Abstract
The effects of simulated acid fog (SAF) and ozone (O3) stress on the growth and physiology of beech (Fagus crenata) saplings were investigated. Three-year-old beech saplings were exposed to SAFs of pH 3 and pH 5 (control) during May 2007 to July 2008. In each SAF treatment group, half of the saplings were exposed to 60 ppb of O3 during September 2007 to July 2008. In comparison to the control saplings, those from the pH 3 treatment had lower total plant biomasses, epicuticular wax amounts, Ca2+ concentrations in their leaves, and lower starch concentrations in their leaves and roots. The effect of O3 was significant only for the starch concentration in the roots, but the O3 exposure also negatively affected the growth and physiology of beech saplings. Results show that acid fog exerts various severe effects, and that both chronic acid fog and O3 exposure suppressed the physiological functions of beech saplings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Tanzawa Mountains in the Tanzawa–Oyama Quasi-National Park, Japan, are located 60 km southwest of the Tokyo Metropolitan area. Beech (Fagus crenata) trees growing on the southwest-facing slope have shown symptoms of decline since the 1980s, but those on the northeast-facing slope are healthy at present.
To clarify the cause of the decline in the forest on the Tanzawa Mountains, several studies have conducted field observations and chamber experiments. Around the Hinokiboramaru (1601 m asl) summit located on the northwestern ridgeline of the Tanzawa Mountains, Maruta and Usui (1997) collected fog water using an active fog water collector and reported that the pH was lower in samples collected on the southwest-facing slope than in samples from the northeast-facing slope. Acid fog may be one cause of the decline, but limited information related to the effects of simulated acid fog (SAF) on growth and physiological responses of beech saplings is available (e.g., on plant height, stem diameter, number of leaves, leaf area, biomass, and starch accumulation in the stem) (Shigihara et al. 2008a).
Ozone (O3) is a widespread phototoxic air pollutant, and Totsuka et al. (1997) have reported the frequent occurrence of higher concentrations of ambient O3 (>100 ppb) in declining forest areas than in a control plot in the Tanzawa Mountains. Recently, Takeda and Aihara (2007) examined beech saplings exposed to unfiltered air or charcoal-filtered air in an open-top chamber on the Tanzawa Mountains and reported that their total biomass was reduced considerably in the unfiltered air. As described, air pollutants such as acid fog and O3 are common environmental stresses for beech seedlings (Shigihara et al. 2008a; Yonekura et al. 2004), but the effects of acid fog and O3 have been considered separately thus far. Although few studies to date have examined the interactive effects of acid fog and O3 on vegetation, such studies must produce more accurate predictions of future ecosystem responses to the changing environment. In particular, no information is available on the combined effects of SAF and O3 on the growth and physiological responses of beech seedlings, although a synergistic effect of SAF and O3 has been reported for fir (Abies firma) seedlings (Yoshida et al. 2004).
The objectives of this study are as follows:
-
1.
To determine how both SAF and O3 simultaneously influence the growth and physiology of beech saplings, the saplings’ responses were examined by measuring total biomass and starch concentrations in roots, leaves, and winter buds. O3 is well known to have a strong impact on the central processes of carbon metabolism, such as photosynthesis and carbohydrate formation (McLaughlin et al. 1982; Meier et al. 1990). The major biomass product of photosynthesis is starch (Gifford et al. 1984).
-
2.
To determine whether much leaching of metal ions from the leaves occurs when beech saplings are exposed to both SAF and O3, the cuticular layer—which protects the leaf surface from loss of moisture, inorganic ions, and gas from vegetation—was examined (Klemm 1989). The cuticular layer, especially epicuticular wax, is reportedly eroded by acid deposition (Mengel et al. 1989) or O3 (Percy et al. 1992). The amount of base cations (K+, Mg2+, and Ca2+) that are leached from the leaves depends on the decrease in wax on the surface.
Materials and methods
Five months before the start of the SAF and O3 exposure treatments conducted at the experimental site of Kanagawa University in Yokohama, Japan (35°28′N, 139°38′E), 32 three-year-old beech saplings (Fagus crenata, height ca. 85.8 cm) were planted individually in four-liter pots containing a 1:2 mixture of leaf mold and red clay. Saplings were treated with a mineral fertilizer (2 g; nitrogen 8%, phosphorus pentoxide 8%, potassium oxide 8%) once annually in mid-March and irrigated with tap water during the study, as described in a previous report (Shigihara et al. 2008a). All saplings were kept under natural environmental conditions. The soil surface was not covered, and the elements leached from leaves and litter entered the soils in which the beech saplings were planted. The meteorological data presented in Table 1 were obtained from Yokohama local meteorological observatory. Additionally, the mean atmospheric concentrations of O3 and nitrogen oxide (NO X ) were, respectively, 15.6 and 43.6 ppb (Kanagawa University observational data).
Prior to the experiment, all saplings were divided into two groups: one group was exposed to SAF at pH 3 (AF3, 16 seedlings each); the other group was exposed to SAF at pH 5 as a control (AF5, 16 seedlings each). The SAF exposure experiments were performed over three periods: April–July 2007, September–December 2007, and April–July 2008. SAF exposure was halted in August 2007 and during January–March 2008 because the frequency of acid fog events is extremely low at Mt. Oyama in the Tanzawa Mountains. The SAF solution for AF3 contained ions typical of fog samples found in Mt. Oyama at the following concentrations (mequiv. L−1): H+ 0.675; Na+ 1.38; NH4 + 1.75; Cl− 0.526; NO3 − 0.943; SO4 2− 0.911. The SAF solution for AF5 was prepared by diluting the SAF of pH 3 with ultrapure water (18.2 MΩ cm resistivity at 25°C and <5 ppb total organic carbon). The SAF solutions were generated using a dry-fog humidifier (Akimist D; H. Ikeuchi and Co. Ltd.), which produced droplet sizes of approximately 20 μm at the rate of 3 L h−1 with compressed air using a compressor in exposure chambers (2.4 × 2.4 × 1.8 m high, 10.4 m3) that consisted of a steel frame and polyvinyl chloride sheeting on all sides, except for the floor. The saplings were exposed to the SAF for 2 h and left in the chamber for 20 min after exposure. During the exposure period, the chamber was closed off with sheets. The exposure treatments were conducted on clear or cloudy days during 1300–1700 JST and twice a week at three-day or four-day intervals. The total exposure duration was 172 h (86 times) in this experiment.
After four months’ exposure to SAF, half of the saplings within each acid fog exposure chamber were exposed to 60 ppb O3 four times a week for 7 h each (during 1000–1700 JST) until July 2008, except during January–March (114 day) in the O3 chamber. The O3 concentrations were determined based on data for atmospheric O3 obtained in the Tanzawa Mountains, where high concentrations of O3 are detected with a mean O3 concentration of approximately 60 ppb (Yonekura et al. 2004). The chamber was 1.2 × 1.2 × 1.2 m high, with a volume of 1.73 m3, and was made of an aluminum framework with polyvinyl chloride sheeting on all sides except for the floor. The O3 exposure experiments were performed over two periods: September–December 2007 and April–July 2008. During the period of January to March, O3 exposure was also stopped because not only acid fog but also high concentration O3 events rarely occur in winter. The O3 was generated with two O3 generators (air essence VR-40; Ohnit Co. Ltd.) from atmospheric air filtered through fiberglass. The ozone was distributed uniformly in the chamber using an electric fan; its concentration was monitored continuously at 10-min intervals using a personal O3 monitor (OC-300; GE Medical Systems) which showed a good correlation with the results obtained using an ultraviolet absorption O3 photometer (UVAD-1000A; Shimadzu Corp.). The accumulated exposure to O3 above a threshold of 40 ppb (AOT40) was 16.6 ppm h during the experimental period. Here the AOT40 of O3 is the sum of the differences between the hourly mean O3 concentration and 40 ppb for each hour when the O3 concentration became greater than 40 ppb. We designated the two treatments as follows: SAF at pH 3 with O3 exposure, “AF3–O3;” SAF at pH 5 with O3 exposure, “AF5–O3.” The respective exposures of SAF and O3 were repeated.
In late August 2008, all saplings were harvested from the pots and divided into winter buds, leaves, branches, stem, and roots. To quantitatively estimate the epicuticular wax, extracts of 15 leaves per sapling were placed in chloroform for 15 s (Sase et al. 1998), and then the chloroform was filtered and evaporated. The residual epicuticular wax was determined gravimetrically, and the amount of epicuticular wax was expressed on a dry mass (mg g−1 dry leaves weight (DW)) basis. After measuring the wax, the plant materials (including the leaves) were dried in a forced-air oven at 80°C for 48 h to obtain the dry weight. Their weights were measured using an electrobalance.
To measure mineral ions, dried leaves were ashed in a muffle furnace at 500°C for 2 h. Ten milligrams of ash were heated in 5 ml of 1% nitric acid at 80°C for 2 h. After cooling and filtering with a membrane filter (0.45 μm), the concentrations of K+, Mg2+, and Ca2+ were determined using atomic spectrometry (AA-6700; Shimadzu Corp.). The starch contents in dry materials were also measured according to the method described by Sugiyama and Oshiro (2001).
Analyses of variance (ANOVA) were performed in this study, and significant differences (P < 0.05 or 0.01) in all-plant biomass, epicuticular wax, metal ion concentrations in leaves, and starch concentrations in plant fractions were determined using Student’s t test in order to evaluate differences among treatment groups. All data presented in this paper are average values and standard errors (SEs) of eight independent replicates.
Results
Because of their large surface areas, leaves are the plant organs that suffer the most exposure to O3 (Lux et al. 1997). Before O3 exposure, no visible injury to the leaves was observed in any of the samplings. Two months after the first exposure to O3, leaf injury symptoms (e.g., bronzing between the leaf veins) appeared in most saplings of AF3–O3 and AF5–O3. The degree of injury was higher for AF3–O3 than for AF5–O3, indicating that chronic acid fog exposure increased the leaves’ sensitivity to O3.
Figure 1 portrays the total plant biomass and top/root dry mass ratio (T/R) of beech saplings in August 2008. The total plant biomass decreased from that observed for pH 5 SAF (control) after exposure to pH 3 SAF and/or O3. Effects of acid fog and/or O3 on the T/R ratio were also observed, indicating suppressed root growth. However, there were no significant differences between the AF3 and AF3–O3 or AF3 and AF5–O3 groups for either index. Acid fog and O3 exerted similar effects, although no significant differences were found between the AF5 and AF5–O3 groups. These two effects (of acid fog and O3) are demonstrated in the ANOVA results presented in Fig. 1.
The amounts of epicuticular wax measured after the harvest are presented in Fig. 2. A large acid fog-induced reduction in epicuticular wax was observed, but no significant effect of O3 was found.
Figure 3 depicts concentrations of metal ions (K+, Mg2+, and Ca2+) in leaves on August 2008. Acid fog significantly reduced the concentrations of K+ and Ca2+. Leaves of saplings exposed to pH 3 SAF had Ca2+ concentrations that were 42% of those of control saplings, although the difference for K+ was less. Mg2+ concentrations were not significantly different between AF3 and AF5.
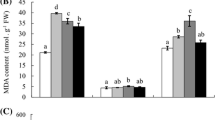
Starch concentrations in the roots, leaves and winter buds are presented in Table 2. In comparison to control saplings, starch concentrations were reduced in the roots and leaves but not in the winter buds of saplings exposed to acid fog. In particular, root starch after exposure to pH 3 SAF was significantly reduced compared to that of the control plants; the effects of acid fog and O3 on the starch concentration appear to be additive. The leaf number presented in Table 1 is the result after harvesting, but we also observed a change in the number of leaves over the experimental period. In September 2007, the mean number of leaves was less for the pH 3 SAF groups (AF3 and AF3–O3) than for the control groups (AF5 and AF5–O3). In May 2008, when the leaves were fully developed, the saplings’ leaves were further suppressed. Acid fog-treated plants showed considerably fewer leaves than control plants, and significant (P < 0.05) antagonistic effects of acid fog and O3 on the mean leaf number were observed.
Discussion
Results obtained after acid fog and/or O3 exposure suggest that these factors had an apparently cumulative impact on the total plant biomass, epicuticular wax and Ca2+ concentration in leaves, and the starch concentration in roots.
The total plant biomass of the beech saplings treated with pH 3 SAF was less than that of the control plants (Fig. 1b); in particular, root growth was significantly reduced (Fig. 1a). Many reports have described that SAF treatment reduces the total plant biomass (Smith et al. 1991; Igawa et al. 2002a; Shigihara et al. 2008a). The root growth impediment reportedly results from aluminum ion dissolution when the soil pH is less than 5.0 (Losno et al. 1993). In our experiment, however, soil pH (H2O) and pH (KCl) were unchanged during the exposure experiment. The reduced number of leaves observed in the experiment reduced the photosynthetic efficiency. Therefore, the decrease in the dry matter weight of the underground part (roots) is considered to be mainly attributable to a reduction in starch pools in the beech saplings. Furthermore, no synergistic effects were found between acid fog and O3; additive effects were found. O3 exposure also decreased the growth of beech saplings in our experiment, as has been reported for O3 exposure in other plant systems (Andersen et al. 1997; Watanabe et al. 2006).
A cuticular layer protects the leaf surface; this helps to prevent the loss of moisture and ions from the plant (Klemm 1989). In a previous study, acid-fog-treated leaves of beech saplings had less epicuticular wax than control leaves, as also shown in Fig. 2. Acid deposition (e.g., fog) and air pollutants accelerate the melting and erosion of the wax (Mengel et al. 1989) and change its chemical composition (Cape 1986). The decreased amount of epicuticular wax enhances the possibility of H+ diffusing to the leaf interior and leaching nutrients from the leaf (Tyree et al. 1991; Shigihara et al. 2008b). Chronic O3 exposure to beech saplings had no effect on the total amount of epicuticular wax. Günthardt-Goerg and Keller (1987) reported that the total amount of wax in Norway spruce needles (Picea abies (L.) Karst.) exposed to O3 at 300 ppb was reduced, but not when exposed to the lower concentration of 100 ppb.
Compared with the control plants, Ca2+ levels in the leaves of beech saplings were reduced substantially by exposure to pH 3 SAF (Fig. 3). Foliar Ca2+ leaching caused by acid fog exposure has been described in reports of both field and laboratory studies (Igawa et al. 2002a, b). The Ca2+ deficiency may strongly affect the physiological functions of plants in terms of, for example, membrane stability and winter cold tolerance (DeHayes et al. 1999). Recently, Shigihara et al. (2008a) reported that fog-induced base cation leaching increased as the pH of the SAF solution decreased. In fact, Ca2+, which is obtained from the soil via the roots, was significantly leached out from the beech foliage in comparison to other conifers such as fir (Abies firma) and cedar (Cryptomeria japonica). The water-soluble components in the soil after harvest were determined, but no significant differences were found between the components of the soil in the pots used for the saplings exposed to pH 3 SAF and those exposed to pH 5 SAF. Therefore, the effect of exposure of acid fog on the water-soluble components of the soil or litter was negligible. These reductions in mineral levels in leaves may result from the reduced uptake in combination with greater mineral retention by other plant parts.
In this study, the concentrations of starch in the roots and leaves of beech saplings were reduced significantly by exposure to pH 3 SAF (Table 2). The greater loss of starch in acid fog-treated plants than in control plants may be caused mainly by the reduced photosynthetic efficiency of leaves. Although we have no tangible proof of this reduction in photosynthesis, we observed a reduced number of leaves, and this is considered to exert a great influence on the photosynthetic efficiency.
In the present study, the combined effect of acid fog and O3 on root starch concentrations was also significant. Braun and Flückiger (1995) reported a significant reduction in root starch concentrations in beech seedlings exposed to ambient O3. These results imply that the ozone generated may impair the biochemical production process. According to Ziegler (1991), starch storage in roots is a sensitive indicator of a tree’s carbohydrate reserves. Therefore, the storage of low amounts of starch in the roots may affect the growth rate of beech saplings.
The results obtained under these experimental conditions underscore that acid fog and O3 exert negative effects on beech saplings in an additive manner, and that these conditions may occur under field conditions in the Tanzawa Mountains.
References
Andersen CP, Wilson R, Plocher M, Hogsett WE (1997) Carry-over effects of ozone on root growth and carbohydrate concentrations of ponderosa pine seedlings. Tree Physiol 17:805–811
Braun S, Flückiger W (1995) Effect of ambient ozone on seedlings of Fagus sylvatica L. and Picea abies L. Karst. New Phytol 129:33–44
Cape JN (1986) Effects of air pollution on the chemistry of surface waxes of Scots pine. Water Air Soil Pollut 31:393–399
DeHayes DH, Schaberg PG, Hawley GJ, Strimbeck GR (1999) Acid rain impacts on calcium nutrition and forest health. BioScience 49:789–800
Gifford RM, Thorne JH, Hitz WD, Giaquinta RT (1984) Crop productivity and assimilate partitioning. Science 225:801–808
Günthardt-Goerg MS, Keller TS (1987) Some effects of long term ozone fumigation on Norway spruce. II. Epicuticular wax and stomata. Trees 1:145–150
Igawa M, Kase T, Satake K, Okochi H (2002a) Severe leaching of calcium ions from fir needles caused by acid fog. Environ Pollut 119:375–382
Igawa M, Okumura K, Okochi H, Sakurai N (2002b) Acid fog remove calcium and boron from fir trees. J For Res 7:213–215
Klemm O (1989) Leaching of cations by acid rain from twigs and single needles. In: Schulze ED, Lange OL, Oren R (eds) Forest decline and air pollution. Springer, Berlin, pp 221–225
Losno R, Colin JL, Le Bris N, Bergametti G, Jickells T, Lim B (1993) Aluminium solubility in rainwater and molten snow. J Atmos Chem 17:29–43
Lux D, Leonardi S, Müller J, Wiemken A, Flückiger W (1997) Effects of ambient ozone concentrations on contents of non-structural carbohydrates in young Picea abies and Fagus sylvatica. New Phytol 137:399–409
Maruta E, Usui N (1997) Forest decline at Hinokiboramaru, Tanzawa (Report on the Natural Environment of Tanzawa–Oyama). Kanagawa Prefecture, Japan, pp 78–80 (in Japanese)
McLaughlin SB, McConathy RK, Duvick D, Mann LK (1982) Effects of chronic air pollution stress on photosynthesis, carbon allocation and growth of white pine trees. For Sci 28:60–70
Meier S, Grand LF, Schoeneberger MM, Reinert RA, Bruck RI (1990) Growth, ectomycorrhizae and nonstructural carbohydrate of loblolly pine seedlings exposed to ozone and soil water deficit. Environ Pollut 64:11–27
Mengel K, Hogrebe AMR, Esch A (1989) Effect of acidic fog on needle surface and water relations of Picea abies. Physiol Plant 75:201–207
Percy KE, Jensen KF, McQuattie CJ (1992) Effects of ozone and acidic fog on red spruce needle epicuticular wax production, chemical composition, cuticular membrane ultrastructure and needle wettability. New Phytol 122:71–80
Sase H, Takamatsu T, Yoshida T (1998) Variation in amount and elemental composition of epicuticular wax in Japanese cedar (Cryptomeria japonica) leaves associated with natural environmental factors. Can J For Res 28:87–97
Shigihara A, Matsumoto K, Sakurai N, Igawa M (2008a) Growth and physiological responses of beech seedlings to long-term exposure of acid fog. Sci Total Environ 391:124–131
Shigihara A, Matsumoto K, Sakurai N, Igawa M (2008b) Leaching of cell wall components caused by acid deposition on fir needles and trees. Sci Total Environ 398:185–195
Smith CR, Vasilas BL, Banwart WL, Walker WM (1991) Physiological response of two soybean cultivars to simulated acid rain. New Phytol 119:53–60
Sugiyama Y, Oshiro A (2001) A simple and rapid analysis of starch content in roots and shoots of satsuma mandarin using colorimetric determination. Soil Sci Plant Nutr 72:81–84 (in Japanese)
Takeda M, Aihara K (2007) Effects of ambient ozone concentrations on beech (Fagus crenata) seedlings in the Tanzawa Mountains, Kanagawa Prefecture, Japan. J Jpn Soc Air Pollut 42:107–117
Totsuka T, Aoki M, Izuta T, Horie K, Shima K (1997) Comparison of the air pollutant concentration and soil conditions on south-slope of damaged beech stands and north-slope of healthy beech stands at Mt. Hinokiboramaru (Report on the Natural Environment of Tanzawa–Oyama). Kanagawa Prefecture, Japan, pp 93–96 (in Japanese)
Tyree MT, Wescott CR, Tabor CA (1991) Diffusion and electric mobility of ions within isolated cuticules of Citrus aurantium. Plant Physiol 97:273–279
Watanabe M, Yamaguchi M, Iwasaki M, Matsuo N, Naba J, Tabe C, Matsumura H, Kohno Y, Izuta T (2006) Effects of ozone and/or nitrogen load on the growth of Larix kaempferi, Pinus densiflora and Cryptomeria japonica seedlings. J Jpn Soc Air Pollut 41:320–334
Yonekura T, Yoshidome M, Watanabe M, Honda Y, Ogiwara I, Izuta T (2004) Carry-over effects of ozone and water stress on leaf phonological characteristics and bud forest hardiness of Fagus crenata seedlings. Trees 18:581–588
Yoshida K, Shibasaki R, Takami C, Takenaka C, Yamamoto K, Tezuka T (2004) Response of gas exchange rates in Abies firma seedlings to various additional stresses under chronic acid fog stress. J For Res 9:195–203
Ziegler P (1991) Starch metabolism in plants: an overview. In: Bonnemain JL, Delrot S, Lucas WJ, Dainty J (eds) Advances in phloem transport and assimilate compartmentation. Quest Editions/Presses Academiques, Nantes, pp 196–203
Acknowledgments
This research was supported as a “Scientific Frontier Research Project” of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shigihara, A., Matsumura, Y., Kashiwagi, M. et al. Effects of acidic fog and ozone on the growth and physiological functions of Fagus crenata saplings. J For Res 14, 394–399 (2009). https://doi.org/10.1007/s10310-009-0144-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-009-0144-6