Abstract
Cases of mast seeding, involving highly variable seed production synchronized over large geographic areas, provide dramatic examples of resource pulses that have been documented for most major world land masses. Here, we compare the dynamic behavior of two of these systems, with the goal of understanding differences in the long-term consequences of masting events to their respective communities. Responses to mast events in deciduous oak forests in eastern North America are characteristically complex and of low resilience. That is, each event produces long-lasting cascading effects in the community, ultimately influencing not only seed consumers such as rodents and deer, but also the parasites and prey of those consumers, diseases transmitted by those parasites, and outbreaks of insect herbivores. In contrast, despite more extreme resource pulsing in New Zealand Nothofagus forests, responses to mast events there are less complex and more resilient, i.e., producing only short-term (<2 years), smoothly damped, numerical responses by a few species, except after (rare) ‘double-mast’ events in consecutive years. A detailed examination of the two systems suggests some tentative explanations for the strongly contrasting dynamics of these systems. Firstly, the higher number of species involved in North America seems to reduce resilience by increasing food chain length, lags, and alternative prey, all of which increase the dynamic complexity compared with that in New Zealand. Secondly, lack of a shared evolutionary history among species did not necessarily reduce resilience. Some exotic species showed well-damped fluctuations (e.g., stoats (Mustela erminea L.) in New Zealand), while other exotics showed complex dynamics (e.g., gypsy moths (Lymantria dispar L.) in North America). Thirdly, recent extinctions of species such as the passenger pigeon (Ectopistes migratorius L.), once the dominant acorn predator in eastern North American forests, have likely produced qualitative changes in system dynamics in both communities. Fourthly, the North American community has more lags and hysteresis, which probably contribute to the greater dynamic complexity in eastern North America than in New Zealand Nothofagus forests. However, positive feedback loops present in North America seem to have little influence on system dynamics. Because the massive perturbations induced by masting events are major recurring challenges to an ecosystem, disentangling the causes of different system responses is likely to lead us to a better understanding of ecosystem function, resilience and stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast seeding, the phenomenon describing highly variable seed production by a geographically definable population of plants, provides a dramatic example of a pulsed resource available only ephemerally and, in most cases, quite irregularly. Entire forests can be swamped with seeds in ‘mast’ years, contrasting dramatically with a nearly total absence of seeds in some other years. The ecological and evolutionary causes of this phenomenon are complex and not entirely understood (Kelly and Sork 2002). One factor shown to select for masting behavior is ‘predator satiation’: infrequent large crops satiate seed eaters, so some seeds survive uneaten. In the intervening years, smaller or no crops keep seed predator populations low, reducing the numbers of animals available to eat all the seeds produced in a subsequent mast year (Janzen 1971; Silvertown 1980). A corollary of the predator satiation hypothesis is that seed predator populations are expected to vary considerably with the annual seed crop, opening up the potential for ‘trophic cascades’ involving both ‘top-down’ and ‘bottom-up’ influences involving multiple trophic levels (Ostfeld and Keesing 2000).
Mast seeding is taxonomically and geographically widespread (Kelly and Sork 2002). However, the trophic consequences of mast seeding have, thus far, been comprehensively studied in only a few systems. Here, we compare the dynamic behavior of two mast seeding systems with the goal of understanding differences in the long-term consequences of masting events to their respective communities.
In making this comparison, we start from several hypotheses about the nature of interactions and their likely effects on system stability (Table 1). We consider these systems to be more stable when they exhibit greater resilience, i.e., a rapid and smoothly damped return to previous conditions following a large pulse of resources (Nakajima 1992; Grimm and Wissel 1997). In contrast, we define less stable systems to be those that show low resilience: slow return, overshoots, hysteresis, alternative stable states, cyclic or chaotic behavior. Our four starting hypotheses are based, in part, on systems theory (Holling 1973). (1) Systems with fewer species will be more resilient (van Nes and Scheffer 2004). With masting we expect more resilience with fewer species, because, with shorter food chains, pulses of resources will move through the entire system more rapidly. Increased numbers of species also increase the chances of having some species that are food-limited, which reduces resilience (Holt 2008) and increases the numbers of alternative prey, which are susceptible to lagged impacts (Schmidt and Ostfeld 2008). (2) Systems with co-evolved species, such as specialized seed predators, will be more resilient, because the species are more likely to have adaptations to cope with the variation in resources, such as food storage systems (Holt 2008). (3) Predators that are highly mobile and move among communities will make systems less resilient, because the composition of the local community species may vary dramatically from high-seed years to low-seed years (Koenig et al. 2003) and dispersal spreads the indirect effects of the seed pulse out beyond the boundaries of the pulse itself (Ostfeld and Keesing 2000; Holt 2008), [although Holt (2008) recently argued that high mobility could also increase resilience by providing de facto refuges for prey]. (4) The presence of lags, positive feedback loops and hysteresis will all be expected to create more complex, less stable, dynamics (Holling 1973; van Nes and Scheffer 2004; Holt 2008).
Clearly, with only two systems, we cannot empirically settle all these questions. However, like Holt (2008), we seek to identify conceptual insights from the scattered literature, in the hope of guiding future studies of other systems. The pulsed resources provided by mast fruiting determine system dynamics in a variety of other systems worldwide. Notable among these are South American temperate forests, where rodent outbreaks (ratadas) follow bamboo flowering blooms over relatively large geographic areas (Murua et al. 1996; Jaksic and Lima 2003); Indonesian dipterocarp forests, in which 20 or more species of Dipterocarpaceae mast synchronously over large geographic areas, attracting large numbers of nomadic vertebrate predators (Curran et al. 1999; Curran and Leighton 2000); rowan stands in Norway, where variable seed crops suffer predation by apple fruit moths, which are, in turn, attacked by a parasitoid wasp (Satake et al. 2004); and California oak woodlands, where masting provides pulsed resources important to a variety of wildlife (Koenig et al. 1994). Unfortunately, relatively little is yet known in many of these systems about the lags, feedback loops, and potential for hysteresis that we call attention to in this paper.
Eastern North American deciduous oak forests
North America: players
Broadleaf deciduous forests cover, or have covered in recent times, vast areas of the temperate world, but they were, perhaps, most extensive in Eastern North America prior to European colonization. The climate in this region is relatively mesic, and forests are composed of a diverse mixture of over 100 species of trees, including over 25 species of oaks (Quercus spp.) (McWilliams et al. 2002), a northern hemisphere Gondwanan genus found in North America, Europe, and Asia. Oaks have dominated the eastern North American landscape throughout much of the past 10,000 years (Watts 1979), despite fluctuating abundance due to varying climatic conditions, frequent fires caused by indigenous peoples (Abrams 1992), and other changes in disturbance regimes affecting both oaks in general and the geographic ecology of individual oak species.
The key pulsed event in this system is a highly variable seed crop, generated primarily by masting events in oaks but also by other genera of seed-bearing trees (Ostfeld and Keesing 2000). Although the spatial synchrony of seed production in eastern deciduous forests has seldom been examined, data from the literature and work on California oaks suggest that geographical synchrony within species is probably considerable, on the order of at least several hundred kilometers (Koenig et al. 1999; Koenig and Knops 2000). Synchrony among species, however, is probably variable, mainly because there is widespread sympatry in North America between species requiring different numbers of years to mature acorns (Koenig and Knops 1997, see below). Acorns themselves are relatively large, with dry masses of up to several grams. They often have relatively high lipid content, making them energy rich, desirable, food resources for a wide range of vertebrate and invertebrate predators, even though they are relatively low in protein and heavily infused with defensive phenolic chemicals, primarily tannins.
Key animals in this system include three major native mammalian predators of acorns—the white-footed mouse (Peromyscus leucopus [Rafinesque]), the eastern chipmunk (Tamias striatus L.), and the white-tailed deer (Odocoileus virginianus [Zimmermann]). A former major player in this system, now extinct, is the passenger pigeon (Ectopistes migratorius), while an important new invertebrate herbivore, the gypsy moth (Lymantria dispar), was introduced from Europe in the late 1860s. Other species which are also likely to be influenced significantly by the acorn crop include tree squirrels (Sciurus spp.) and insect predators such as filbert weevils (Curculio spp.), but these have not been studied in detail and have yet to be incorporated into the models exploring the interactions contributing to this system.
North America: drivers
In present-day oak ecosystems the thorough consumption of the seed crop in all but particularly large mast years by an impressive diversity of insects and vertebrates suggests that predator satiation is a primary driver of masting in this system. Although the predator satiation hypothesis has yet to be critically tested, it seems reasonable to predict that it was even more important in pre-settlement times, when huge flocks of nomadic passenger pigeons concentrated in areas of mast abundance (Schorger 1955; Blockstein and Tordoff 1985). However, there is at least one strong argument against the notion that predator satiation is the only factor driving masting patterns in North American oak communities: the sympatric distribution of ‘1-year’ and ‘2-year’ species of oaks.
American oaks belong to two major groups, the ‘white’ oaks (subgenus Quercus) and the ‘red’ oaks (subgenus Erythrobalanus). With few exceptions, white oaks mature acorns in a single season (hence ‘1-year’ species), while red oaks require two seasons to mature acorns (‘2-year’ species). Unlike Northern Europe, where the native oak species are all in the white oak subgroup, both white and red oaks, and thus both 1-year and 2-year species, are well represented in eastern North American hardwood forests and generally elsewhere in North America. Both types can exhibit some degree of periodicity (Abrahamson and Layne 2002), with 1-year species often exhibiting strong negative temporal autocorrelations between acorn crops at 1-year time lags, and hence short cycles of two or sometimes 3 years. In contrast, 2-year species can show even stronger negative lags but at longer time intervals, so they tend to have longer cycles. In any case, acorn crops of the two types of oaks are generally asynchronous, because both depend on similar environmental conditions during flowering, but in red oaks the subsequent lag in acorn maturation is longer (Sork et al. 1993; Koenig and Knops 1997). As a result, variability in total mast production is generally lower in North American forests than it would be otherwise (Liebhold et al. 2004).
Such asynchrony contrasts with the high interspecific synchrony in seed production observed among Dipterocarp communities in southeast Asia (Curran et al. 1999) and in masting plants of several families in New Zealand (Schauber et al. 2002), which are all 1-year species synchronized by environmental factors. This asynchrony in the oak system caused by the different maturation rates of acorns in the two subgenera reduces the effectiveness of predator satiation, which would be far more extreme if all species in the community were to produce acorns synchronously. The fact that some of the more important seed predators in oak ecosystems are also major seed dispersal agents may be the primary selective force limiting the overall advantages of synchronous seed production throughout the community.
In the deciduous forests of eastern North America, the proximate environmental drivers of masting in oaks are generally fairly complex. They apparently include climatic factors, dating from the previous spring, summer, and even, at least in the case of 2-year species, the spring of 2 years before (Sork et al. 1993). As in the Nothofagus species discussed below, temporal lag effects demonstrate that resources have to be stored from one year to the next in order to produce large mast crops (Sork et al. 1993; Koenig et al. 1994).
North America: interactions
Recent work has documented some of the more notable present-day trophic effects of large acorn crops in this biome (Ostfeld et al. 1996; Wolff 1996; Jones et al. 1998; McShea 2000; Ostfeld and Keesing 2000; Schmidt and Ostfeld 2003, 2008; Wang et al. 2008) (Fig. 1). There is a strong positive correlation between the acorn crop and rodent densities. There is no suggestion that the rodents are partly responding to an increase in invertebrates, such as caterpillars, feeding on the mast crop, in contrast to the New Zealand system (see below). The correlations between acorn crops and deer densities are less clear, presumably because, in poor acorn years, they can move long distances to find habitats that are not oak dominated (McShea and Schwede 1993).
Interactions among taxa in the eastern North American oak system. Arrows represent directions of the predominant influence between pairs of units. Plus symbols indicate that an increase of the donor level results in an increase of the recipient level; minus symbols indicate a decrease in the recipient level. Dashed lines correspond to feedback loops that may have minor effects in the short-to-medium term (see text). Modified after Ostfeld et al. (1996) and Schmidt and Ostfeld (2008)
In the oak forests of the eastern USA the ecological consequences of these responses are complex and felt in almost all parts of the community. Numerical changes in rodent densities, and habitat shifts by deer, influence the density, distribution, and prevalence of disease in deer ticks (Ixodes scapularis Say), the primary vector of Lyme disease, thereby influencing human infection rates (Ostfeld et al. 1996). Masting events also correlate significantly with increased nest predation rates and reduced numbers of several songbird species susceptible to depredation by rodents, particularly understory birds such as worm-eating warblers (Helmitheros vermivorus [Gmelin]) and hooded warblers (Wilsonia citrina [Boddaert]) (McShea 2000). Schmidt and Ostfeld (2003, 2008) confirm these correlations for several species of thrushes and describe a series of pulsed events set into motion by the variable acorn crop. The numerical response of rodents is followed by a pulse of generalized predators, such as owls, which respond both numerically and sometimes behaviorally through dietary shifts to take advantage of the greater availability of rodent prey.
One of the best-studied interactions in this system involves the introduced gypsy moth (Fig. 2), a species causing widespread defoliation of oaks. In some cases this defoliation leads to catastrophic oak mortality, although, at the regional scale, there have been only relatively minor changes in forest composition (Gansner et al. 1983). When the density of gypsy moth larvae and pupae is low, predation by small mammals causes mortality sufficient to determine whether regional moth populations remain low or erupt to high densities (Campbell and Sloan 1977; Elkinton and Liebhold 1990). Gypsy moth populations increase somewhat synchronously to outbreak levels at roughly decadal intervals, often just after mast failures, which causes declines in gypsy moth predators (Elkinton and Liebhold 1990; Jones et al. 1998; Liebhold et al. 2000) (Fig. 2).
Time-course of community dynamics in the eastern North American system. Note the long x-axis range (cf. Fig. 4) and that abundances of understory birds (worm-eating and hooded warblers) and gypsy moths in panel b are on a log scale, while red oak seedfall and white-footed mice in panel a are on a linear one. For sources, see the text
In its native range in both Europe (Gschwantner et al. 2002) and Asia (Liebhold et al. 1998), the dynamics of the gypsy moth are also influenced by small mammal predators. The quasi-periodic oscillations of either 5-year or 10-year periods (Johnson et al. 2005) in natural gypsy moth populations are similar to those observed in North America (Johnson et al. 2006). These similarities raise the question of whether decadal regional outbreaks of gypsy moths are driven by direct trophic interactions with natural enemies or result indirectly from mast events. The obvious hypothesis, that the numerical relationships between gypsy moths, mice, and mast observed within the gypsy moth’s exotic range in North America (Elkinton et al. 1996; Jones et al. 1998) also dominate the dynamics of this insect in its native ranges in Europe and Asia, cannot be tested at this time, because there are no detailed analyses available from the gypsy moth’s native range.
The converse of the interactions introduced into a system by exotic species such as the gypsy moth are those that have disappeared through extinctions of key species. Within the forest itself, the American chestnut (Castanea dentata [(Marsh.) Borkh.]) has been virtually eliminated from North America by the chestnut blight (caused by the alien fungus species Endothia parasitica [Murrill]) during the mid-1900s. Chestnuts, which tend to produce more constant seed crops from year to year than do oaks (Diamond and Giles 2000), were originally a dominant species in these forests, and their decline has been followed by a general increase in oaks (Vandermast and Van Lear 2002) and, thus, quite possibly with a concomitant increase in masting and magnitude of resource pulses in the eastern North America biome.
An even more dramatic change has been the extinction, during the latter part of the nineteenth century, of the passenger pigeon, formerly the world’s most abundant bird and an acorn pulse specialist. This species wandered widely in search of large acorn crops and settled in vast numbers in deciduous forests during masting events. How the elimination of this key species has altered disturbance regimes in these forests is difficult to reconstruct, but the changes have almost certainly been significant (Blockstein and Tordoff 1985; Ellsworth and McComb 2003). For example, Blockstein (1998) proposed that the considerable numerical increases in rodents, and the current severity of Lyme disease, that go along with the large pulses of resources produced by masting events today, might have been much reduced when passenger pigeons were present to eat a large proportion of the acorn crop before the acorns fell to the ground and became available to the mice.
There are also significant nutritional differences among species of acorns that may be important in this and other systems. In particular, ‘red’ oak acorns generally have much higher lipid content than ‘white’ oak acorns (e.g., Koenig and Benedict 2002). White oak acorns also tend to sprout earlier, often in the fall rather than the following spring. Both these features make red oak acorns potentially more valuable for overwintering populations of consumers, a conclusion supported by recent work indicating that red oak seed pulses may, in some cases, exert a much greater influence on populations of rodents than white oak seed pulses (Wolff 1996; Shimada and Saitoh 2006).
North America: lags
In comparison with the New Zealand system (below), lags in the eastern North American forests are relatively uncomplicated, but they span more years. The ‘trophic cascades’ initiated by a large acorn crop in the fall of year x require at least 2 years to ripple through the system. White-footed mice depend upon mast as a source of over wintering food, but winter survival affects their numbers during the spring and summer of year x + 1, during which time they depredate birds’ nests, subsequently deceasing avian populations the following year (year x + 2) (McShea 2000). These cycles in mice numbers seem particularly pronounced in more northerly regions such as Maine (Wang et al. 2008). The multi-year life cycle of the ticks causes the lag in Lyme disease risk, which peaks in year x + 2. Once released by declines in mouse populations, gypsy moth populations may not reach peak densities until several years later.
The effects of these trophic cascades are dampened to some extent by strong inverse temporal autocorrelation between acorn crops (Sork et al. 1993; Koenig et al. 1994). That is, a relatively good crop in one year tends to be followed by a relatively poor crop the next. Consequently, back-to-back years of good crops are uncommon, and the positive effects of a good acorn crop are generally countered by the negative effects of the poorer crop that follows. This is less true of red oaks, which generally have a less pronounced 2-year on-off cycle than white oaks.
North America: feedback loops and hysteresis
The long (5- or 10-year) cycles of gypsy moths comprise a major hysteresis loop producing changes in gypsy moth numbers between two alternative states (Elkinton and Liebhold 1990; Liebhold et al. 2000). In the first (low-density) state, gypsy moths are maintained at low densities by intense predation on pupae by white-footed mice. The system flips to the second (high-density) state at irregular intervals, after an acorn crop failure reduces the density of over-wintering mice, as described above. Once moths reach high densities, their dynamics are no longer affected by mice, even after masts resume and mouse densities recover, so this state can persist for some years and involve widespread canopy tree defoliation. The system typically flips back to the first stable state when an epizootic spreads through the high-density gypsy moth population, causing high rates of mortality.
This hysteresis loop thus tends to destabilize the system, because the irregular gypsy moth outbreaks, once triggered, can last for several years. However, the hysteresis simultaneously serves to reduce the importance of a positive feedback loop that begins when abundant numbers of gypsy moths defoliate trees, including oaks, reducing future acorn crops and likely future white-footed mice predation on moth pupae. This positive feedback could be far more destabilizing, but it does not, in practice, extend the gypsy moth outbreak, because once gypsy moths reach high densities they are no longer regulated by mice, as explained above.
Ostfeld et al. (1996) hypothesized several feedback loops, including a potentially negative influence of gypsy moths on acorn production, an effect that in the extreme may influence species composition of the forest. Thus, the loop in this case runs from acorns to mice to gypsy moths to acorn production to oak regeneration and, ultimately, to forest composition. There is evidence that gypsy moths raise oak mortality rates and increase regeneration of other hardwoods (Ostfeld et al. 1996), but other factors probably have greater effects on forest composition (see below).
A second possible feedback loop runs from acorns to seed predators (mice, deer, and others) to the survival of the seeds of other species with effects on forest composition. The idea is that high mouse populations could entirely wipe out some seed crops, induce periodic failure in recruitment of some of the non-oak seedlings, and, thereby, affect species composition of the forest understory (Ostfeld et al. 1994). Impacts on alternative prey in general are reviewed in Schmidt and Ostfeld (2008).
The extent to which either of these latter feedback loops significantly influence forest structure in the long-term remains to be determined. In the case of the second loop, running through the seed predators affecting tree regeneration, the negative effects, to the extent they exist, will be expressed only decades later in differences in the regeneration patterns of the various species of forest trees. Hence, this interaction is unlikely to qualify as a true short-term dynamic feedback loop. The loop running through gypsy moths is potentially short-term, but, even when moth populations are high, severe defoliation is typically observed in only a minority of oak stands in a region. Thus, although gypsy moth outbreaks may influence acorn production and (ultimately) regeneration patterns, they probably constitute a relatively small disturbance over a large area. By contrast, browsing by the relatively high numbers of deer now common through most of the region is considered to be a much more important factor limiting oak regeneration (Abrams 1992).
North America: conclusion
There are several striking features about the eastern North American oak forest system. First, it comprises a complex community of masting species that are, to some extent, asynchronized with one another. Nonetheless, the species-specific cycles coincide sufficiently to produce large pulses of resources that instigate an impressive series of trophic effects. Once perturbed by a seed pulse, the system is relatively chaotic and involves hysteresis, lags, and feedback loops that generally destabilize the whole forest ecosystem. That is, the combined effects require several years to pass through the system, and the consequences of the resource pulses are not always predictably the same in different parts of the system.
This general conclusion carries a major caveat, however: the system observed today is very different from the one that was found in this region 1,000 years ago. Several key players have been lost, including a dominant tree species (the American chestnut), whose loss may have increased the inter-annual variability of seeds across all tree species combined, and a major consumer (the passenger pigeon), whose presence may have significantly altered the availability of pulsed resources (Bucher 1992) and, thus, the degree to which they currently influence remaining consumers such as white-footed mice. At least two other species (wild turkeys Meleagris galapavo L. and black bears Ursus americancus [Pallas]) are probably much scarcer than in pre-European times, whereas white-tailed deer are probably now at much higher densities than during pre-settlement times (McShea 2000). Hence, the importance of these three species in this system may now be very different. Furthermore, one of the players currently responsible for the most complex dynamics (the gypsy moth) is exotic, and probably none of the native lepidopteran species played a similar role prior to the arrival of gypsy moths 140 years ago. Unfortunately, there is no way to know what the dynamics of this system looked like prior to these events. It is a reasonable hypothesis, however, that at least part of the dynamic instability of the system is driven by the loss of native species and introduction of exotic species that are now such key destabilizing influences in this system.
New Zealand evergreen Nothofagus forests
New Zealand: players
Evergreen forests dominated by Nothofagus spp. (southern beeches, Fagaceae) are the most widespread forest type in New Zealand: pure beech forest (most of which is on the cooler South Island) makes up 46% of native forest cover nationally, and mixed Nothofagus–podocarp–angiosperm forest contributes another 22% (Wardle 1984). The four species of Nothofagus in New Zealand can be considered functionally interchangeable with respect to masting community dynamics. All four show strong mast seeding, are in synchrony at scales up to several hundreds of kilometers (Schauber et al. 2002), produce relatively small seeds (3–8 mg) two to three orders of magnitude smaller than most acorns in North American forests, and are associated with irruptions of introduced rodents.
The community dynamics outlined here are best known and most pronounced in South Island pure Nothofagus (monogeneric) forests, where most of the work reported here has been carried out. In general, these forests are old-growth, with little direct disturbance to the trees by humans, who arrived in New Zealand only around 1280 AD (Hogg et al. 2003; Wilmshurst et al. 2008). However, indirect human impacts are considerable, primarily via introduced mammals.
New Zealand has no native terrestrial mammals, but 32 introduced mammal species are now established, of which three are particularly important in Nothofagus forests (King 2005; Ruscoe et al. 2006). The house mouse (Mus musculus L.) is distributed throughout both North and South Islands, both in commensal and non-commensal habitats, and is the only small (<50 g) rodent in pure Nothofagus forest. The main predator of mice in these forests, and the most important mammalian carnivore, is the stoat (Mustela erminea). Following mast events, mice undergo irruptions, followed a few months later by increases in the stoats that prey upon them. The ship rat (Rattus rattus L.) is very common in non-beech forests in other parts of New Zealand (King et al. 1996), but it is normally scarce or absent in pure Nothofagus forests, except following mast years.
Masting in the Nothofagus community affects four native hole-nesting birds: three parrots, the kaka Nestor meridionalis [Gmelin], yellow-crowned parakeet Cyanoramphus auriceps [Kuhl], and orange-fronted parakeet C. malherbi [Souancé], and an endemic passerine, the mohua or yellowhead (Mohoua ochrocephala [Gmelin]). All four feed heavily on Nothofagus seed during mast years, and all are declining and considered threatened by predation, primarily from stoats (O’Donnell 1996b; Wilson et al. 1998; Dilks et al. 2003). Likewise, the threatened endemic long-tailed bat (Chalinolobus tuberculatus [Forster]) also roosts in holes and, while not feeding on Nothofagus seed, is affected by masting-related irruptions of stoats and rats that have caused its populations to decrease (Pryde et al. 2005).
It is possible that some of the many now extinct native birds fed heavily on Nothofagus mast crops, such as several of the smaller upland moa species and Finsch’s duck (Worthy and Holdaway 2002), along with the extant but critically endangered kakapo (Strigops habroptilus [Grey]). Unfortunately, most of them had disappeared before European colonization, so information about their diets and roles in Nothofagus forests is limited. Other species, such as native insectivorous birds and introduced deer, are unresponsive to masting—possibly, at least in the latter case, because of the small size of Nothofagus seeds.
In summary, the Nothofagus community has relatively few species that respond to the pulsed resources provided by a masting event, and the community is a recent assemblage without a long history of interaction among the presently resident species.
New Zealand: drivers
At the evolutionary level, individual Nothofagus trees gain substantial benefits from masting through more efficient wind pollination (Kelly et al. 2001). For example, in mountain beech at 1,340 m altitude at Craigieburn, if the plants did not mast but instead had constant flowering at the mean seed output each year, mean pollination levels would be around 18%, whereas masting increases this to over 40%.
The economies of scale conferred by wind pollination do not exclude the possibility that masting in Nothofagus was also selectively beneficial through predator satiation (Kelly et al. 2001; Koenig et al. 2003). Unfortunately, the extent of historical extinctions in the native avifauna makes it impossible to test whether there was, in fact, predator satiation of native seed predators in the past. All we can say is that, currently, masting efficiently satiates mice, which are voracious and highly efficient consumers of Nothofagus seeds (Choquenot and Ruscoe 2000; Ruscoe et al. 2005). Indeed, if Nothofagus did not mast, the introduction of mice could have severely affected tree regeneration.
The proximate climatic drivers for masting in Nothofagus are well understood (Schauber et al. 2002; Richardson et al. 2005; Monks and Kelly 2006). Generally, all Nothofagus species flower heavily in the early austral summer, 8 months after a warm late summer/early fall (January–April). There are also weaker effects of frosts and rainfall during the time of flowering/pollination, and important lag effects of previous seedfall, indicating that resources must be saved up before a massive seeding effort can be attempted.
New Zealand: interactions
The most important interactions in Nothofagus forest are summarized in Fig. 3. Note the lack of feedback loops, where a later species in the sequence can feed back to influence an earlier species (Ruscoe et al. 2006).
Interactions among taxa in Nothofagus masting communities. Arrows represent directions of the predominant influence between pairs of units. Plus symbols indicate that an increase of the donor level results in an increase of the recipient level; minus symbols indicate a decrease in the recipient level. Heavy lines represent the most important connections emphasized in this article; light lines represent other connections
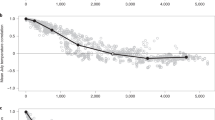
The normal course of a mast year is summarized in Fig. 4. The Nothofagus community is dynamically stable, characterized by rapid, smoothly dampened, short-lived, increases in seeds, mice, and stoats. In each case, densities usually decrease considerably within a year of their respective peaks (Wilson et al. 1998; King 2002; Fitzgerald et al. 2004; Purdey et al. 2004). Mice have a short lifespan and high reproductive rate, so they increase in numbers rapidly during a mast seedfall then decrease equally rapidly. During mast years in the South Island (but not in at least one forest in the North Island: Fitzgerald et al. 2004) they continue to breed into winter, and by the following spring (September) 6 months after seedfall, when the seeds that have not been depredated germinate, mice are abundant and breeding rapidly. Reproduction usually continues throughout the first summer after seedfall, but, after November, recruitment is extremely low (King 1982), and, by the end of the first summer (February), food shortage and recruitment failure have usually reduced densities back to pre-mast levels (Fig. 4). Stoats respond both functionally and numerically to the high numbers of mice in spring, and, in turn, stoats reach high densities in the summer following a mast year. Depredation by stoats may contribute to reduction of mouse numbers, but the evidence for this is mixed (Ruscoe et al. 2003; Kelly et al. 2005).
Time course of a single Nothofagus—house mice—stoat cycle. Note short x-axis range (cf. Fig. 2) and the log scale for seedfall. By two summers after the mast event (x + 2), the system is back to its pre-mast state (except for mohua). Units for house mice are animals caught per 100 kill-trap nights, for stoats, numbers caught/500 trap nights, and for mohua, arbitrary units (densities are continually declining in this species). Based on Wardle (1984) for seed, King (2002) for mice and stoats, and O’Donnell (1996a) for mohua
Hence, within 1 to 2 years the system returns largely to its pre-mast state, with two exceptions. The first exception is native hole-nesting birds. Over at least the past 20 years, two species (mohua and orange-fronted parakeets) have been ratcheting downwards in density with each mast/stoat cycle, with the result that they are now at grave risk of global extinction (Elliott 1996; Elliott et al. 1996; O’Donnell 1996a, 1996b; Dilks et al. 2003). Yellow-crowned parakeets are still widespread, but their populations cycle widely (increasing during a beech mast year, then decreasing the following summer when stoat numbers peak), so that there is concern about their long-term persistence. Kaka are large long-lived parrots (~550 g, lifespan >20 years), but in these Nothofagus forests they are declining, to the extent that where stoats are present kaka are expected to become locally extinct without intensive management (Wilson et al. 1998). Native birds that are not hole-nesting also suffer heavier stoat predation during a mast/stoat cycle, as shown, for example, in bellbirds (Anthornis melanura [Sparrman]; Kelly et al. 2005), but apparently not to the extent that their populations are threatened.
The second element that does not show a predictable, rapid, return to pre-pulse state element is rats. Although ship rats are typically absent or very rare in pure Nothofagus forests, they sometimes do increase after mast seeding events. Over the past 30 years or so in beech (N. solandri [(Hook. f.) Oerst.]) forests on higher-altitude mountains, rats have been almost absent, regardless of masting events (King 1983; Kelly et al. 2005), whereas in mixed red (N. fusca [(Hook. f.) Oerst.])–silver (N. menziesii [(Hook. f.) Oerst.]) beech–podocarp forests at lower altitudes, ship rats increased sevenfold (to modest densities) after a moderate Nothofagus mast year in 1976 (King and Moller 1997) before decreasing again within a year. However, following an unusual Nothofagus double-mast event in 1999 and 2000 (back-to-back mast years), ship rats reached high densities in Nothofagus forests throughout the South Island for 2 years (Pryde et al. 2005), with severe consequences for native birds and bats, especially mohua (Dilks et al. 2003). While it is possible that, at some sites, stoat trapping to protect threatened birds assisted the increase in rats during the double-mast event, it was not the sole cause, because rats also increased at sites where there was no stoat trapping (Dilks et al. 2003).
New Zealand: lags
The fluctuations caused by pulsed inputs of Nothofagus seed are rapid and well dampened, probably because there are few lags in the system and also because at least three key species (mice, stoats, kaka) show anticipatory responses, in which species change their numbers or behavior before the large seed crop is available.
(1) Mice. Even though the beech seed is shed from the trees only in the fall (February–May), breeding in mice has already been accelerated during the previous spring (November–December; Fig. 4), because, in the spring of a mast year, large quantities of spent male flowers fall as litter (Alley et al. 2001; Fitzgerald et al. 2004). This litter supports increases in invertebrates such as litter-feeding caterpillars and predatory spiders, both of which are eaten by mice and may be as important an explanation for their irruptions as the seeds themselves (Murphy and Dowding 1995; Fitzgerald et al. 1996; Alley et al. 2001; Fitzgerald et al. 2004). As a result, the mice increase in numbers rapidly through the spring and summer, before a seedfall, in response to increased invertebrate food, as well as the autumn of the seedfall year itself, in response to seeds.
(2) Stoats. Although the breeding biology of stoats includes two factors that prevent an immediate response to increased food (fixed single-birth season, delayed implantation), the effect is countered by a third factor that permits high reproductive success by female stoats producing young conceived before a seedfall. The seasons of estrus and implantation in stoats are short and controlled by day length, so the animals are unable to breed over winter, even when food is abundant after a high-seed year. Moreover, stoats ovulate in late spring (November) but implantation of the blastocysts is delayed until the following late winter (July), so the maximum number of offspring following a mast year has already been set the year before (King et al. 2003). However, females always produce many more blastocysts (average 8–10, maximum 20) than they can rear in a poor season. Six months after a mast event, when food is abundant from elevated densities of mice and seed-feeding birds and juvenile survival from implantation to independence is good, very large litters of young stoats can be successfully weaned in early summer (mid-December). Consequently, although the increase of stoat numbers lags 6–9 months behind the mouse increase, stoat numbers then increase by an order of magnitude in one breeding season.
Lags in this system are further reduced as a consequence of the unusual relationship in New Zealand between stoat numbers, mouse numbers, and bird predation. The common northern hemisphere experience is that, when rodents (voles or lemmings) are abundant, the strong functional response by mustelids to rodents reduces predation on birds, and conversely, after the decline of a rodent population, prey-switching by mustelids causes high predation on birds no longer protected by the abundance of alternative prey (King and Powell 2007). Hence, the period of greatest risk to birds is after the rodents decline. The same does not happen in New Zealand, because the only rodents in Nothofagus forests are introduced feral house mice and ship rats, which almost never reach densities high enough to meet the nutritional needs of the large numbers of young stoats invading the forest after a seedfall (White and King 2006). On the contrary, in summer after the seedfall, New Zealand stoats show a large numerical response to the extra mice but inadequate prey-switching away from birds, so the greatest risk to birds is during the peaks in both rodents and stoats (in December, 9 months after seedfall; see Fig. 4) and is usually proportional to stoat density, regardless of the density of mice. The only exceptional cases found so far are the few very heavy masting events when mice do reach extremely (for New Zealand) high densities, at which time birds obtain temporary protection from predation until mice densities drop (White and King 2006).
Stoat densities also decrease rapidly. The cohort of juveniles produced in the spring following a mast seedfall are larger than usual (King 2002), but there is no ‘silver spoon effect’; that is, they do not benefit from their privileged upbringing during a time of abundant food. On the contrary, they have unusually high mortality rates through their first year (Powell and King 1997; Wittmer et al. 2007), and most survivors do not successfully breed the following summer (Fig. 4). Very high population growth rates in the seedfall years are matched by large decreases in the following 2 years (Wittmer et al. 2007). The result is that 20 months later, by the second summer after seedfall, stoat densities in areas not subject to control (Murphy and Dowding 1995; O’Donnell et al. 1996) may be back to pre-mast levels.
At least in the South Island, rats sometimes (but not always) exhibit winter breeding after a heavy seedfall, just as mice do (Fitzgerald et al. 2004; Efford et al. 2006). Rat numbers increase over winter, so the risk of predation by rats on birds such as mohua may already be higher than usual at the start of the breeding season in August/September, several months before stoat numbers increase dramatically in December/January (O’Donnell et al. 1996; King and Moller 1997; Pryde et al. 2005).
(3) Kaka. Kaka are long-lived parrots which, in Nothofagus forest, do not even attempt to breed in non-mast seeding years, regardless of supplementary feeding of other foods (Wilson et al. 1998). Remarkably, kaka anticipate a mast seeding and begin to nest in October–November, before or during flowering, fully 6 months before seedfall (Beggs and Wilson 1991). It is not known how they can anticipate mast years, although the birds (especially females) feed on Nothofagus tree sap, so they may be able to detect plant signals associated with heavy flowering (Wilson et al. 1998).
This anticipatory nesting has two consequences, both favorable for kaka. First, the fledglings make their maximum energy demands in late summer, as green seeds are swelling and offer abundant nutritious food (Beggs 1999; White 2007). Second, kaka nest during the summer before seedfall, when stoat densities are still low. Kaka young will have fledged well before the following summer, when stoat numbers peak. Female kaka are extremely vulnerable to stoats when nesting, but unless there is an unusual double-mast event, kaka do not attempt to nest during the summer after seedfall, so their risk of predation is minimized, Indeed, without this unusual behavior (Kelly and Sork 2002, p. 436), which reduces exposures of nests to predation, kaka would probably already be extinct in South Island beech forests.
New Zealand: feedback loops and hysteresis
We know of no hysteresis in the Nothofagus masting system. The inexorable ratcheting down of hole-nesting birds is not hysteresis, as there is no compensating reversal driven by a second process. In fact, these bird declines are probably the last slow adjustment of the system to the introduction of stoats (and rodents) more than a century ago, with a stable end-point being the local or global extinction of the birds in the absence of active conservation management. In present-day forests, increases of most animal species are driven by increased food supply, and decreases are driven largely by decreased food supply (with small contributions from elevated densities of predators). There are no feedback loops in the Nothofagus system, apart from normal predator–prey interactions driven by food availability.
New Zealand: conclusion
Two features of New Zealand Nothofagus communities are noteworthy. First, the systems experience very large pulses, because, over large areas, the canopy is composed entirely of Nothofagus species showing synchronous seed crops which are among the most variable in the world (Kelly et al. 2000). Despite these massive pulses, the system is surprisingly dynamically stable; after each pulse, it returns rapidly to pre-pulse conditions. Resilience seems to be enhanced by anticipatory behavior, short lags, and the lack of hysteresis.
Second, as in the North American system, there have been important species introductions and extinctions in Nothofagus communities, so their contemporary dynamic behavior may be very different from that existing before human colonization. Unfortunately, with regard to the seed consumers before human colonization, we again have little information from which to infer the original behavior of this system.
Discussion
Pulsed inputs from masting plants are observed in many communities globally, but the implications for community dynamics have generally not been fully explored. Drawing on extensive published data for the well-studied Quercus and Nothofagus systems, we suggest some tentative conclusions about the features that affect the dynamic stability in such systems, in relation to our original hypotheses (Table 1).
First, system stability appears to be decoupled from evolutionary history. The Nothofagus system is relatively resilient, despite several of the key species having been in New Zealand for fewer than 200 years. House mice and stoats are not sympatric in Europe, where mice are mostly commensal rather than feral as in New Zealand. House mice are seldom eaten by stoats anywhere but in New Zealand; stoats prefer voles, lemmings and rabbits throughout the northern hemisphere (King and Powell 2007). Both native kaka and introduced house mice show anticipatory responses to Nothofagus mast events, although the mice are probably just responding after an increase in litter-feeding invertebrates. In contrast, the widely oscillatory population behavior of gypsy moths in the North American system demonstrates that exotic species can also be impressively unstable.
A corollary of this conclusion is that many of the key interactions found in these systems appear to be idiosyncratic in the sense that they are the consequences of relationships peculiar to the particular species present in the system. A good example of this is the relationship between white-footed mouse densities, ticks, and Lyme disease that is a key component of eastern North American oak forests. Key components of this system are also present in western North American oak forests. However, Lyme disease is considerably less important in those forests because the western fence lizard (Sceloporus occidentalis [Baird and Girard]), rather than mice, is the primary host of ticks. This species of lizard happens to be a much less competent host for the causative agent of Lyme disease (Borrelia), because its blood contains factors that neutralize Borrelia (Lane and Quistad 1998).
Second, our two case studies emphasize that, with historical extinctions and recent introductions, the dynamics of many masting communities are likely to be qualitatively different now from their previous states. This is most clearly illustrated by the oak forest system of eastern North America, where previously vast numbers of passenger pigeons might have so efficiently harvested particularly large oak mast crops (Bucher 1992) to which other consumers would have shown much smaller responses, and the system might have been much more resilient than it is today. Similarly, in Nothofagus forests, in pre-human times, native birds were more abundant and might have consumed a much larger fraction of mast seed crops.
Third is the importance of short lags, and especially of anticipatory responses, in rapid community responses to pulsed inputs. Such anticipatory responses may be widespread in masting communities, including extended diapause by invertebrate seed predators (Brockerhoff and Kenis 1997; Kelly et al. 2000; McKone et al. 2001; Kolesik et al. 2007). Similar anticipatory breeding has been suggested for several species of red squirrels in advance of conifer cone crops (Boutin et al. 2007), although White (2007) raises doubts as to whether it is physically possible for the breeding to be literally anticipatory. Anticipatory responses and short lags allow pulsed resources to feed through the community in the shortest possible time, thus speeding the system’s return to the pre-pulse state.
Fourth, we stress the likely importance of hysteresis and rare events in creating long-duration, multi-year ripples through communities from mast events. Gypsy moth dynamics in North America show major outbreaks on either a 5-year or 10-year cycle, possibly driven by the long time intervals between crop failures in red oaks that free gypsy moths from regulation by white-footed mice. Once a population has been released, it climbs to outbreak levels before the over-compensatory response of pathogens or other natural enemies can cause populations to crash back to levels where mice once again maintain populations in check. In contrast, in the simple food-limited Nothofagus community, increases in the comparable herbivore (house mice) last for a much shorter time. Similarly, rare Nothofagus double-mast events can stimulate responses in ship rats that have important ramifications for bird densities.
Fifth, positive feedback loops are potentially destabilizing but actually seem to have little practical effect, even in the eastern North American system where several such putative loops are present, because they seem to have, at best, only weak, long-delayed, effects.
Sixth, our hypotheses on the effects of species diversity and predator mobility were only partly supported. The first hypothesis, that the lack of alternative foods in the simple Nothofagus forests seems to contribute to the rapid, presumably food-limited, declines in mice and stoats after they peak during a mast event, still stands. However, our second hypothesis, that food-limited declines would be more rapid and the system more resilient if consumers were immobile rather than highly mobile, would appear to be falsified by the former role of passenger pigeons in the deciduous forests of eastern North America. These birds could move to and consume the bulk of even large mast crops and then move on, thus potentially enhancing, rather than decreasing, resilience. On reflection, mobility by vertebrate seed predators might best be regarded as an evolutionary adaptation to the pulsed resources that minimizes fluctuation in the vertebrate’s numbers by averaging resources over large spatial scales; exactly this point was recently also made by Yang et al. (2008).
However, these conclusions are based on only two well-studied examples of the many mast seeding communities in different parts of the globe. Although many other such systems remain to be discovered, four are known to have significant effects on at least some aspects of their communities. These include the mass flowering of bamboo in South America; synchronous mast seeding of dipterocarp communities in Indonesia; widespread synchronous seeding by rowan across Norway; and masting of oaks in California. All of these systems share the critical feature: pulses of resources spatially synchronized over sufficiently large geographic areas, on the order of tens to hundreds of thousands of square kilometers, to have significant numerical effects on seed predators on a regional scale. At least one—that of the Dipterocarpaceae in Indonesia—involves synchronous seed production by dozens of species apparently cued by El Niño events, as is the taxonomically widespread masting synchrony observed among species in New Zealand. Further study of these, as well as other mast seeding communities, should yield new insights into the importance of evolutionary history vs short-term stochastic factors in determining the complexity and stability of ecological systems.
References
Abrahamson WG, Layne JN (2002) Relation of ramet size to acorn production in five oak species of xeric upland habitats in south-central Florida. Am J Bot 89:124–131. doi:10.3732/ajb.89.1.124
Abrams MD (1992) Fire and the development of oak forests. BioScience 42:346–353. doi:10.2307/1311781
Alley JC, Berben PH, Dugdale JS, Fitzgerald BM, Knightbridge PI, Meads MH et al (2001) Responses of litter-dwelling arthropods and house mice to beech seeding in the Orongorongo Valley, New Zealand. J R Soc N Z 31:425–452
Beggs JR (1999) Comparison of the quality of red and silver beech (Nothofagus) seeds in Nelson Lakes National Park, New Zealand. N Z J Bot 37:495–501
Beggs JR, Wilson PR (1991) The Kaka Nestor meridionalis meridionalis, a New Zealand parrot endangered by introduced wasps and mammals. Biol Conserv 56:23–38. doi:10.1016/0006-3207(91)90086-O
Blockstein DE (1998) Lyme disease and the passenger pigeon? Science 279:1831. doi:10.1126/science.279.5358.1831c
Blockstein DE, Tordoff H (1985) Gone forever: a contemporary look at the extinction of the passenger pigeon. Am Birds 39:845–851
Boutin S, Dhondt AA, Wauters LA, Tosi G, Humphries MM, McAdam A (2007) Anticipatory reproduction and population growth in seed predators feeding on pulsed resources. Science 314:1928–1930. doi:10.1126/science.1135520
Brockerhoff EG, Kenis M (1997) Oviposition, life cycle, and parasitoids of the spruce cone maggot, Strobilomyia anthracian (Diptera: Anthomyiidae), in the Alps. Bull Entomol Res 87:555–562
Bucher EH (1992) The causes of extinction of the passenger pigeon. In: Power DM (ed) Current ornithology, vol 9. Plenum Press, New York, pp 1–36
Campbell RW, Sloan RJ (1977) Natural regulation of innocuous gypsy moth populations. Environ Entomol 6:315–322
Choquenot D, Ruscoe WA (2000) Mouse population eruptions in New Zealand forests: the role of population density and seedfall. J Anim Ecol 69:1058–1070. doi:10.1046/j.1365-2656.2000.00462.x
Curran LM, Leighton M (2000) Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol Monogr 70:101–128
Curran LM, Caniago I, Paoli BD, Astianti D, Kusneti M, Leighton M et al (1999) Impact of El Niño and logging on canopy tree recruitment in Borneo. Science 286:2184–2188. doi:10.1126/science.286.5447.2184
Diamond SJ, Giles RH (2000) Hard mast production before and after the chestnut blight. South J Appl For 24:196–201
Dilks PJ, Williams M, Pryde M, Fraser I (2003) Large scale stoat control to protect mohua (Mohoua ochrocephala) and kaka (Nestor meridionalis) in the Eglinton Valley, Fiordland, New Zealand. N Z J Ecol 27:1–9
Efford MG, Fitzgerald BM, Karl BJ, Berben PH (2006) Population dynamics of the ship rat Rattus rattus L. in the Orongorongo Valley, New Zealand. N Z J Ecol 33:273–297
Elkinton JS, Liebhold AM (1990) Population dynamics of gypsy moth in North America. Annu Rev Entomol 35:571–596
Elkinton JS, Healy WM, Buonaccorsi JP, Boettner GH, Hazzard A, Liebhold AM et al (1996) Interactions among gypsy moths, white-footed mice, and acorns. Ecology 77:2332–2342. doi:10.2307/2265735
Elliott GP (1996) Productivity and mortality of mohua (Mohoua ochrocephala). N Z J Zool 23:229–237
Elliott GP, Dilks PJ, O’Donnell CFJ (1996) The ecology of yellow-crowned parakeets (Cyanoramphus auriceps) in Nothofagus forest in Fiordland, New Zealand. N Z J Zool 23:249–265
Ellsworth JW, McComb BC (2003) Potential effects of passenger pigeon flocks on the structure and composition of presettlement forests of Eastern North America. Conserv Biol 17:1548–1558. doi:10.1111/j.1523-1739.2003.00230.x
Fitzgerald BM, Daniel MJ, Fitzgerald AE, Karl BJ, Meads MJ, Notman PR (1996) Factors affecting the numbers of house mice (Mus musculus) in hard beech (Nothofagus truncata) forest. J R Soc N Z 26:237–249
Fitzgerald BM, Efford MG, Karl BJ (2004) Breeding of house mice and the mast seeding of southern beeches in the Orongorongo Valley, New Zealand. N Z J Ecol 31:167–184
Gansner DA, Herrick OW, DeBald PS, Acciavatti RE (1983) Changes in forest condition associated with gypsy moth. J For 81:155–157
Gschwantner T, Hock G, Schopf A (2002) Impact of predators on artificially augmented populations of Lymantria dispar L. pupae (Lep., Lymantriidae). J Appl Entomol 126:66–73. doi:10.1046/j.1439-0418.2002.00626.x
Grimm V, Wissel C (1997) Babel, or the ecological stability discussions: an inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 109:323–334. doi:10.1007/s004420050090
Hogg AG, Higham TFG, Lowe DJ, Palmer JG, Reimer PJ, Newnham RM (2003) A wiggle-match date for Polynesian settlement of New Zealand. Antiquity 77:116–125
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23. doi:10.1146/annurev.es.04.110173.000245
Holt RD (2008) Theoretical perspectives on resource pulses. Ecology 89:671–681. doi:10.1890/07-0348.1
Jaksic FM, Lima M (2003) Myths and facts on ratadas: bamboo blooms, rainfall peaks and rodent outbreaks in South America. Aust Ecol 28:237–251. doi:10.1046/j.1442-9993.2003.01271.x
Janzen DH (1971) Seed predation by animals. Annu Rev Ecol Syst 2:465–492. doi:10.1146/annurev.es.02.110171.002341
Johnson DM, Liebhold AM, Bjørnstad ON, McManus ML (2005) Circumpolar variation in periodicity and synchrony among gypsy moth populations. J Anim Ecol 74:882–892. doi:10.1111/j.1365-2656.2005.00980.x
Johnson DM, Liebhold AM, Bjørnstad O (2006) Geographical variation in the periodicity of gypsy moth outbreaks. Ecography 29:367–374. doi:10.1111/j.2006.0906-7590.04448.x
Jones CG, Ostfeld RS, Richard MP, Schauber EM, Wolff JO (1998) Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 279:1023–1026. doi:10.1126/science.279.5353.1023
Kelly D, Sork VL (2002) Mast seeding in perennial plants: why, how, where? Annu Rev Ecol Syst 33:427–447. doi:10.1146/annurev.ecolsys.33.020602.095433
Kelly D, Harrison AL, Lee WG, Payton IJ, Wilson PR, Schauber EM (2000) Predator satiation and extreme mast seeding in 11 species of Chionochloa (Poaceae). Oikos 90:477–488. doi:10.1034/j.1600-0706.2000.900306.x
Kelly D, Hart DE, Allen RB (2001) Evaluating the wind-pollination benefits of mast seeding. Ecology 82:117–126
Kelly D, Brindle C, Ladley JJ, Robertson AW, Maddigan FW, Butler J et al (2005) Can stoat (Mustela erminea) trapping increase bellbird (Anthornis melanura) populations and benefit mistletoe (Peraxilla tetrapetala) pollination? N Z J Ecol 29:69–82
King CM (1982) Age structure and reproduction in feral New Zealand populations of the house mouse (Mus musculus), in relation to seedfall of southern beech. N Z J Ecol 9:467–479
King CM (1983) The relationships between beech (Nothofagus sp.) seedfall and populations of mice (Mus musculus), and the demographic and dietary responses of stoats (Mustela erminea) in three New Zealand forests. J Anim Ecol 52:141–166. doi:10.2307/4593
King CM (2002) Cohort variation in the life-history parameters of stoats Mustela erminea in relation to fluctuating food resources: a challenge to boreal ecologists. Acta Theriol (Warsz) 47:225–244
King CM (2005) Handbook of New Zealand mammals, 2nd edn. Oxford University Press, Melbourne
King CM, Moller H (1997) Distribution and response of rats Rattus rattus, R. exulans to seedfall in New Zealand beech forests. Pac Conserv Biol 3:143–155
King CM, Powell RA (2007) The natural history of weasels and stoats: ecology, behavior, and management, 2nd edn. Oxford University Press, New York
King CM, Innes JG, Flux M, Kimberley MO, Leathwick JR, Williams DS (1996) Distribution and abundance of mammals in relation to habitat in Pureora Forest Park. N Z J Ecol 20:215–240
King CM, White PCL, Purdey DC, Lawrence B (2003) Matching productivity to resource availability in a small predator, the stoat (Mustela erminea). Can J Zool 81:662–669. doi:10.1139/z03-042
Koenig WD, Benedict LS (2002) Size, insect parasitism, and energetic value of acorns stored by acorn woodpeckers. Condor 104:539–547. doi:10.1650/0010-5422(2002)104[0539:SIPAEV]2.0.CO;2
Koenig WD, Knops JMH (1997) Patterns of geographic synchrony in growth and reproduction of oaks within California and beyond. In: Pillsbury NH, Verner J, Tietje WD (eds) Proceedings of the Symposium on Oak Woodlands: ecology, management, and urban interface issues. Pacific SW Forest & Range Exp. Stn Gen. Tech. Rep. PSW-GTR-160. San Luis Obispo, CA, pp 101–108
Koenig WD, Knops JMH (2000) Patterns of annual seed production by northern hemisphere trees: a global perspective. Am Nat 155:59–69. doi:10.1086/303302
Koenig WD, Mumme RL, Carmen WJ, Stanback MT (1994) Acorn production by oaks in central coastal California: variation in and among years. Ecology 75:99–109. doi:10.2307/1939386
Koenig WD, Knops JMH, Carmen WJ, Stanback MT (1999) Spatial dynamics in the absence of dispersal: acorn production by oaks in central coastal California. Ecography 22:499–506
Koenig WD, Kelly D, Sork VL, Duncan RP, Elkinton JS, Peltonen MS et al (2003) Dissecting components of population-level variation in seed production, and the evolution of masting. Oikos 102:581–591. doi:10.1034/j.1600-0706.2003.12272.x
Kolesik P, Sarfati M, Brockerhoff EG, Kelly D (2007) Description of Eucalyptodiplosis chionochloae sp. nov., a cecidomyiid feeding on inflorescences of Chionochloa (Poaceae) in New Zealand. N Z J Zool 34:107–115
Lane RS, Quistad GB (1998) Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J Parasitol 84:29–34. doi:10.2307/3284524
Liebhold AM, Higashiura Y, Unno A (1998) Forest type affects predation on gypsy moth (Lepidoptera: Lymantriidae) pupae in Japan. Environ Entomol 27:858–862
Liebhold AM, Elkinton JS, Williams D, Muzika R-M (2000) What causes outbreaks of the gypsy moth in North America? Popul Ecol 42:257–266. doi:10.1007/PL00012004
Liebhold AM, Koenig WD, Bjørnstad O (2004) Spatial synchrony in population dynamics. Annu Rev Ecol Evol Syst 35:467–490. doi:10.1146/annurev.ecolsys.34.011802.132516
McKone MJ, Thom AL, Kelly D, Cone AJ (2001) Phenology and identification of two flies (Chloropidae: Diplotoxa similis Cecidomyiidae: undescribed species) that feed in the inflorescences of Chionochloa pallens (Poaceae). N Z J Zool 28:89–101
McShea WJ (2000) The influence of acorn crops on annual variation in rodent and bird populations within oak dominated forests. Ecology 81:228–238
McShea WJ, Schwede G (1993) Variable acorn crops: responses of white-tailed deer and other mast consumers. J Mammal 74:999–1006. doi:10.2307/1382439
McWilliams WH, O’Brian GC, Reese GC, Waddell KL (2002) Distribution and abundance of oaks in North America. In: McShea WJ, Healy WM (eds) Oak forest ecosystems. Johns Hopkins University Press, Baltimore, pp 13–33
Monks A, Kelly D (2006) Testing the resource matching hypothesis in the mast seeding tree Nothofagus truncata (Fagaceae). Aust Ecol 31:366–375. doi:10.1111/j.1442-9993.2006.01565.x
Murphy EC, Dowding JE (1995) Ecology of the stoat in Nothofagus forest: home range, habitat use and diet at different stages of the beech mast cycle. N Z J Ecol 19:97–109
Murua R, Gonzalez LA, Gonzalez M, Jofre YC (1996) Flowering effects of the shrub Chusquea quila Kunth (Poaceae) on demography of rodent populations of the temperate rain forest in southern Chile. Bol Soc Biol Concepcion 67:37–42
Nakajima H (1992) Sensitivity and stability of flow networks. Ecol Model 62:123–133. doi:10.1016/0304-3800(92)90085-S
O’Donnell CFJ (1996a) Monitoring mohua (yellowhead) populations in the South Island, New Zealand, 1983–1993. N Z J Zool 23:221–228
O’Donnell CFJ (1996b) Predators and the decline of New Zealand forest birds: an introduction to the hole-nesting bird and predator programme. N Z J Zool 23:213–219
O’Donnell CFJ, Dilks PJ, Elliott GP (1996) Control of a stoat (Mustela erminea) population irruption to enhance mohua (yellowhead) (Mohoua ochrocephala) breeding success in New Zealand. N Z J Zool 23:279–286
Ostfeld RS, Keesing F (2000) Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol Evol 15:232–237. doi:10.1016/S0169-5347(00)01862-0
Ostfeld RS, Lewin N, Schnurr J, Canham CD, Pickett STA (1994) The roles of small rodents in creating patchiness. Pol Ecol Stud 20:265–276
Ostfeld RS, Jones CG, Wolff JO (1996) Of mice and mast: ecological connections in eastern deciduous forests. BioScience 46:323–330. doi:10.2307/1312946
Powell RA, King CM (1997) Variation in body size, sexual dimorphism and age-specific survival in stoats, Mustela erminea (Mammalia: Carnivora), with fluctuating food supplies. Biol J Linn Soc Lond 62:165–194. doi:10.1006/bijl.1996.0154
Pryde MA, O’Donnell CFJ, Barker RJ (2005) Factors influencing survival and long-term population viability of New Zealand long-tailed bats (Chalinolobus tuberculatus): implications for conservation. Biol Conserv 126:175–185. doi:10.1016/j.biocon.2005.05.006
Purdey DC, King CM, Lawrence B (2004) Age structure, dispersion and diet of a population of stoats (Mustela erminea) in southern Fiordland during the decline phase of the beechmast cycle. N Z J Zool 31:205–225
Richardson SJ, Allen RB, Whitehead D, Carswell FE, Ruscoe WA, Platt KH (2005) Climate and net carbon availability determine temporal patterns of seed production by Nothofagus. Ecology 86:972–981. doi:10.1890/04-0863
Ruscoe WA, Choquenot D, Heyward R, Yockney I, Young N, Drew K (2003) Seed production, predators and house mouse population eruptions in New Zealand beech forests. In: Singleton GR, Hinds LA, Krebs CJ, Spratt DM (eds) Rats, mice and people: rodent biology and management. Australian Centre for International Agricultural Research, Canberra
Ruscoe WA, Elkinton JS, Choquenot D, Allen RB (2005) Predation of beech seed by mice: effects of numerical and functional responses. J Anim Ecol 74:1005–1019. doi:10.1111/j.1365-2656.2005.00998.x
Ruscoe WA, Norbury G, Choquenot D (2006) Trophic interactions among native and introduced animal species. In: Allen RB, Lee WG (eds) Biological invasions in New Zealand. Springer, Berlin, pp 247–263
Satake A, Bjornstad ON, Kobro S (2004) Masting and tropic cascades: interplay between rowan trees, apple fruit moth, and their parasitoid in southern Norway. Oikos 104:540–550. doi:10.1111/j.0030-1299.2004.12694.x
Schauber EM, Kelly D, Turchin P, Simon C, Lee WG, Allen RB et al (2002) Synchronous and asynchronous masting by 18 New Zealand plant species: the role of temperature cues and implications for climate change. Ecology 83:1214–1225
Schmidt KA, Ostfeld RS (2003) Songbird populations in fluctuating environments: nest predator responses to pulsed resources. Ecology 84:406–415. doi:10.1890/0012-9658(2003)084[0406:SPIFEP]2.0.CO;2
Schmidt KA, Ostfeld RS (2008) Numerical and behavioral effects within a pulse-driven system: consequences for direct and indirect interactions among shared prey. Ecology 89:635–646. doi:10.1890/07-0199.1
Schorger A (1955) The passenger pigeon. University of Wisconsin Press, Madison
Shimada T, Saitoh T (2006) Re-evaluation of the relationship between rodent populations and acorn masting: a review from the aspect of nutrients and defensive chemicals in acorns. Popul Ecol 48:341–352. doi:10.1007/s10144-006-0012-6
Silvertown JW (1980) The evolutionary ecology of mast seeding in trees. Biol J Linn Soc Lond 14:235–250. doi:10.1111/j.1095-8312.1980.tb00107.x
Sork VL, Bramble J, Sexton O (1993) Ecology of mast-fruiting in three species of North American deciduous oaks. Ecology 74:528–541. doi:10.2307/1939313
van Nes EH, Scheffer M (2004) Large species shifts triggered by small forces. Am Nat 164:255–266. doi:10.1086/422204
Vandermast DB, Van Lear DH (2002) Riparian vegetation in the southern Appalachian mountains (USA) following chestnut blight. For Ecol Manage 155:97–106
Wang G, Wolff JO, Vessey SH, Slade NA, Witham JW, Merritt JF, et al. Comparative population dynamics of Peromyscus leucopus in North America: influences of climate, food, and density dependence. Popul Ecol (in press). doi:10.1007/s10144-008-0094-4
Wardle JA (1984) The New Zealand beeches: ecology, utilization and management. New Zealand Forest Service, Christchurch
Watts WA (1979) Late Quaternary vegetation of the central Appalachian and New Jersey coastal plain. Ecol Monogr 49:427–469. doi:10.2307/1942471
White PCL, King CM (2006) Predation on native birds in New Zealand beech forests: the role of functional relationships between stoats Mustela erminea and rodents. Ibis 148:765–771. doi:10.1111/j.1474-919X.2006.00579.x
White TCR (2007) Mast seeding and mammal breeding: can a bonanza food supply be anticipated? N Z J Zool 34:179–183
Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH (2008) Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc Natl Acad Sci 105:7676–7680
Wilson PR, Karl BJ, Toft RJ, Beggs JR, Taylor RH (1998) The role of introduced predators and competitors in the decline of kaka (Nestor meridionalis) populations in New Zealand. Biol Conserv 83:175–185. doi:10.1016/S0006-3207(97)00055-4
Wittmer HU, Powell RA, King CM (2007) Understanding contributions of cohort effects to variation in population growth of fluctuating populations. J Anim Ecol 76:946–956. doi:10.1111/j.1365-2656.2007.01274.x
Wolff JO (1996) Population fluctuations of mast-eating rodents are correlated with production of acorns. J Mammal 77:850–856. doi:10.2307/1382690
Worthy TH, Holdaway RN (2002) The lost world of the moa. Indiana University Press, Bloomington
Yang LH, Bastow JL, Spence KO, Wright AN (2008) What can we learn from resource pulses? Ecology 89:621–634. doi:10.1890/07-0175.1
Acknowledgments
We thank C.M. (Kim) King, Wendy Ruscoe, Ken Schmidt, and Louie Yang for their helpful comments. Support came from the N Z Foundation for Research, Science and Technology’s OBI ‘Ecosystems Resilience’ and the Royal Society of New Zealand’s Marsden Fund grant UOC0403 (DK), and from the National Science Foundation and the Integrated Hardwoods Range Management Program (WDK).
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript was submitted for the special feature based on the symposium in Jozankei, Hokkaido, held on 21 October 2007.
Rights and permissions
About this article
Cite this article
Kelly, D., Koenig, W.D. & Liebhold, A.M. An intercontinental comparison of the dynamic behavior of mast seeding communities. Popul Ecol 50, 329–342 (2008). https://doi.org/10.1007/s10144-008-0114-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-008-0114-4