant effects on both specialist and generalist and high matrix-tolerant butterfly species richness. However, the negative effects of isolation on species richness were only found in specialist and low matrix-tolerant species. Our results demonstrate that patch isolation only affects specialist species. This suggests that when applying IBT to terrestrial fragmented landscapes, researchers should be careful not to overlook patch area and isolation effects on specialists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human domination of the Earth’s ecosystems has led to a severe loss of natural habitat and isolation of habitat remnants. Habitat loss and fragmentation are the most likely major causes of the increased rate of species extinction observed in recent decades (Fahrig 2003; Foley et al. 2005). Fragmentation has resulted in many habitat remnants (“islands”) becoming isolated from continuous habitat (“mainland”). By considering habitat fragments as islands embedded in an ocean, many researchers have applied the theory of island biogeography (IBT, MacArthur and Wilson 1967) to predict and understand species loss and distribution in fragmented ecosystems (Zschokke et al. 2000; Magura et al. 2001; Brose 2003). Many of these studies have found species richness to decrease with decreasing habitat area and increasing habitat isolation (Lomolino et al. 1989; Davies and Margules 1998; Magura et al. 2001). Over the past four decades, IBT has been used to highlight the importance of patch size and isolation for maintaining species diversity in fragmented ecosystems.
However, many previous studies applying IBT to fragmented landscapes have so far failed to recognize one crucial fact: the matrix is not usually completely inhospitable to movement. For oceanic islands, the surrounding marine environment is completely inhospitable; however, for terrestrial habitat fragments, the effect of the surrounding matrix environment on the reproduction and dispersal of organisms is less well understood (e.g., Gustafson and Gardner 1996). Recently, many empirical studies have reported strong influences of the matrix composition on species distribution and colonization–extinction dynamics in fragmented landscapes (Ricketts 2001; Tischendorf et al. 2003; Kupfer et al. 2006; Dormann et al. 2007; Prugh et al. 2008; Umetsu et al. 2008; Watling et al. 2011). In a meta-analysis, Watling et al. (2011) compared the effects of matrix composition (composition of surrounding land cover) with those of habitat connectivity (based only on the most suitable, original “habitat”) on the occurrence and abundance of animals in fragmented habitats across 184 taxa. They concluded that the matrix composition has comparable effects to (and sometimes stronger effects than) habitat connectivity in fragmented landscapes. Recently, fragmentation research has changed focus from patch-based study (i.e., measuring only patch-level factors such as patch size and isolation) to landscape mosaic-based study (Kupfer et al. 2006).
In general, the definition of a “patch” becomes less obvious if species are able to use the matrix as a feeding or nesting habitat (Sisk et al. 1997). Recent empirical and modeling studies (Cook et al. 2002; Bender and Fahrig 2005; Guldemond and van Aarde 2010) demonstrated that the effects of forest patch size and isolation affect only forest-dependent species, suggesting that IBT predictions were a better fit to the data when matrix-dwelling species were removed from the analysis. Cook et al. (2002) pointed out that spillover of generalist species from matrix into patches may obscure patch size and distance effects on specialists. Therefore, it is difficult to predict community and population dynamics in fragmented landscapes without understanding the different effects of patch area and isolation on species diversity between specialists and generalists. However, the mechanisms underlying such different area and isolation effects between specialists and generalists are mostly unknown.
Butterflies can be classified into generalists or specialists based on their resource use pattern in two fundamental niche dimensions: niche width (range of host plant species used by larvae) and voltinism (number of generations per year) (Kitahara and Fujii 1994). Using this approach, we can regard species with feeding specialization as “feeding specialists” and uni- and bivoltine species as “seasonal specialists.” In addition to these species’ traits, tolerance to the matrix strongly affects species dispersal ability (Kupfer et al. 2006). Therefore, for butterflies, tolerance to the matrix may be another important criteria of habitat occurrence in fragmented landscapes. The availability of resources in the matrix is a key factor determining the presence of fragment-dependent species in fragmented landscapes (e.g., Jokimäki and Huhta 1996). Thus, we can assume that species whose larval host plants are cultivated by humans in the matrix (e.g., in gardens, on roadsides, and in agricultural areas) are species with high tolerance to the matrix. In contrast, species whose larval host plants are not cultivated in the matrix are species with low tolerance to the matrix. Ries and Debinski (2001) found a high matrix permeability for generalist butterfly species, whereas specialists were unlikely to emigrate from a fragment. Considering their low dispersal and matrix tolerance capabilities, the effects of fragment size and isolation may be stronger for species with a low tolerance to the matrix than those with a high tolerance to the matrix.
In the present study, we tested the classical predictions of IBT for the butterfly assemblages of Tama, central Japan. The Tama area lies at the foot of Mount Takao in Tokyo, and was covered by deciduous secondary forests until the 1950s. Because of intense housing construction, the former large forest was replaced by many small, isolated urban forest fragments. In 1970, 56.6 % of the forest fragments exceeded 100 ha, and 5.2 % were smaller than 20 ha. After 1970, the number of small forests gradually increased and by 2000, 60.9 % of the forests were smaller than 20 ha (Kataoka and Tamura 2005). This particular situation provides an excellent model system to test IBT. In this study, we tested the following hypotheses: (1) total butterfly species richness increases with increasing habitat area and decreasing isolation (distance to the mainland), and (2) specialist and low matrix-tolerant species are more affected by fragment size and isolation than generalists and low matrix-tolerant species. As mentioned previously, we classified butterfly species into specialists and generalists based on two classifications (i.e., feeding and seasonal) and according to the tolerance to the matrix, and tested IBT for each group.
Methods
Study area and sampling sites
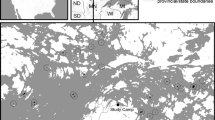
The forests were all located in a 10 × 15 km area in southwestern Tokyo, central Japan (N35.61 and E139.38) (Fig. 1). The western boundary of the study area is a mountain range dominated by Mount Takao (599 m a.s.l.), and contains a forest referred to as the “mainland” in this study. In this study area, the mainland forest was more diverse in terms of both plant and animal species than the urban forest fragments, and all 53 species recorded in this study also occur in this mainland forest (Nagase 2004). As mentioned previously, we can assume that the mainland is the source area from which species disperse and colonize islands—the forest fragments in our case. The forest fragments were dominated by secondary forests of two deciduous oaks, Quercus serrata Thunb. ex Murray and Q. acutissima Carruthers with Pinus densiflora Sieb. et Zucc., Abies firma Sieb.et Zucc, and evergreen oaks, Q. glauca Thunb. ex Murray and Q. acuta Thunb. ex Murray also present (Kataoka and Tamura 2005). A forest fragment was considered to be any forest area containing any of these tree species. The border of a forest fragment was defined as any treeless belt with an open canopy of more than 10 m, following Kurosawa and Askin (2003).
A total of 20 forest fragments that ranged in area from 1.1 to 121.6 ha were selected. Forest fragments were separated from each other by varying distances (Fig. 1). To avoid pseudoreplication, forest fragments separated by at least 800 m were selected. To minimize the effects of the matrix type on the butterfly community, forest fragments that were mostly surrounded by residential areas were selected. ArcView geographic information system software (ver. 3.2, ESRI, CA, USA) was used with aerial photographs (1:25,000) to quantitatively assess the forest fragment area and isolation (nearest distance to the mainland). Other authors have stressed the strong influence of other patch factors such as shape (Laurance 1991) and connectivity (Vos and Stumpel 1995) on the number of species. Therefore, we also measured the shape index (Laurance and Yensen 1991) and connectivity (Hanski, 1994). In this study, however, these two factors had no significant effect on butterfly species richness, so we removed these two factors from the analysis.
Butterfly survey and classification
Butterfly communities were monitored using the line transect method (Pollard 1977). Transect counts were conducted once a month between 0930 and 1430 hours during the adult flight season (early April–early October 2009) if the weather was suitable. No butterfly surveys were conducted in August because several butterfly species have been reported to aestivate at this time (Fukuda et al. 1983). Butterflies within a 10 m radius of a position along the transect were recorded while walking at a steady pace (10 m/min). We randomly set a 500 m long transects (from edge to interior) and spent 50 min at each transect. In every six observations, we surveyed the same transect routes. Individuals that could not be identified by sight were caught, identified, and released (e.g., skippers and lycaenids). Since it is impossible to distinguish between Pieris metele and P. rapae in the field, these two species were treated as Pieris spp. for the analysis.
We classified all 53 butterflies that were observed into specialist or generalist species based on the following two dimensions. First, we used a seasonal dimension to define uni- and bivoltine (≤2 brood/generations per year) as “seasonal specialists” and multivoltine species (≥3 brood/generations per year) as “seasonal generalists.” Second, we used a feeding dimension to define mono- and oligophagous species (larvae feeding on a maximum of ten plant species from the same family) as “feeding specialists” and polyphagous species (larvae feeding on more than ten plant species from the same family or more than two families) as “feeding generalists,” following Kitahara and Fujii (1994). In the present study, I applied these classifications following Kitahara and Fujii (1994) with minor modifications. Third, we used the “tolerance to the matrix” dimension to classify species whose larval host plants (at least one species) were found in gardens, on roadsides, and in agricultural areas in the matrix as “high matrix-tolerant species” (i.e., matrix-dwelling species). On the other hand, species whose larval host plants were not found in gardens, on roadsides, or in agricultural areas in the matrix were classified as “low matrix-tolerant species” (i.e., fragment-dependent species). These classifications were based on data from Fukuda et al. (1982, 1983, 1984a, b), the Tokyo Metropolitan Government (2000), and the Ministry of Agriculture, Forestry and Fisheries (2010).
Statistical analysis
We used an information theoretic approach for model selection and to assess model performance. This involved the use of generalized linear models with a Poisson distribution and a log-link function. Species richness was used as the dependent variable, and fragment size (ha, log-transformed) and isolation were used as explanatory variables. To select the best models among the candidate models, we used the small-sample corrected version of Akaike’s information criterion (AICc, Burnham and Anderson 2002). The AICc value for each model quantifies its parsimony (based on a trade-off between the model’s goodness of fit and the number of parameters included) relative to the other models considered. We used the “dredge” function from the MuMIn package (ver. 1.0.0) (Barton 2009) to test models defined by all possible variable combinations and rank them by their AICc-based model weights (ΔAICc j = AICc i − AICcmin, where AICcmin and AICc i represent the AICc of the best model and that of the ith supported model in the model subsets, respectively). The plausibility of each model was quantified by its relative likelihood, which was proportional to the exponent of −0.5 × ΔAICc given our data. Then, for each candidate model, the normalized Akaike weights were compiled as evidence that the model is the best of the set (Burnham and Anderson 2002). Because the ΔAICc values were less than 2 in our models, indicating that the other candidate models had substantial support as explanations of butterfly species richness (Burnham and Anderson 2002), we performed model averaging with the “model.avg” function. Model averaging provides unconditional model variances and more reliable parameter estimates for each predictor. To determine the reliability of the predictor estimates from averaging, we calculated the weighted unconditional standard error along with its associated confidence interval (95 % CI). To assess the relative importance of each predictor, we also used the relative importance value (RIV) for each. To calculate the RIV, the calculated Akaike weights for each model that contained the parameter of interest were summed. Generalized linear models and AICc values were calculated using the R software package (v.2.12.0, R Development Core Team 2010).
Results
In total, we found 1,625 butterfly individuals belonging to 53 species [mean of 24 species per site (SD = 7.3)] (Table 1). Twenty-four species were classified into seasonal specialists and 29 were seasonal generalists, 26 species were feeding specialists and 27 were feeding generalists, and 32 were low matrix-tolerant species and 21 were high matrix-tolerant species (Table 1).
Island biogeography theory of total butterfly species
There was a strong support for an increase in species richness with increasing fragment size. Fragment size had the highest RIV (1.00) and a high, positive parameter estimate that did not include 0 within the 95 % CI (Table 2A). Compared with fragment size, isolation had relatively weak, negative effects on butterfly species richness. The parameter estimate for isolation overlapped with 0 within the 95 % CI and had a low RIV (0.49).
Island biogeography theory for specialists versus generalists
For all three butterfly groups (feeding, seasonal, and tolerance to the matrix groups), there was a strong relationship between fragment size and species richness for both the specialist and generalist species and for both the high and low matrix-tolerant species (Table 2B, C, D; Fig. 2). In addition, for all groups, fragment size had a high, positive parameter estimate that did not include 0 within the 95 % CI. However, the effect of fragment size on species richness did not differ between specialists and generalists or between high and low matrix-tolerant species, because the 95 % CIs overlapped within each group (Table 2B, C, D; Fig. 2).
Species richness for three butterfly groups in relation to fragment size and isolation (distance to mainland). Filled circles indicate specialists and low matrix-tolerant species and open circles indicate generalist and high matrix-tolerant species; solid lines indicate the average models for specialists and low matrix-tolerant species and broken lines indicate those of generalist and high matrix-tolerant species (Table 2)
For the two specialist groups and the low matrix-tolerant species, there was a negative relationship between isolation and species richness (Table 2B, C, D; Fig. 2). The negative effects of isolation on generalist species and high matrix-tolerant species were weaker than those on specialists and low matrix-tolerant species (Table 2B, C, D), because the RIVs for isolation in generalists and high matrix-tolerant species were smaller than those for specialists and low matrix-tolerant species (feeding 0.74 > 0.20, seasonal 0.70 > 0.23, matrix 0.87 > 0.20).
Discussion
Species–area relationships
As predicted by MacArthur and Wilson (1967), we found a significant positive relationship between fragment size and the total number of butterfly species. This result was consistent with those of previous studies that examined the effects of patch size on species assemblages (e.g., birds, Bellamy et al. 1996; butterflies, Öckinger and Smith 2006; plants, Kohn and Walsh 1994). The significant and positive relationship observed between forest size and butterfly species richness is considered to be due to large areas typically having lower extinction rates and higher immigration rates than small areas. Both of these mechanisms have been described by the theories of IBT (MacArthur and Wilson 1967) and metapopulations (Hanski and Gyllenberg 1997). Another reason for the higher species richness in large fragments is the higher habitat heterogeneity in large areas compared with small areas (Connor and McCoy 1979; Forman and Godron 1986).
In this study, we did not find any difference in the effects of size on species richness between specialists and generalists or between high and low matrix-tolerant species (Table 2). Cook et al. (2002) and Guldemond and van Aarde (2010) reported that the effects of patch size on matrix-dwelling species (i.e., high matrix-tolerant species) were weaker than those on fragment-dependent species (i.e., low matrix-tolerant species). In their study, the matrix was a good-quality environment where many generalist bird and plant species persisted, meaning that the boundaries between patch and matrix were obscure for high matrix-tolerant species. On the contrary, the matrix of our study area was completely urbanized, which means that even high matrix-tolerant species cannot live in the matrix due to habitat degradation and increased air and soil pollution (e.g., Bastin and Thomas 1999; McKinney 2002). Therefore, we found a positive and significant relationship between fragment size and species richness even for generalists and high matrix-tolerant species. In general, the functional notion of fragments and matrix is dependent on the landscape type, scale, and organism (Kupfer et al. 2006). Our approach should be expanded to analyze a broader range of organisms and ecosystems.
Species–isolation relationships
One of the most important findings of the present study is that a negative relationship between isolation and butterfly species richness was only found in specialists and low matrix-tolerant species (Table 2; Fig. 2). There are possible reasons why responses to isolation differed between specialists and generalists or between high and low matrix-tolerant species in our study area. Generally, the host plant resources of feeding generalists are distributed over a wide range of cover types, so they may be able to move between patches regardless of the underlying environmental heterogeneity. In contrast, monophagous and oligophagous species are less likely to move across the boundary of one habitat patch to another patch like stepping stones. Therefore, the negative relationship between feeding specialist diversity and isolation may reflect their limited dispersal ability. In general, we can expect that species with multiple host plants will be more successful in fragmented landscapes than monophagous species (e.g., Zimmerman et al. 2005). Phenological parameters may also be associated with dispersal ability (Kotze and O’Hara 2003). The intrinsic rate of natural increase (r) is positively correlated with voltinism, so multivoltinism and univoltinism are associated with higher and lower values of r, respectively (e.g., Shapiro 1975). In general, species with high intrinsic rates of natural increase (r strategy) have greater ability to disperse offspring widely because they are “early successional species” (Pianka 1978). This indicates that seasonal specialist species could be less likely to move from the mainland and successfully establish in fragments, which may explain the negative relationship between species richness and distance to the mainland. The response of species richness to isolation was slightly clearer in low matrix-tolerant species than in high matrix-tolerant species (Fig. 2f; Table 2d). Since the resources of high matrix-tolerant species existed in both the fragments and the matrix, they may have been able to easily disperse from the mainland and colonize fragments.
Management implications
Based on the findings of our study, we are able to draw two important conclusions. First, we showed that, in addition to fragment size and isolation, the availability of resources in the matrix is a particularly important influence on the butterfly species distribution. Over the past four decades, IBT has had an enormous impact on conservation biology in fragmented landscapes. As a result, the value of large and well-connected forest fragments has been emphasized. Recent empirical studies reported that some species may be able to compensate for a loss of their natural forest habitat by moving into other habitat types (e.g., plantations or other modified habitats) (Norton et al. 2000; Cook et al. 2002). Gascon et al. (1999) also suggested that species that avoid the matrix tend to decline or disappear in fragmented landscapes, while those that can move within and use the matrix often remain stable or increase. Therefore, characteristics of the matrix may reduce the effect of habitat loss and fragmentation. In the present study, patch area had a stronger effect on butterfly diversity than patch isolation, which means that larger nature reserves are of greater value for the conservation of butterflies. However, the maintenance of large nature reserves incurs great costs in highly fragmented urban ecosystems (Franklin 1993), and so managing the matrix environment may be an effective and important approach for conservation. Although some natural life-history traits of the butterfly are likely to be fixed (e.g., voltinism and food range), tolerance to the matrix could easily be changed by human activities.
Second, our results suggest that patch isolation only affects specialist species and low matrix-tolerant species. This suggests that the distribution and population dynamics of butterfly species will vary among different functional groups in our study area. It has also led to a concern that if fragments distant from the mainland have high species diversity, this will mostly comprise generalist or high matrix-tolerant species. However, in general, species prone to extinction in fragmented landscapes are specialist species (Koh et al. 2004; Soga and Koike 2012). Therefore, when preserving biodiversity, conservation managers should prioritize action for specialist and low matrix-tolerant species and not only focus on all of the species. To do this, it is important to understand which local and landscape factors are necessary for the conservation of habitat specialists. Based on our results, fragment size and isolation may be important for species persistence, so we recommend conserving large and well-connected fragments for butterfly specialists. This will be particularly important for the long-term survival of habitat specialists in highly fragmented urban forests. In conclusion, we argue that when applying IBT to terrestrial fragmented landscapes, researchers should be careful not to overlook patch area and isolation effects on specialists.
References
Barton K (2009) MuMIn: multi-model inference (R package version 1.0.0). http://r-forge.r-project.org/projects/mumin/
Bastin L, Thomas CD (1999) The distribution of plant species in urban vegetation fragments. Landsc Ecol 14:493–507
Bellamy PE, Hinsley SA, Newton I (1996) Factors influencing bird species numbers in small woods in south-east England. J Appl Ecol 33:249–262
Bender DJ, Fahrig L (2005) Matrix structure obscures the relationship between interpatch movement and patch size and isolation. Ecology 86:1023–1033
Brose U (2003) Island biogeography of temporary wetland carabid beetle communities. J Biogeogr 30:879–888
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach. Springer, New York
Connor EF, McCoy ED (1979) The statistics and biology of the species–area relationship. Am Nat 113:791–833
Cook WM, Lane KT, Foster BL, Holt RD (2002) Island theory, matrix effects and species richness patterns in habitat fragments. Ecol Lett 5:619–623
Davies KF, Margules CR (1998) Effects of habitat fragmentation on carabid beetles: experimental evidence. J Anim Ecol 67:460–471
Dormann CF, Schweiger O, Augenstein I, Bailey D, Billeter R, de Blust G, DeFilippi R, Frenzel M, Hendrickx F, Herzog F, Klotz S, Liira J, Maelfait J, Schmidt T, Speelmans M, van Wingerden WKRE, Zobel M (2007) Effects of landscape structure and land-use intensity on similarity of plant and animal communities. Glob Ecol Biogeogr 16:774–787
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Forman RTT, Godron M (1986) Landscape ecology. Wiley, New York
Franklin JF (1993) Preserving biodiversity: species, ecosystems, or landscapes? Ecol Appl 3:202–205
Fukuda H, Hama E, Kuzuya T, Takahashi A, Tahakashi M, Tanaka B, Tanaka H, Wakabayashi M, Watanabe Y (1982) The life histories of butterflies in Japan, vol I. Hoikusha, Osaka (in Japanese)
Fukuda H, Hama E, Kuzuya T, Takahashi A, Tahakashi M, Tanaka B, Tanaka H, Wakabayashi M, Watanabe Y (1983) The life histories of butterflies in Japan, vol II. Hoikusha, Osaka (in Japanese)
Fukuda H, Hama E, Kuzuya T, Takahashi A, Tahakashi M, Tanaka B, Tanaka H, Wakabayashi M, Watanabe Y (1984a) The life histories of butterflies in Japan, vol III. Hoikusha, Osaka (in Japanese)
Fukuda H, Hama E, Kuzuya T, Takahashi A, Tahakashi M, Tanaka B, Tanaka H, Wakabayashi M, Watanabe Y (1984b) The life histories of butterflies in Japan, vol IV. Hoikusha, Osaka (in Japanese)
Gascon C, Lovejoy TE, Bierregaard RO Jr, Malcolm JR, Stouffer PC, Vasconcelos HL, Laurance WF, Zimmerman B, Tocher M, Borges S (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91:223–229
Guldemond RAR, van Aarde RJ (2010) Forest patch size and isolation as drivers of bird species richness in Maputaland, Mozambique. J Biogeogr 37:1884–1893
Gustafson EJ, Gardner RH (1996) The effect of landscape heterogeneity on the probability of patch colonization. Ecology 77:94–107
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63:151–162
Hanski I, Gyllenberg M (1997) Uniting two general patterns in the distribution of species. Science 275:397–400
Jokimäki J, Huhta E (1996) Effects of landscape matrix and habitat structure on a bird community in northern Finland: a multi-scale approach. Ornis Fenn 73:97–113
Kataoka T, Tamura N (2005) Effects of habitat fragmentation on the presence of Japanese squirrels, Sciurus lis, in suburban forests. Mamm Stud 30:131–137
Kitahara M, Fujii K (1994) Biodiversity and community structure of temperate butterfly species within a gradient of human disturbance: an analysis based on the concept of generalist vs. specialist strategies. Popul Ecol 36:187–199
Koh LP, Sodhi NS, Brook BW (2004) Ecological correlates of extinction proneness in tropical butterflies. Conserv Biol 18:1571–1578
Kohn DD, Walsh DM (1994) Plant species richness—the effect of island size and habitat diversity. J Ecol 82:367–377
Kotze DJ, O’Hara RB (2003) Species decline—but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 135:138–148
Kupfer JA, Malanson GP, Franklin SB (2006) Not seeing the ocean for the islands: the mediating influence of matrix-based processes on the forest fragmentation effects. Glob Ecol Biogeogr 15:8–20
Kurosawa R, Askin RA (2003) Effects of habitat fragmentation on birds in deciduous forests in Japan. Conserv Biol 17:695–707
Laurance WF (1991) Edge effects in tropical forest fragments: application of a model for the design of nature reserves. Biol Conserv 57:205–219
Laurance WF, Yensen E (1991) Predicting the impacts of edge effects in fragmented habitats. Biol Conserv 55:77–92
Lomolino MV, Brown JH, Davis R (1989) Island biogeography of montane forest mammals in the American southwest. Ecology 70:180–194
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Magura T, Ködövöcz V, Tóthmérész B (2001) Effects of habitat fragmentation on carabids in forest patches. J Biogeogr 28:129–138
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
Ministry of Agriculture, Forestry and Fisheries (2010) Homepage. http://www.maff.go.jp/j/tokei/index.html
Nagase T (2004) Takao, Jinbasan no tyou. Keyaki, Tokyo (in Japanese)
Norton MR, Hannon SJ, Schmiegelow FKA (2000) Fragments are not islands: patch vs landscape perspectives on songbird presence and abundance in a harvested boreal forest. Ecography 23:209–223
Öckinger E, Smith HG (2006) Landscape composition and habitat area affects butterfly species richness in semi-natural grasslands. Oecologia 149:526–534
Pianka ER (1978) Evolutionary ecology. Harper and Row, New York
Pollard E (1977) A method for assessing changes in the abundance of butterflies. Biol Conserv 12:116–134
Prugh LR, Hodges KE, Sinclair ARE, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. Proc Nat Acad Sci USA 105:20770–20775
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Ries L, Debinski DM (2001) Butterfly responses to habitat edges in the highly fragmented prairies of Central Iowa. J Anim Ecol 70:840–852
Shapiro AM (1975) The temporal component of butterfly species diversity. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Belknap, Harvard University, Cambridge, pp 181–195
Sisk TD, Haddad NM, Ehrlich PR (1997) Bird assemblages in patchy woodlands: modeling the effects of edge and matrix habitats. Ecol Appl 7:1170–1180
Soga M, Koike S (2012) Life-history traits affect vulnerability of butterflies to habitat fragmentation in urban remnant forests. Ecoscience 19(1). doi:10.2980/19-1-3455
Tischendorf L, Bender DJ, Fahrig L (2003) Evaluation of patch isolation metrics in mosaic landscapes for specialist vs. generalist dispersers. Landsc Ecol 18:41–50
Tokyo Metropolitan Government (2000) Tokyo road side tree map. Tokyo Metropolitan Government, Tokyo (in Japanese)
Umetsu F, Metzger JP, Pardini R (2008) Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic Forest small mammals. Ecography 31:359–370
Vos CC, Stumpel HP (1995) Comparison of habitat isolation parameters in relation to fragmented distribution patterns in the tree frog (Hylea arborea). Landsc Ecol 11:203–214
Watling JI, Nowakowski AJ, Donnelly MA, Orrock JL (2011) Meta-analysis reveals the importance of matrix composition for animals in fragmented habitat. Grob Ecol Biogeogr 20:209–217
Zimmerman K, Fric Z, Filipová L, Konvička M (2005) Adult demography, dispersal and behaviour of Brenthis ino (Lepidoptera: Nymphalidae): how to be a successful wetland butterfly. Eur J Entomol 102:699–706
Zschokke S, Dolt C, Rusterholz HP, Oggier P, Braschler B, Thommen GH, Ludin E, Erhardt A, Baur B (2000) Short-term responses of plants and invertebrates to experimental small-scale grassland fragmentation. Oecologia 124:559–572
Acknowledgments
We thank Y. Yamaura and two anonymous referees for fruitful suggestions. This study was funded by the Urban Green Tech Japan, and Fuji Film Green Foundation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Soga, M., Koike, S. Patch isolation only matters for specialist butterflies but patch area affects both specialist and generalist species. J For Res 18, 270–278 (2013). https://doi.org/10.1007/s10310-012-0349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-012-0349-y