Abstract
To clarify the effects of asynchronous seed production among tree species on the population of seed predators, we investigated the relationship between the annual variation in production of mature acorns and the insect damage in those acorns of two sympatric oak species, Quercus variabilis Blume and Quercus serrata Thunb. ex Murray, over 4 years at two study sites. The annual variation in acorn production was noticeable, with a coefficient of variation (CV) at the two sites of 1.05 and 0.80 for Q. variabilis and 0.87 and 0.73 for Q. serrata. Annual fluctuation in acorn production by Q. serrata was synchronized between the two sites. Since annual fluctuation in acorn production was not synchronized between the two species, the CVs for the total acorn production by both oak species (0.83 and 0.62 at the two sites) were lower than those for Q. variabilis and Q. serrata alone. The rate of predation by the specialist predators (Curculio weevils) on the acorns of both species was not related to the annual acorn crop size. Prolonged diapause of Curculio weevils might stabilize their populations. The rate of acorn predation by the generalist predators (tortricid moths) was also not related to the annual crop size. Asynchronous acorn production by the two oak species would help to stabilize the population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acorns, the seeds of Quercus species, are a rich source of food for many animals. Of these predators, insects are the most important for both pre- and post-dispersal phases of acorn development, and have large effects on the regeneration process of Quercus species (Matsuda 1982; Fujii 1993; Maeto 1993; Fukumoto and Kajimura 2005; Pulido and Díaz 2005).
Large fluctuations among years in acorn production occur in the genus Quercus (Sork et al. 1993; Koenig et al. 1994; Crawley and Long 1995; Maeto and Ozaki 2003; Espelta et al. 2008); years with particularly high acorn production are referred to as “mast seeding” years (Janzen 1971; Silvertown 1980; Kelly 1994). For insect predators, acorns are therefore unpredictable food resources with large annual fluctuations. The magnitude of the annual fluctuation in acorn production can be associated with the dynamics of insect predators. Quercus species often co-occur in temperate forests, and share the same insect seed predators (Ueda 2000; Shibata et al. 2002; Ueda and Osumi 2003). Synchronous acorn production between populations of sympatric tree species that share the same seed predators has the potential advantage of predator satiation (Janzen 1971; Silvertown 1980), thereby ensuring that at least some seeds will survive. Recently, asynchronous acorn production was reported in North America between 1-year species, which mature their acorns in the year after flowering, and 2-year species, which mature their acorns in the next year after flowering (Sork et al. 1993; Koenig et al. 1994; Liebhold et al. 2004; Kelly et al. 2008). However, we found no information in the research literature on how asynchronous acorn production by sympatric species affected resource utilization by acorn-feeding insects.
Quercus variabilis Blume (a 2-year species) and Quercus serrata Thunb. ex Murray (a 1-year species) co-occur in the temperate deciduous forests of central Japan (Fukumoto and Kajimura 2005). Detailed studies of the biology of acorn-feeding insects have been carried out previously (Fukumoto and Kajimura 1999, 2001). Here, we investigated the relationships between the annual variation in the production of mature acorns and insect damage to the acorns of both oak species over 4 years at two study sites. We analyzed the annual synchrony of acorn production between the two species and the yearly changes in acorn predation by each insect. On the basis of the results of this monitoring, we discuss potential mechanisms capable of stabilizing the insect population size as a function of the biology of acorn-feeding insects.
Materials and methods
Study site and methods
We carried out our study in the secondary forests in the Nagoya University Campus (site A) and in Higashiyama Park (site B), both in Nagoya City, Aichi Prefecture, central Japan (35°10′N, 136°58′E, 50 m a.s.l.). The forests consist mainly of deciduous oaks (Q. variabilis and Q. serrata) and Japanese red pine (Pinus densiflora Sieb. et Zucc). Table 1 summarizes the tree density, diameter at breast height, and total basal area of Q. variabilis and Q. serrata at the two study sites. The mean annual precipitation and temperature at the nearby Nagoya Weather Station are about 1,500 mm and 15.1°C (the highest monthly mean is 32.3°C in August and the lowest, 0.1°C in January), respectively (National Astronomical Observatory 2001). The two sites lie only about 1.5 km apart, and have similar climatic conditions.
We selected five trees of Q. variabilis and five of Q. serrata at site A, and five Q. variabilis and 14 Q. serrata at site B. We chose trees whose canopies did not overlap with trees of the same species, but one pair of neighboring Q. serrata at site B overlapped their canopies slightly. We established four 0.5-m2 seed traps (0.25 m2) under each tree canopy. The seed traps at site A were plastic containers raised about 0.2 m above the ground, whereas those at site B were made from nylon cloth and were positioned about 0.8 m above the ground. Our previous research (Fukumoto and Kajimura 2005) has demonstrated that there is no difference in the efficiency of acorn collection between the two types of trap. Sampling periods at site A were 31 May–21 December 1997, 11 April–20 December 1998, 9 April–17 December 1999, and 13 April–23 December 2000; at site B they were 15 June–19 December 1997, 12 April–25 December 1998, 15 April–19 December 1999, and 18 April–22 December 2000.
We collected the contents of the traps once every 1 or 2 months. Then, we dissected the acorns and classified their internal condition as sound (a mature acorn with sound cotyledons), aborted (an immature acorn with undeveloped cotyledons), insect-infested (an acorn with penetrating holes or the presence of eggs, larvae, adults, or larval feces), or degenerated (a mature acorn with evidence of desiccation or fungal attack). Aborted acorns and immature insect-damaged acorns were removed from our sample. Insects were identified to the species level from their eggs, larvae, adults, or larval feces. We identified tortricid moths and Curculio weevils only to the family and genus level, respectively, because their eggs, larvae, and larval feces were too similar to let us confirm finer taxonomic distinctions in these groups.
Data analysis
The rate of acorn predation by each insect species or group was the number of infested acorns divided by the total number of mature acorns in our sample. In this study, acorns damaged by two insects or insect groups simultaneously were counted twice. To assess whether mast seeding in Q. variabilis and Q. serrata would be an effective strategy against generalist acorn-feeding insects, we calculated the rate of combined acorn predation as the total number of infested acorns divided by the sum of mature acorns of Q. variabilis and Q. serrata combined. Because the total basal area differed between the two oak species at both sites, we weighted the number of mature acorns of Q. variabilis by multiplying the number of mature acorns of this species by the ratio of total basal area of Q. variabilis to that of Q. serrata at each study site (see Table 1). To measure the magnitude of the annual variation in acorn production, we used the coefficient of variation (CV) as an index of masting (Silvertown 1980; Kelly 1994), with our calculation based on the mean number of mature acorns for all years. The acorn predation rate was arcsine-transformed and the number of mature acorns produced was log-transformed before statistical analysis. All statistical tests were performed using version 11.5 of the SPSS software (SPSS 2002).
Results
Annual fluctuations in acorn production
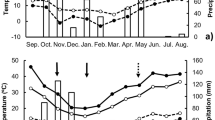
The annual variation in production of mature acorns by Q. variabilis was noticeable (Fig. 1), with a coefficient of variation (CV) of 1.05 at site A and 0.80 at site B. Large numbers of Q. variabilis acorns were produced in 1998 at site A and in both 1997 and 2000 at site B (Fig. 1). The annual fluctuation in acorn production was not synchronized between the two sites (r = −0.352, P = 0.648).
Quercus serrata produced no mature acorns in 1997 at either site, but acorn production increased thereafter, except in 2000 at site A (Fig. 2). The CVs for the annual variation in Q. serrata acorn production were 0.87 at site A and 0.73 at site B. The annual fluctuation in acorn production was synchronized between two sites (r = 0.994, P = 0.006).
There was no synchrony in the annual acorn production between Q. variabilis and Q. serrata (r = −0.400, P = 0.600 at site A; r = −0.472, P = 0.528 at site B). The CVs for the annual variation in their combined acorn production were 0.83 at sites A and 0.62 at site B. The annual fluctuation in the combined acorn production by the two tree species was synchronized between the two sites (r = 0.981, P = 0.019).
Yearly changes in insect damage
Predation of Q. variabilis acorns by Curculio weevils, with C. robustus (Coleoptera: Curculionidae) assumed to be dominant (Fukumoto and Kajimura 2001), ranged from 33.3 to 79.4% (Fig. 1). The rates of predation by Coccotrypes cardamomi (Coleoptera: Scolytidae) and tortricid moths, mainly Cydia glandicolana (Lepidoptera: Tortricidae) (Fukumoto and Kajimura 2001), were <40% at both sites. The rate of predation by each insect group was independent of the annual fluctuations in the production of mature acorns (Pearson’s correlation, P > 0.05).
Predation of Q. serrata acorns by Curculio weevils, with C. sikkimensis (Coleoptera: Curculionidae) assumed to be dominant (Fukumoto and Kajimura 2001), ranged from 3.2 to 10.2% (Fig. 2). The rates of predation by tortricid moths, mainly C. glandicolana (Fukumoto and Kajimura 2001), increased throughout the study period at both sites, and reached levels >20% in 2000 (Fig. 2). The rates of predation by C. cardamomi and Mechoris ursulus (Coleoptera: Attelabidae) were both <15% throughout the study period at both sites. The rate of predation by each insect group was independent of the annual fluctuation in the production of mature acorns (Pearson’s correlation, P > 0.05).
Of the insects identified at our study sites, C. robustus was specific to Q. variabilis, and C. sikkimensis and M. ursulus were specific to Q. serrata (Fukumoto and Kajimura 1999, 2001). On the other hand, C. glandicolana and C. cardamomi were known to be generalists at our study sites (Fukumoto and Kajimura 1999, 2001). Among the generalist predators, the rates of predation by tortricid moths and C. cardamomi on the combined acorn production of both tree species ranged from 4.7 to 22.6% and from 0 to 13.4%, respectively (Fig. 3). The rates of predation by tortricid moths and C. cardamomi were independent of the annual fluctuation in the combined acorn production (Pearson’s correlation, P > 0.05).
Discussion
Asynchrony of the annual fluctuation of acorn production between Q. variabilis and Q. serrata
Large fluctuations in acorn production among years occur in the genus Quercus (Sork et al. 1993; Koenig et al. 1994; Crawley and Long 1995; Maeto and Ozaki 2003; Espelta et al. 2008). The predator-satiation hypothesis, a powerful hypothesis for explaining the selective advantages of mast seeding, proposes that mast seeding is an adaptation against predators: satiating predators by producing more seeds than they can consume during occasional mast years ensures that some seeds will survive, whereas starving predators by producing fewer seeds than the predator population requires for a high survival rate in the intervening periods prevents predator populations from being sustained at high levels (Janzen 1971; Silvertown 1980). Synchronous acorn production between populations of sympatric species sharing the same seed predators would, in theory, offer the advantage of increasing the likelihood of predator satiation (Janzen 1971; Silvertown 1980). However, we observed no annual synchrony in acorn production between Q. variabilis (a 2-year species) and Q. serrata (a 1-year species) at our study sites (Fig. 3). Sork et al. (1993) and Liebhold et al. (2004) also reported asynchronous acorn production between 1-year species and 2-year species of North American oaks that shared the same acorn predators. One probable explanation for asynchronous acorn production between 1-year and 2-year species relates to differences in their endogenous reproductive dynamics (i.e., differences in the duration of their reproductive cycle). Sork et al. (1993) also clarified that past acorn production had a major impact on the size of the current acorn crop because oak species have an inherent reproductive cycle. For example, Quercus alba (a 1-year species) had a 4-year cycle, Quercus velutina (a 2-year species) had a 2-year cycle, and Quercus rubra (a 2-year species) had a 4-year cycle. To clarify the masting frequencies of Q. variabilis and Q. serrata in our study area, we should collect a longer time series of data on annual acorn production of these species.
Resource utilization by acorn-feeding insects in relation to their biology
In our study sites, M. ursulus was a specialist predator of the acorns of Q. serrata (Fig. 2). Acorn damage by M. ursulus in 1998 was low at both sites. Since the low production of Q. serrata acorns in 1997 would have starved some of the M. ursulus that would otherwise have emerged in 1998 (Fukumoto and Kajimura 2001), the population of M. ursulus was greatly reduced in 1998. Thus, mast seeding by Q. serrata would be an effective means of controlling M. ursulus populations.
In contrast, the population of Curculio weevils on Q. serrata did not decrease in 1998, although the dominant species (C. sikkimensis) was a specialist on Q. serrata in our study sites (Fukumoto and Kajimura 2001). Since not a few individuals of Curculio weevils spend two or more winters in diapause before emergence (Menu 1993; Menu and Debouzie 1993; Menu et al. 2000; Maeto and Ozaki 2003), prolonged diapause would allow some Curculio weevils to avoid starvation in years with poor acorn production, thus stabilizing the weevil population.
As most tortricid moths do not exhibit prolonged diapause (Fukumoto and Kajimura 2001; Maeto and Ozaki 2003), starvation would occur in years with poor acorn production. However, there was no synchrony in annual acorn production between Q. variabilis and Q. serrata at either of our study sites, and the CVs for the combined acorn production of the two Quercus species were lower than those for the individual Quercus species. Thus, asynchronous acorn production of these sympatric species would tend to stabilize populations of generalist insects such as the tortricids.
The rate of predation by C. cardamomi was also independent of the annual fluctuation in total acorn production of the two tree species (Fig. 3). The rates of predation by this beetle in 1997 and 1998 were extremely low, even though this species is a generalist acorn predator. C. cardamomi also attacks post-dispersal Quercus acorns (Ueda 1995) and has a wide range of alternative host plant species, such as bracken fern (Pteridium aquilinum L.) (Gray 1972), the crown of strawberry, and the bark of Japanese red pine (Nobuchi 1981). Ueda (1995) also reported that C. cardamomi could produce two generations within a year. Thus, predation patterns by C. cardamomi could not be explained by annual fluctuations in production of mature acorns, since the presence of alternative food resources would also affect the dynamics of C. cardamomi populations.
References
Crawley MJ, Long CR (1995) Alternate bearing, predator satiation and seedling recruitment in Quercus robur L. J Ecol 83:683–696
Espelta JM, Cortés P, Molowny-Horas R, Sánchez-Humanes B, Retana J (2008) Masting mediated by summer drought reduces acorn predation in Mediterranean oak forests. Ecology 89:805–817
Fujii S (1993) Studies on acorn production and seed predation in Quercus serrata—growth, falling phenology, estimation of production, and insect seed predators. Bull Osaka Mus Nat Hist 47:1–17
Fukumoto H, Kajimura H (1999) Seed-insect fauna of pre-dispersal acorns and acorn seasonal fall patterns of Quercus variabilis and Q. serrata in central Japan. Entomol Sci 2:197–203
Fukumoto H, Kajimura H (2001) Guild structures of seed insects in relation to acorn development in two oak species. Ecol Res 16:145–155
Fukumoto H, Kajimura H (2005) Cumulative effects of mortality factors on reproductive output in two co-occurring Quercus species: which mortality factors most strongly reduce reproductive potential? Can J Bot 83:1151–1158
Gray B (1972) Observations on Poecilips cardamomi (Schaufuss) the second species of Scolytidae to be found in bracken fern (Col.). Entomol Tidskr 93:229–237
Janzen DH (1971) Seed predation by animals. Annu Rev Ecol Syst 2:465–492
Kelly D (1994) The evolutionary ecology of mast seeding. Trends Ecol Evol 9:465–470
Kelly D, Koenig WD, Liebhold AM (2008) An intercontinental comparison of the dynamics behavior of mast seeding communities. Popul Ecol 50:329–342
Koenig WD, Mumme RL, Carmen WJ, Stanback MT (1994) Acorn production by oaks in central coastal California: variation within and among years. Ecology 75:99–109
Liebhold AM, Sork VL, Peltonen M, Koenig WD, Bjørnstad ON, Westfall R, Elkinton J, Knops JMH (2004) Within-population spatial synchrony in mast seeding of North American oaks. Oikos 104:156–164
Maeto K (1993) Acorn insects of Quercus mongolica var. grosseserrata in Hitsujigaoka natural forest, Hokkaido—life history of the principal species and their impacts on seed viability. Trans Annu Meet Hokkaido Br Jpn For Soc 41:88–90
Maeto K, Ozaki K (2003) Prolonged diapause of specialist seed-feeders makes predator satiation unstable in masting of Quercus crispula. Oecologia 137:392–398
Matsuda K (1982) Studies on the early phase of the regeneration of a konara oak (Quercus serrata Thunb.) secondary forest 1. Development and premature abscissions of konara oak acorns. Jpn J Ecol 32:293–302
Menu F (1993) Strategies of emergence in the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 96:383–390
Menu F, Debouzie D (1993) Coin-flipping plasticity and prolonged diapause in insects: example of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 93:367–373
Menu F, Roebuck JP, Viala M (2000) Bet-hedging diapause strategies in stochastic environments. Am Nat 155:724–734
National Astronomical Observatory (2001) Chronological Scientific Tables. Maruzen, Tokyo
Nobuchi A (1981) Poecilips cardamomi (Schaufuss) (Coleoptera, Scolytidae) injurious to crown of strawberry and chestnut (Studies on Scolytidae XXIV). Jpn J Appl Entomol Zool 25:294–296
Pulido FJ, Díaz M (2005) Regeneration of Mediterranean oak: a whole-cycle approach. Ecoscience 12:92–102
Shibata M, Tanaka H, Iida S, Abe S, Masaki T, Niiyama K, Nakashizuka T (2002) Synchronized annual seed production by 16 principal tree species in a temperate deciduous forest, Japan. Ecology 83:1727–1742
Silvertown JW (1980) The evolutionary ecology of mast seeding in trees. Biol J Linn Soc 14:235–250
Sork VL, Bramble J, Sexton O (1993) Ecology of mast-fruiting in three species of North American deciduous oaks. Ecology 74:528–541
SPSS (2002) SPSS for Windows, release 11.5J [computer program]. SPSS, Chicago
Ueda A (1995) Field study of two spermathophagous scolytid beetles, Coccotrypes graniceps Eichhoff and C. cardamomi (Schaufuss), in acorns of Quercus myrsinaefolia Blume. In: Hain FP, Salom SM, Ravlin WF, Payne TL, Raffa KF (eds) Behavior, population dynamics and control of forest insects. The Ohio State University, Wooster, pp 610–626
Ueda A (2000) Pre- and post-dispersal damage to the acorns of two oak species (Quercus serrata Thunb. and Q. mongolica Fischer) in a species-rich deciduous forest. J For Res 5:169–174
Ueda A, Osumi K (2003) Insect attack on acorns of 4 deciduous oaks, Quercus serrata, Q. aliena, Q. acutissima, and Q. variabilis, at the same site. Appl For Sci 12:129–135
Acknowledgments
We thank the members of the Laboratory of Forest Protection, Nagoya University, for their helpful suggestions and for their assistance during the field surveys. This study was supported in part by a Grant-in-Aid for Scientific Research from JSPS (KAKENHI) (No. 20405025), and by funding from the Inamori Foundation in 2005, the IFO (Institute for Fermentation, Osaka) Foundation in 2007, and the Shouwahoukoukai (Ito Chube’e) Foundation in 2008.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fukumoto, H., Kajimura, H. Effects of asynchronous acorn production by co-occurring Quercus trees on resource utilization by acorn-feeding insects. J For Res 16, 62–67 (2011). https://doi.org/10.1007/s10310-010-0208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-010-0208-7