Abstract
Tropospheric ozone (O3) has become one of the main urban air pollutants. In the present study, we assessed impact of ambient and future ground-level O3 on nine commonly growing urban tree species under Free Air Ozone Enrichment (FAOE) condition. During the study period, mean ambient and elevated ozone (EO3) concentrations were 48.59 and 69.62 ppb, respectively. Under EO3 treatment, stomatal density (SD) significantly decreased and guard cell length (GCL) increased in Azadirachta indica, Bougainvillea spectabilis, Plumeria rubra, Saraca asoca and Tabernaemontana divaricata, while SD increased and GCL decreased in Ficus benghalensis and Terminalia arjuna. Proline levels increased in all the nine plant species under EO3 condition. EO3 significantly reduced photosynthetic rate, stomatal conductance (gs), and transpiration rates (E). Only A. indica and N. indicum showed higher gs and E under EO3 treatment. Water use efficiency (WUE) significantly increased in F. benghalensis and decreased in A. indica and T. divaricata. Air Pollution Tolerance Index (APTI) significantly increased in Ficus religiosa and S. asoca whereas it decreased in B. spectabilis and A. indica. Of all the plant species B. spectabilis and A. indica were the most sensitive to EO3 (high gs and less ascorbic acid content) while S. asoca and F. religiosa were the most tolerant (lowgs and more ascorbic acid content). The sensitivity of urban tree species to EO3 is a cause of concern and should be considered for future urban forestry programmes. Our study should guide more such studies to identify tolerant trees for urban air pollution abatement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid pace of industrialization and urbanization has resulted in high levels of air pollution and this problem is more common and serious in fast developing countries like China and India than the rest of the world (Health Effects Institute, 2019). Air pollution is generally caused by industries, power plants, automobiles using fossil fuel, and other agricultural practices including crop residue burning. Urban air pollution is mainly caused by automobiles and roadside dust. Main pollutants include oxides of nitrogen (NOx), carbon monoxide (CO), sulfur dioxide (SO2), ozone (O3), and suspended particulate matter (PM2.5 and PM10). In most of the Indian cities including Lucknow (capital city of Uttar Pradesh), two main urban pollutants of concern are O3 and particulate matter. Both of these pollutants have caused one of the highest mortality in India (Health Effects Institute, 2019). In India, there are reports of decrease in productivity of rice, wheat, and maize crops due to rising O3 in Indo-Gangetic Plains (IGP) (Oksanen et al., 2013). Using GEOS-Chem model, Lu et al. (2018) predicted that in future O3 pollution problem in India may become much severe.
Ambient O3 is mainly produced by the photochemical reactions of air pollutants such as volatile organic compounds, carbon monoxide, and oxides of nitrogen in the presence of sunlight (Ainsworth, 2017). In humans, exposure to high level of O3 may result in respiratory and cardiovascular diseases, and premature mortality (Levy et al., 2005). In plants, EO3 causes reduced photosynthesis and accelerated senescence (Ainsworth, 2017; Wittig et al., 2009). Stomata play crucial role in gas exchange between plants and atmosphere. Plants control the amount of O3 entering into the leaves through stomatal responses (Hoshika et al., 2015). Various leaf morphological characteristics (e.g., stomatal density and pore size, leaf weights and areas) are related to sensitivity/tolerance of plants to O3.
Plants play a vital role in air pollution remediation (Nowak et al., 2014). Plants growing in urban environments mitigate significant amount of air pollution through filtering, intercepting and absorbing pollutants (Nowak et al., 2014). Manes et al. (2016) reported a monetary value of USD 47 and 297 million for PM10 and ozone removal, respectively by urban and peri-urban trees in 10 cities of Italy. Nowak et al. (2014) reported that trees and forests removed 14.4 million tons of air pollution in 2010 in the USA, with human health effects valued at 6.8 billion USD. Generally native/introduced plants are grown around roads and dividers depending upon their height, growth architecture, functional group (deciduous/evergreen), leaf structure etc. But plants respond differently to ambient air pollution. There have been reports of responses of tree species to air pollutants in terms of their relative tolerance or sensitivity (Gao et al., 2016; Wen et al., 2004). However, information on the impacts of current and future levels of O3 on trees is scarce in India. We recently studied impact of long term (2 years) exposure of elevated ozone (+ 20 ppb above the ambient) on photosynthetic traits and anti-oxidative defense system of Leucaena leucocephala, a tree of great economic importance (Singh et al., 2021). It was found that there were several negative long lasting physiological and biochemical impacts on Leucaena. The results revealed that Leucaena exhibited greater sensitivity to O3 during initial stage of exposure, i.e., up to 12 months than the later stage (Singh et al., 2021).
Air Pollution Tolerance Index (APTI) is used to evaluate relative sensitivity/tolerance levels of plants to air pollution. APTI uses four parameters, viz., leaf extract pH, relative water content (RWC), total chlorophyll, and ascorbic acid content. These parameters are estimated and computed to obtain a numerical APTI of a plant (Singh et al., 1991). Large numbers of studies have been carried out to evaluate APTI of different plant species (Kaur et al., 2020). It is to be noted, however, that all the APTI related studies have been done on plant species that were growing in urban environment with current levels of pollution. However, it is necessary to explore and understand the responses of common plants to future levels of pollution to identify pollution tolerant plants, which in turn would provide useful information/suggestions to policy makers and urban planners.
Therefore, the present study evaluated the impact of EO3 on APTI, and physiological and stomatal characteristics of nine common tropical plants, grown in Free Air Ozone Enrichment (FAOE) facility.
Materials and methods
The experimental site was located in the garden of CSIR-National Botanical Research Institute (CSIR-NBRI), Lucknow along the southern bank of river Gomati at 26°55′ N latitude, 80°59′ E longitude and 113 m asl altitude.
FAOE setup
CSIR-NBRI FAOE setup consists of 4 rings each of 10 m diameter; two rings each for ambient and EO3. The O3 exposure system in FAOE ring consists of horizontal and vertical pipes and nozzle systems, with a maximum height of 5 m. Ozone was produced from pure oxygen using an ozone generator, and dispensed from the vertical and horizontal pipes through nozzles. O3 concentrations were constantly monitored from the centre of each ring using UV photometric ozone analyzers (Model O3 LEDM, Automatikprodukter, AP). More details of FAOE are given in Singh et al. (2021). The O3 exposure was done for 8 h day−1 (from 09:00 to 17:00 h) during study period. In the EO3 ring, O3 concentration was kept + 20 ppb above the ambient levels. Hourly recorded readings were averaged for each day. Ozone exposure started on 1 January 2018 and ended on 31 March 2018. AOT40 (Accumulated Ozone exposure over a Threshold of 40 ppb (=80 µg/m³)) values were calculated (Fig. 1).
Plant material and growing condition
Nine dominant tropical plant species which commonly grow in urban landscapes outside the forest were selected for the study. They were Azadirachta indica, Bouganvillia spectibilis, Ficus benghalensis, Ficus religiosa, Nerium indicum, Plumeria rubra, Saraca asoca, Tabernaemontana divaricata and Terminalia arjuna (Table 1). Approximately 1-year-old seedlings were planted in plastic pots of 12″ diameter (filled with garden soil) and grown under ambient conditions. Fifteen days before start of ozone exposure, seedlings with similar height and basal diameter were transferred to FAOE rings. In every ring, there were 5 replicate plants of each of the nine plant species. So for one particular plant species, there were total 20 seedlings in four rings. Inside the ring, pots were kept at a distance of 60 cm from one another. All plants were watered as and when required to avoid water stress. Triplicate samples were collected for each plant species from each treatment for determining different parameters in the last week of the experiment. For leaf gas measurements and stomatal characteristics, replicate numbers are given is respective figure legends.
Measurement of pH of leaf extract
0.5 g of fresh leaf was crushed in 50 ml of distilled water and pH of the extract was determined after calibrating pH meter (digital) with buffer solutions of pH values 4, 7, and 10 (Singh & Rao, 1983).
Relative water content estimation
Fresh leaves were plucked and fresh weight was taken. The leaf petioles were then immersed in water wrapping the leaf lamina with an aluminum foil for 4 h. The leaf was dried using a blotting paper and then weighed again to obtain the turgid weight. The leaves were then dried in an oven at 70 °C for 72 h and the dry weight was recorded (Barrs & Weatherley, 1962).
Relative water content (RWC) was calculated as per the following equation (Evans & Ting, 1974):
where
FW = Fresh weight (g)
DW = Dry weight (g)
TW = Turgid weight (g)
Total chlorophyll content estimation
For estimation of total chlorophyll, 0.5 g fresh leaves, from each plant representing ambient and O3 treated, were homogenized in chilled 80% acetone and homogenates were centrifuged at 10,000 g for 10 min. The absorbance of the clear supernatant was taken at 663 and 646 nm on a UV–VIS spectrophotometer (Spectra Max Plus, Molecular devices, USA). The concentration of total chlorophyll was calculated and expressed as mg g−1 fresh weight of leaves following Lichtenthaler (1987):
where A663 and A646 represent optical density at specific wavelengths
v = final volume (ml) of chl extract
w = fresh weight (g) of leaves
d = distance of light path (cm)
Ascorbic acid content estimation
Ascorbic acid (AA) was determined following Omayl et al. (1979). The leaf sample of 0.5 g was crushed in 2.5 ml of 10% TCA followed by centrifugation at 3500 g for 20 min. Re-extracted and the volume was made up to 5 ml. Of this 0.5 ml aliquot was taken and 1 ml DTC reagent (prepared by 3 g DNPH + 0.4 g thiourea and 0.05 g CuSO4 in 100 ml of 9 N H2SO4) was added. Subsequently it was incubated for 3 h at 37 °C in water bath. After that 0.750 ml of ice cold 65% H2SO4 was added and left for 30 min. The spectrophotometer reading at 520 nm OD was recorded. Ascorbic acid content was calculated using standard curve of ascorbic acid and expressed as mg g -1 fresh weight.
Calculation of air pollution tolerance index
The air pollution tolerance index (APTI) for various plant species from ambient and EO3 rings was calculated as per the equation given by Singh and Rao (1983). It uses four parameters namely ascorbic acid content, total chlorophyll content, relative water content, and pH of leaf extract.
where A = Ascorbic acid content (mg g−1 fresh weight)
T = Total chlorophyll (mg g−1 fresh weight)
P = pH of leaf extract
R = Relative water content of leaf (%)
Determination of leaf gas exchange parameters
Gas exchange parameters viz., the rate of net photosynthesis (PN), stomatal conductance (gs), and transpiration (E) were measured in 3rd or 4th fully expanded leaves with a portable photosynthesis system (Li-6800, LI-COR, Lincoln, NE, Nebraska, USA). The photosynthetically active radiation (PAR) was maintained at 1000 μmol (photons) m−2 s−1, the level of CO2 in the leaf chamber was maintained at 400 µmol CO2 mol−1. The vapor pressure deficit level was less than 3 kPa, the leaf temperature was 30 °C and the relative humidity was 60―65%. All the gas exchange parameters were measured between 8:00 and 11:00 h on four plants per treatment.
Measurement of stomatal density and guard cell length
Stomatal density (SD) and guard cell length (GCL) were measured on the same leaves which were used for gas exchange analysis. Canada balsam was used to create impressions on the abaxial leaf surface. Later, cello tape was applied to take negative impressions of leaves and the impressions were placed on glass slides. Images were captured using a Leica DM2500 microscope attached to a Leica DFC450C camera (Leica Microsystems, Wetzlar, Germany). Twelve images (3 images per leaf from 4 independent plants) were analyzed for stomatal density and guard cell length. SD was expressed as number of stomata/mm2.
Estimation of proline content
Proline content was estimated following a modified protocol of Bates et al. (1973). 500 mg of leaf tissue was homogenized in 1 ml of 3% (w/v) aqueous sulfosalicylic acid. Then 96% acetic acid and 3% sulfosalicylic acid (2:1) were added, followed by ninhydrin reagent. The mixture was set aside in a water bath at 100 °C for 1 h and then it was allowed to cool at room temperature. Toluene was added and the absorbance of a fraction with toluene aspired from the liquid phase was measured at a wavelength of 520 nm. Proline concentration was estimated using a standard curve and expressed as µmol proline g−1 fresh weight of leaves.
Statistical analysis
All the bar graphs were prepared using Sigma Plot 11.0 (Systat Software, Inc., Richmond, CA, USA). A pairwise comparison was done using Student’s t-test to examine the level of significance between the ambient and elevated ozone (EO3) exposed plants. All data are means ± standard deviation (SD) from independent measurements made on four individual plants. The significance levels are shown as *P < 0.05, **P < 0.01, and ***P < 0.001.
Results and discussion
Ozone concentration and AOT 40 values
The mean ozone concentrations during the experimental period were 48.59 ppb in ambient ozone (AO3) ring and 69.62 ppb in EO3 ring, indicating about 1.4 times enhancement in O3 levels due to ozone treatment (Fig. 1). Calculated AOT40 (accumulated exposure over a threshold 40 ppb) values for ambient and EO3 rings were 2.47 ppm h and 8.53 ppm h, respectively (Fig. 1).
During the period under study, maximum ambient O3 levels were recorded in the month of March, followed by February and January (Fig. 1). High ambient O3 concentrations in Indo-gangetic region of India were also reported by Tiwari et al. (2008) and Singh et al. (2010). This high concentration has been attributed to longer sun-shine hours, hot weather and lower relative humidity (Sharma et al., 2016). We have earlier reported high ambient O3 and AOT40 values for Lucknow city (Maurya et al., 2020; Singh et al., 2021).
Impact of EO3 on pH of leaf extract
The pH of the cell is an important factor for intracellular trafficking of vesicles and proteins, and the transport of small molecules like hormones apart from maintaining optimum environment for various biochemical reactions (Verweij et al., 2008). The pH of leaf extract of F. religiosa was 7.32 at ambient condition while that of T. arjuna and S. asoca were below 6.0 at ambient condition. Other plant species had pH between 6.0 and 7.0 under both the treatments. Most plant species showed decreasing pH values under EO3 as compared to ambient O3 (Table 2). Only in Ficus religiosa, pH significantly (p < 0.05) increased from 7.32 (ambient O3) to 8.72 (EO3). In fact, it was an important contributor to high APTI value of F. religiosa under EO3 condition. Zhang et al. (2016) reported that the greater is the value of leaf extract pH, the stronger is the ability of plants to absorb SO2 and NOx. The pH can also affect the reactivities of ascorbic acid towards ozone. Chang et al. (2021) have shown that higher acidity in airway-lining fluids could cause the lower reactivity of ascorbic acid to ozone. This implies that antioxidant ability of ascorbic acid against O3 may be significantly suppressed at low pH of leaf extract.
Impact of EO3 on Relative Water Content (RWC)
RWC is the water present in leaves relative to the full turgidity. Decreased RWC is associated with protoplasmic permeability, loss of water and dissolved nutrients, and early senescence of leaves (Agrawal & Tiwari, 1997). RWC in a plant body helps maintaining its physiological balance under stress conditions such as exposure to air pollution (Zhang et al., 2016). RWC was significantly greater (p < 0.05) in ambient conditions in comparison to EO3 condition in F. benghalensis, S. asoca, P. rubra, F. religiosa and N. indicum, where it ranged between 90 and 97%. It ranged between 80 and 90% in T. arjuna, T. divaricata, and B. spectabilis (Table 2). RWC was minimum in A. indica. Exposure to EO3 resulted in significant decline in RWC in B. spectabilis (15%), and A. indica (14%) in comparison to their respective controls. Previous studies have shown reduced leaf RWC due to drought (Deeba et al., 2012), high temperature (Duan et al., 2017), and air pollution (Zhang et al., 2016). Thus, in an urban environment, high leaf RWC helps plants maintain their physiological balance and enhance their tolerance ability to stress conditions.
Impact of EO3 on total chlorophyll content
EO3 caused significant decrease in chlorophyll content in most plant species (Table 2). In comparison to their respective control plants, maximum decrease was recorded in F. benghalensis (54.5%) followed by A. indica (47.35%), T. arjuna (42.24%), T. divaricata (29.15%), P. rubra (28.16%), B. spectabilis (13.55%) and F. religiosa (9.86%). N. indicum did not show any significant difference. Degradation of chlorophyll pigment has been widely used as an indicator of air pollution (Ninave et al., 2001). Reduced chlorophyll content due to EO3 is due to destruction of chlorophyll (Saitanis et al., 2001), or damage to thylakoid membranes (Kivimäenpää et al., 2005). Moreover, O3 indirectly affects chlorophyll content by accelerating leaf senescence (Donnelly et al., 2000). S. asoca was the only plant which showed enhanced chlorophyll content (15.78%) in response to EO3 treatment. Higher chlorophyll content provides tolerance to plants against air pollutants (Prajapati & Tripathi, 2008).
Impact of EO3 on ascorbic acid content
Ascorbic acid (AA) is a natural antioxidant and a strong reducing agent. It plays an important role in pollution tolerance and protects the plant against oxidative damage (Castagna & Ranieri, 2009).
In response to EO3 treatment, all the plants had greater levels of AA content compared to their respective controls except B. spectabilis (Table 2). The increase was more than 100% in S. asoca, 41% in T. divaricata, 40% in T. arjuna, 36% in N. indicum, 28% in A.indica and P. rubra, 26% in F. benghalensis, and 11% in F. religiosa. In contrast, in B. spectabilis, AA content was reduced by 30% in comparison to the control (Table 2).
AA is the most abundant water-soluble antioxidant in plants (Gallie, 2013) and is considered as an important molecule for plants’ tolerance to various abiotic stresses, e.g., metal stress (Dixit et al., 2001), heat stress (Kumar et al., 2013), and drought stress (Dolatabadian et al., 2009). In our study, a direct relationship was obtained between AA content of plants and ozone concentration, suggesting that greater Reactive Oxygen Species (ROS) production due to O3 induces higher production of AA (Bellini & De Tullio, 2019). Potential mechanisms include direct detoxification of O3-generated ROS by AA or alteration of redox signaling pathways that regulate O3-induced responses (Bailey et al., 2019). Plants use several strategies to combat O3 stress, including O3 avoidance (stomatal control) or O3 tolerance (detoxification/repair system). The protective role of AA, a ROS-scavenger is also supported by the enhanced O3-sensitivity shown by mutants deficient in AA synthesis (Conklin et al., 1996) and by transgenic plants overexpressing ascorbate oxidase (Sanmartin et al., 2003).
Air Pollution Tolerance Index (APTI) in response to EO3
APTI of F. religiosa, T. arjuna, and T. divaricata were greater in EO3 condition as compared to the ambient condition. Highest APTI values of 29.34 ± 0.24 (ambient) and 26.32 ± 0.41 (elevated) were in F. religiosa, and were lowest in T. arjuna (15.29 ± 0.25 and 15.26 ± 0.77). APTI values of T. divaricata were 20.88 ± 0.43 and 19.30 ± 0.79 in elevated and ambient O3, respectively (Table 2). APTI values for F. benghalensis were 27.07 and 25.05, for A. indica 20.88 and 16.70, for S. asoca 18.97 and 21.52, respectively in elevated and ambient O3. The APTI values in Nerium indicum were16 ± 1.15 and 18 ± 0.48, and the least were in Bougainvillea spectabilis i.e. 16 ± 1.15 and 11 ± 0.48 in ambient and EO3, respectively. Thus, the most significant change in APTI was in Bougainvillea spectabilis whereas, the least change was in Nerium indicum.
It is evident that APTI values of plants changed under EO3 condition. Some plants showed better APTI values under EO3 and some showed lower APTI values under EO3 treatment. Higher Greater APTI values are associated with higher tolerance of plants to air pollutants (Jyothi & Jaya, 2010; Pandey et al., 2016). According to a classification by Singh et al. (1991), plants having APTI values less than 12 are regarded as sensitive, between 13–16 are intermediate tolerants, between 17–20 are considered moderately tolerants and those with APTI value of more than 20 are tolerant to air pollutants. The variation in APTI values can be attributed to the variation in any of the four parameters used for computation of the index. In the present study, Bougainvillea APTI values reduced maximally in EO3 treatment, which may be attributed to less ascorbate, less chlorophyll content and low RWC. S. asoca APTI values increased maximally under EO3, which could be due to increased ascorbate content, more chlorophyll, and constant RWC. Das et al. (2010) categorized A. indica, growing around a steel manufacturing factory, as sensitive due to its low APTI value. Similar response was also observed in A. indica in the present study. Mukherjee and Agrawal (2018) reported A. indica to be sensitive to air pollutants (SO2, NO2 and O3). Although this plant maintained its antioxidant level but failed to maintain its water balance and resource utilization. Similarly in the present study, A. indica showed increase in antioxidants but had reduced RWC and total chlorophyll content which probably affected its photosynthetic performance under EO3 treatment.
The linear regression plots of individual variables with APTI in both ambient and EO3 condition showed a high positive correlation between APTI score and ascorbic acid content (Fig. 2a, b). However, total chlorophyll content was weakly and negatively correlated with APTI score. A positive correlation between RWC and APTI score was also obtained (Fig. 2a, b).
Impact of EO3 on leaf stomatal density (SD) and guard cell length (GCL)
Stomata play a crucial role in determining the O3 influx to leaves, because the majority of the O3 enters the leaf via stomatal pores (Vainonen & Kangasjärvi, 2015). Since O3 enters leaves of the plant through stomata, O3 influx are highly dependent on stomatal density and conductance (Shang et al., 2021). Stomatal density decreased significantly in A. indica, P. rubra, S. asoca, and T. divaricata by 35%, 37%, 33%, and 23%, respectively, while it significantly increased in F. benghalensis and. T. arjuna by 14% and 19%, respectively (Fig. 3a). Neufeld et al. (2019) reported that 100 ppb of O3 tended to reduce stomatal density, suggesting structural changes in plants. On the other hand, some studies have reported increased stomatal densities in trees due to high O3 (Baek et al., 2018; Frey et al., 1996).
GCL significantly increased in A. indica, B. spectabilis, P. rubra, S. asoca and T. divaricata by 18.5%, 9%, 19%, 22%, and 13.7%, respectively, while it significantly decreased in F. benghalensis, F. religiosa and in T. arjuna by 13.3%, 11.6%, and 11.4%, respectively under EO3 treatment as compared to the ambient conditions (Fig. 3b). Light micrographs (Fig. 4) and scanning electron micrographs (Fig. 5) also show the changes as mentioned above. Guard cells control the rates of CO2/O3 entry by regulating the apertures of the stomatal pores in the leaf epidermis. Reduced stomatal conductance is commonly observed after O3 exposure (Pearson & Mansfield, 1993), and it is possible that these reductions reflect a primary response of the guard cells to O3 (Maier-Maercker, 1999). Torsethaugen et al. (1999) showed in Vicia faba that O3 directly affects guard cells, targeting inward K+ channels, and inhibits stomatal opening. One possible mechanism for the observed O3 effect on guard cells, was direct oxidation of the channel proteins. Alternatively, elevation of cytosolic Ca2+ levels after O3 exposure is another plausible mechanism to explain these responses.
Light micrographs of the abaxial surface stomata in the leaves of Azadirachta indica A ambient ozone (AO3) and B elevated ozone (EO3); Bouganvillea spectabilis C, AO3 and D, EO3; Ficus benghalensis E, AO3 and F, EO3; Ficus religiosa G, AO3 and H, EO3; Plumeria rubra I, AO3 and J, EO3; Saraca asoca K, AO3 and L, EO3; Tabernaemontana divaricata M, AO3 and N, EO3; Terminalia arjuna O, AO3 and P, EO3. Scale bar is 50 μm
Scanning electron microscopy of abaxial surface of the leaves of Azadirachta indica A, Ambient Ozone (AO3) and B, Elevated Ozone (EO3); Bouganvillea spectabilis C, AO3 and D, EO3; Ficus benghalensis E, AO3 and F, EO3; Ficus religiosa G, AO3 and H, EO3; Plumeria rubra I, AO3 and J, EO3; Saraca asoca K, AO3 and L, EO3; Tabernaemontana divaricata M, AO3 and N, EO3; Terminalia arjuna O, AO3 and P, EO3. Scale is 100 μm
The effects of air pollutants on stomatal responses have been reported extensively, and the responses are very complex among the plants that vary with the environmental stressors. Ambient O3 enters into the leaves of the plant through stomata and its influx is greatly dependent on the stomatal density and stomatal conductance (Bertolino et al., 2019; Lee et al., 2021). Decrease in SD and GCL limits gs and transpiration (E), showing a shift towards minimum use of water and avoidance of O3 influx (Hoshika et al., 2015). Elagoz et al. (2006) reported significantly higher O3 induced gs rates in O3 sensitive bush bean than tolerant ones. This indicates that stomatal movement plays an important role in plants’ response to O3.
Higher stomatal densities and larger aperture size were found in O3 sensitive bush bean cultivars exposed to 60 ppb O3 (Elagoz et al., 2006).
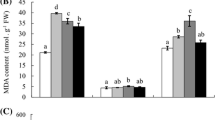
Impact of EO3 on proline content
Proline level in all the species increased more in EO3 than the ambient condition. Proline increased in leaves of A. indica, B. spectabilis, F. religiosa, N. indicum, P. rubra, S. asoca and T. arjuna by 3.7 2.05, 3.7, 1.81, 2.3, 2.19 and 2.98 fold, respectively. The increase was not significant in F. benghalensis and T. divaricata under EO3 as compared to ambient condition (Fig. 6).
Ozone is a powerful oxidant that produces ROS when it enters into the leaves. The response of various species and cultivars to O3 varies, and it is dependent on the evolved defense against ozone stress (Chaudhary & Rathore, 2021). Furthermore, the duration and degree of the stress affect the plant’s sensitivity to O3. One of the goals of this research was to determine whether plant’s antioxidative potential confers ozone tolerance to the selected tree species. Higher concentration of proline is generally considered as an indicator of induction of defense mechanism against different stresses (Verbrugeen & Hermans, 2008). Tropospheric O3 in the substomatal cavity forms ROS and also initiates a series of reactions which alters level of anions and leads to increase in proline content (Mansfield & Freer-Smith, 1984). Proline protects membranes and proteins by acting as a protein-compatible hydrotope (Srinivas & Balasubramanian, 1995) and it also acts as a hydroxyl radical scavenger (Smirnoff & Cumbes, 1989). The significant increase in proline accumulation in A. indica and F. religiosa followed by T. arjuna, P. rubra, S. asoca, B. spectabilis and N. indicum might be important in maintaining water potential equilibrium inside cells, which in turn acted as an adaptive mechanism against EO3 (Fig. 5) (Calzone et al., 2019).
Impact of EO3 on photosynthetic performance
The gas-exchange parameters of net photosynthesis (PN), stomatal conductance (gs), and transpiration (E) were monitored in ambient and elevated ozone (EO3) conditions. PN significantly decreased by 64.4%, 48.4%, 18.4%, 26.0%, 21.3%, 49.6%, 31.8%, and 15.4% in A. indica, B. spectabilis, F. benghalensis, F. religiosa, P. rubra, S. asoca, T. divaricata and T. arjuna, respectively due to EO3 conditions (Fig. 7).
Photosynthetic characteristics of different plant species grown under ambient and elevated O3 (+ 20 ppb above ambient) conditions. A Net photosynthesis, PN; B transpiration rate, E; C stomatal conductance, gs; D water use efficiency, WUE. Values are mean +/– S.D., n = 5. The significance levels are shown as *P < 0.05, **P < 0.01, and ***P < 0.001 according to Student’s t – test
Except A. indica, transpiration (E) declined in all the species under EO3 condition. F. benghalensis and S. asoca recorded the highest decline i.e. reduction by 62% and 61%, respectively (Fig. 6b). E increased only in A. indica. The stomatal conductance (gs) significantly reduced in F. benghalensis, F. religiosa, S. asoca, T. divaricata, and T. arjuna by 57.6%, 52.8%, 67.1%, 25.2%, and 37.3%, respectively under EO3 condition compared to the ambient condition. On the other hand, gs increased significantly in A. indica and N. indicum (Fig. 7c). Water use efficiency (WUE), which is the ratio of photosynthesis over transpiration, increased significantly under EO3 condition only in F. benghalensis in comparison to the ambient condition. It significantly decreased in A. indica and T. divaricata by more than 1.5-fold (Fig. 7d). McLaughlin et al. (2007) reported that spikes in ambient O3 significantly increased water loss of trees, suggesting that O3-induced aberrations in the stomatal dynamics differ among the species and the environmental conditions. Hoshika et al. (2012) reported that in poplar clone, although O3 stress resulted in loss of stomatal control over water loss, it was compensated by lower stomatal conductance and premature leaf shedding. Lower rates of stomatal conductance indicates a role for stomatal exclusion as a contributing factor towards reduced O3 sensitivity as observed by Bailey et al. (2019) in contrasting soybean genotypes. Thus lower stomatal conductance seems to be an important trait associated with O3 tolerance. This shows that stomatal exclusion is only one of the several possible tolerance mechanisms present in different plants. The lower rates of stomatal conductance along with lower transpiration rates suggest a potential link between O3 tolerance and drought tolerance. This assumes significance as almost all avenue or roadside plants also experience drought stress in an urban environment.
On exposure to EO3, the decrease or increase in SD and GCL alters the gas exchange traits. Usually, stomatal closure is stimulated by O3, leading to lower O3 uptake by plants and water usage due to less transpiration (Hoshika et al., 2015). In the present study, the change in stomatal conductance (gs) in B. spectabilis and P. rubra was not significant while significant decrease was observed in T. divaricata, N. indicum and T. arjuna followed by A. indica, F. religiosa, F. benghalensis and S. asoca in ozone treated plants. This response may be linked to a plants’ counter-measure mechanism for limiting pollutant entry by restricting its stomata. However, such a mechanism may also lower CO2 entry and ultimately lead to reduced rate of photosynthesis and transpiration (Shang et al., 2020). In plants, photosynthesis is the most basic and important physiological process. Under stressful conditions, physiological activities of the plants usually decrease. One of the most essential metrics for monitoring plant physiological activity under stress conditions is photosynthesis. Significant decrease in photosynthesis was observed in EO3 treated plants, viz., T. arjuna, F. benghalensis, F. religiosa, and P. rubra followed by T. divaricata, S. asoca, B. spectabilis, and A. indica compared to the ambient ozone condition. However, PN did not vary significantly in N. indicum (Fig. 7). Shang et al. (2017) reported reduction in photosynthesis in Poplar clone 546 and 107 exposed to ambient + 40 ppb and ambient + 60 ppb compared to charcoal filtered air. Poplar clone 546 reported maximum reduction in photosynthesis with significant reduction in its stomatal conductance and total chlorophyll. In A. indica, reduced PN was correlated with maximum reduction in its stomatal conductance as well as stomatal density which suggests that closure of stomata restricted uptake of O3 which might have affected photosynthesis as well. In the present study, low stomatal conductance was correlated with low stomatal density as well as increased guard cell length in all the tree species except in F. benghalensis and T. arjuna which suggests that stomatal closure took place under EO3 and it also affected its PN rate. Lee et al. (2021) reported reduction in stomatal density in Brassica juncea exposed to EO3 (105.6 ppb for 8 h). In F. benghalensis and T. arjuna, increase in stomatal density and reduced guard cell length along with increased WUE and reduced E could be an O3 tolerance mechanism as it represents a shift towards maximum water utilization in CO2 assimilation. The factors such as SD and GCL have the ability to affect stomatal mobility and the resultant gs, and WUE (Drake et al., 2013). This flexible modulation of stomatal size and number enables plants to shift the maximum and minimum gas exchange by modifying the stomatal size in response to the environmental stress (Hetherington & Woodward, 2003).
Conclusion
The present study provides empirical evidence that pollution tolerant trees could be a viable strategy to improve air quality. Among the tree species studied, S. asoca and F.religiosa were the most tolerant, and B. spectabilis and A. indica were the most sensitive to EO3. The APTI was useful, but to a limited extent in selecting tolerant plants. Therefore, it is time that a more robust and inclusive index is developed, as indicated in the present study. WUE, stomatal traits and photosynthesis rates varied among different plant species and therefore, these should be taken as O3 responsive parameters. The species screened for O3 tolerance in this study, though limited in number, should be useful for plantation in the areas experiencing high O3 concentration such as many pockets of peninsular India and Indo-Gangetic plains.
References
Ainsworth, E. A. (2017). Understanding and improving global crop response to ozone pollution. The Plant Journal, 90(5), 886–897.
Agrawal, S. K., & Tiwari, S. L. (1997). Susceptibility level of few plants on the basis of air pollution tolerance index. Indian Forester, 123, 319–322.
Baek, S. G., Park, J. H., Na, C. S., Lee, B., Cheng, H. C., & Woo, S. Y. (2018). The morphological characteristics of Pterocarpus indicus induced by elevated ozone under well-watered and drought conditions. Forest Science and Technology, 14(3), 105–111.
Bailey, A., Burkey, K., Taggart, M., & Rufty, T. (2019). Leaf traits that contribute to differential ozone response in ozone-tolerant and sensitive soybean genotypes. Plants, 8(7), 235. https://doi.org/10.3390/plants8070235
Barrs, H. D., & Weatherley, P. E. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences, 15(3), 413–428.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207.
Bellini, E., & De Tullio, M. C. (2019). Ascorbic acid and ozone: Novel perspectives to explain an elusive relationship. Plants, 8, 122. https://doi.org/10.3390/plants8050122
Bertolino, L. T., Caine, R. S., & Gray, J. E. (2019). Impact of stomatal density and morphology on water-use efficiency in a changing world. Frontiers in Plant Science, 10, 225.
Calzone, A., Podda, A., Lorenzini, G., Maserti, B. E., Carrari, E., Deleanu, E., Hoshika, Y., Haworth, M., Nali, C., Badea, O., & Pellegrini, E. (2019). Cross-talk between physiological and biochemical adjustments by Punica granatum cv. Dente di cavallo mitigates the effects of salinity and ozone stress. Science of the Total Environment, 656, 589–597.
Castagna, A., & Ranieri, A. (2009). Detoxification and repair process of ozone injury: From O3 uptake to gene expression adjustment. Environmental Pollution, 157, 1461–1469.
Chang, Y. P., Wu, S. J., Lin, M. S., Chiang, C. Y., & Huang, G. G. (2021). Ionic-strength and pH dependent reactivities of ascorbic acid toward ozone in aqueous micro-droplets studied using aerosol optical tweezers. Physical Chemistry Chemical Physics, 23(16), 10108–10117.
Chaudhary, I. J., & Rathore, D. (2021). Assessment of ozone toxicity on cotton (Gossypium hirsutum L.) cultivars: Its defensive system and intraspecific sensitivity. Plant Physiology and Biochemistry, 166, 912–927.
Conklin, P. L., Williams, E. H., & Last, R. L. (1996). Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proceedings of the National Academy of Sciences, 93(18), 9970–9974.
Das, S., Mallick, S. N., Padhi, S. K., Dehury, B. C., Acharya, B. C., & Prasad, P. (2010). Air Pollution Tolerance Index (APTI) of various plant species growing in industrial areas of Rourkela. Indian Journal of Environmental Protection, 30, 563–567.
Deeba, F., Pandey, A. K., Ranjan, S., Mishra, A., Singh, R., Sharma, Y. K., Shirke, P. A., & Pandey, V. (2012). Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiology and Biochemistry, 53, 6–18. https://doi.org/10.1016/j.plaphy.2012.01.002
Dixit, V., Pandey, V., & Shyam, R. (2001). Differential anti-oxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). Journal of Experimental Botany, 52(358), 1101–1109.
Donnelly, A., Jones, M. B., Burke, J. I., & Schnieders, B. (2000). Elevated CO2 provides protection from O3 induced photosynthetic damage and chlorophyll loss in flag leaves of spring wheat (Triticum aestivum L., cv.‘Minaret’). Agriculture, Ecosystems & Environment, 80(1–2), 159–168.
Dolatabadian, A., Modarres-Sanavy, S. A. M., & Sharifi, M. (2009). Alleviation of water deficit stress effects by foliar application of ascorbic acid on Zea mays L. Journal of Agronomy and Crop Science, 195, 347–355. https://doi.org/10.1111/j.1439-037X.2009.00382.x
Duan, H., Wu, J., Huang, G., Zhou, S., Liu, W., Liao, Yang, X., Xiao, Z., & Fan, H. (2017). Individual and interactive effects of drought and heat on leaf physiology of seedlings in an economically important crop. AoB Plants, 9(1). https://doi.org/10.1093/aobpla/plw090
Drake, P. L., Froend, R. H., & Franks, P. J. (2013). Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany, 64, 495–505.
Elagöz, V., Han, S. S., & Manning, W. J. (2006). Acquired changes in stomatal characteristics in response to ozone during plant growth and leaf development of bush beans (Phaseolus vulgaris L.) indicate phenotypic plasticity. Environmental Pollution, 140(3), 395–405.
Evans, L. S., & Ting, I. P. (1974). Ozone sensitivity of leaves: Relationship to leaf water content, gas transfer resistance, and anatomical characteristics. American Journal of Botany, 61(6), 592–597.
Frey, B., Scheidegger, C., Gunthardt-Goerg, M. S., & Matyssek, R. (1996). The effects of ozone and nutrient supply on stomatal response in birch (Betula pendula) leaves as determined by digital image-analysis and X-ray microanalysis. New Phytologist, 132(1), 135–143.
Gallie, D. R. (2013). L-ascorbic acid: A multifunctional molecule supporting plant growth and development. Scientifica, 2013, 795964. https://doi.org/10.1155/2013/795964
Gao, F., Calatayud, V., García-Breijo, F., Reig-Armiñana, J., & Feng, Z. (2016). Effects of elevated ozone on physiological, anatomical and ultrastructural characteristics of four common urban tree species in China. Ecological Indicators, 67, 367–379.
Health Effects Institute. (2019). State of Global Air 2019, www.stateofglobalair.org (accessed on 10 February 2022).
Hetherington, A. M., & Woodward, F. I. (2003). The role of stomata in sensing and driving environmental change. Nature, 424, 901–908.
Hoshika, Y., Katata, G., Deushi, M., Watanabe, M., Koike, T., & Paoletti, E. (2015). Ozone-induced stomatal sluggishness changes carbon and water balance of temperate deciduous forests. Scientific Reports, 5(1), 1–8.
Hoshika, Y., Omasa, K., & Paoletti, E. (2012). Whole-tree water use efficiency is decreased by ambient ozone and not affected by O3-induced stomatal sluggishness. PLoS ONE, 7(6), e39270.
Jyothi, S. J., & Jaya, D. S. (2010). Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvananthapuram, Kerala. Journal of Environmental Biology, 31(3), 379-386.
Kaur, M., Sharma, A., Katnoria, J., & Nagpal, A. (2020). Air Pollution Tolerance Index (APTI): An important determinant for the development of green space in and around industrial/urban area. Bioremediation: Current Research and Applications; Rathoure, AK, Ed.; IK International Publishing House Pvt. Ltd.: New Delhi, India, 422–449.
Kumar, R., Goswami, S., Gadpayle, K., Singh, K., Sharma, S. Singh, G., Pathak, S., & Rai, R. (2013). Ascorbic acid at pre-anthesis modulate the thermotolerance level of wheat (Triticum aestivum) pollen under heat stress. Journal of Plant Biochemistry and Biotechnology. 23. https://doi.org/10.1007/s13562-013-0214-x
Kivimäenpää, M., Sellden, G., & Sutinen, S. (2005). Ozone-induced changes in the chloroplast structure of conifer needles, and their use in ozone diagnostics. Environmental Pollution, 137(3), 466–475.
Lee, J. K., Kwak, M. J., Park, S. H., Kim, H. D., Lim, Y. J., Jeong, S. G., Choi, Y. S., & Woo, S. Y. (2021). Ozone response of leaf physiological and stomatal characteristics in Brassica juncea L. at Supraoptimal temperatures. Land, 10(4), 357.
Levy, J. I., Chemerynski, S. M., & Sarnat, J. A. (2005). Ozone exposure and mortality: An empiric bayes meta regression analysis. Epidemiology, 16(4), 458–468.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic light stress on primary processes of photosynthesis. Journal of Plant Physiology, 138, 92–96.
Lu, X., Zhang, L., Liu, X., Gao, M., Zhao, Y., & Shao, J. (2018). Lower tropospheric ozone over India and its linkage to the South Asian monsoon. Atmospheric Chemistry and Physics, 18(5), 3101–3118.
Maier-Maercker, U. (1999). Predisposition of trees to drought stress by ozone. Tree Physiology, 19(2), 71–78.
Manes, F., Marando, F., Capotorti, G., Blasi, C., Salvatori, E., Fusaro, L., Cianncarella, L., Mircea, M., Marchetti, M., Chirici, G., & Munafo, M. (2016). Regulating ecosystem services of forests in ten Italian metropolitan cities: Air quality improvement by PM10 and O3 removal. Ecological Indicators, 67, 425–440.
Mansfield, T. A., & Freer-Smith, P. H. (1984). The role of stomata in resistance mechanisms. In M. J. Koziol & F. R. Whatley (Eds.), Gaseous Air Pollutants and Plant Metabolism (pp. 131–146). Butterworth.
Maurya, V. K., Gupta, S. K., Sharma, M., Majumder, B., Deeba, F., Pandey, N., & Pandey, V. (2020). Growth, physiological and proteomic responses in field grown wheat varieties exposed to elevated CO2 under high ambient ozone. Physiology and Molecular Biology of Plants, 26(7), 1437–1461. https://doi.org/10.1007/s12298-020-00828-9
Mukherjee, A., & Agrawal, M. (2018). Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicology and Environmental Safety, 152, 42–54.
McLaughlin, S. B., Nosal, M., Wullschleger, S. D., & Sun, G. (2007). Interactive effects of ozone and climate on tree growth and water use in a southern Appalachian forest in the USA. New Phytologist, 174(1), 109–124.
Neufeld, H. S., Sullins, A., Sive, B. C., & Lefohn, A. S. (2019). Spatial and temporal patterns of ozone at Great Smoky Mountains National Park and implications for plant responses. Atmospheric Environment: X, 2, 100023.
Ninave, S. Y., Chaudhari, P. R., Gajghate, D. G., & Tarar, J. L. (2001). Foliar biochemical features of plants as indiators of air pollution. Bulletin Environmental Contamination & Toxicology, 67, 133–140.
Nowak, D. J., Hirabayashi, S., Bodine, A., & Greenfield, E. (2014). Tree and forest effects on air quality and human health in the United States. Environmental Pollution, 193, 119–129.
Omayl, S. T., Turubull, J. D., & Saubexilich, H. E. (1979). Selected methods for determination of ascorbic acid in animal cells, tissues and fluids. Methods in enzymology (pp. 3–11). Acadmic press.
Oksanen, E., Pandey, V., Pandey, A. K., Keski-Saari, S., Kontunen-Soppela, S., & Sharma, C. (2013). Impacts of increasing ozone on Indian plants. Environmental Pollution, 177, 189–200.
Pandey, A. K., Pandey, M., & Tripathi, B. D. (2016). Assessment of air pollution tolerance index of some plants to develop vertical gardens near street canyons of a polluted tropical city. Ecotoxicology and Environmental Safety, 134, 358–364.
Pearson, M., & Mansfield, T. A. (1993). Interacting effects of ozone and water stress on the stomatal resistance of beech (Fagus sylvatica L.). New Phytologist, 123(2), 351–358.
Prajapati, S. K., & Tripathi, B. D. (2008). Anticipated Performance Index of some tree species considered for green belt development in and around an urban area: A case study of Varanasi city. India. Journal of Environmental Management, 88(4), 1343–1349.
Saitanis, C. J., Riga-Karandinos, A. N., & Karandinos, M. G. (2001). Effects of ozone on chlorophyll and quantum yield of tobacco (Nicotiana tabacum L.) varieties. Chemosphere, 42(8), 945–953.
Sanmartin, M., Drogoudi, P. D., Lyons, T., Pateraki, I., Barnes, J., & Kanellis, A. K. (2003). Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta, 216(6), 918–928.
Shang, B., Feng, Z., Gao, F., & Calatayud, V. (2020). The ozone sensitivity of five poplar clones is not related to stomatal conductance, constitutive antioxidant levels and morphology of leaves. Science of the Total Environment, 699, 134402.
Shang, B., Xu, Y., Peng, J., Agathokleous, E., & Feng, Z. (2021). High nitrogen addition decreases the ozone flux by reducing the maximum stomatal conductance in poplar saplings. Environmental Pollution, 272, 115979.
Shang, B., Zhaozhong, F., Pin, L., Xiangyang, Y., Yansen, X., & Calatayud, V. (2017). Ozone exposure- and flux-based response relationships with photosynthesis, leaf morphology and biomass in two poplar clones. Science of the total Environment, 603–604, 185–195.
Sharma, S., Sharma, P., Khare, M., & Kwatra, S. (2016). Statistical behavior of ozone in urban environment. Sustainable Environment Research, 26, 142–148.
Singh, P., Kannaujia, R., Narayan, S., Tewari, A., Shirke, P. A., & Pandey, V. (2021). Impact of chronic elevated ozone exposure on photosynthetic traits and anti-oxidative defense responses of Leucaena leucocephala (Lam.) de wit tree under field conditions. Science of the Total Environment, 782, 146907.
Singh, S., Agrawal, S. B., Singh, P., & Agrawal, M. (2010). Screening three cultivars of Vigna mungo L. against ozone by application of ethylenediurea (EDU). Ecotoxicology and Environmental Safety, 73, 1765–1775.
Singh, S. K., & Rao, D. N. (1983). Evaluation of plants for their tolerance to air pollution. In Proceedings of symposium on air pollution control (Vol. 1, No. 1, 218–224).
Singh, S. K., Rao, D. N., Agrawal, M., Pandey, J., & Naryan, D. (1991). Air pollution tolerance index of plants. Journal of Environmental Management, 32(1), 45–55.
Smirnoff, N., & Cumbes, Q. J. (1989). Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry, 28(4), 1057–1060.
Srinivas, V., & Balasubramanian, D. (1995). Proline is a protein-compatible hydrotrope. Langmuir, 11(7), 2830–2833.
Tiwari, S., Rai, R., & Agrawal, M. (2008). Annual and seasonal variations in tropospheric ozone concentrations around Varanasi. International Journal of Remote Sensing, 29, 4499–4514.
Torsethaugen, G., Pell, E. J., & Assmann, S. M. (1999). Ozone inhibits guard cell K+ channels implicated in stomatal opening. Proceedings National Academy of Sciences, 96(23), 13577–13582.
Vainonen, J. P., & Kangasjärvi, J. (2015). Plant signalling in acute ozone exposure. Plant, Cell & Environment, 38(2), 240–252.
Verbruggen, N., & Hermans, C. (2008). Proline accumulation in plants: A review. Amino Acids, 35(4), 753–759.
Verweij, W., Spelt, C., Di Sansebastiano, G. P., Vermeer, J., Reale, L., Ferranti, F., Koes, R., & Quattrocchio, F. (2008). An H+P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nature Cell Biology, 10(12), 1456–1462. https://doi.org/10.1038/NCB1805
Wen, D., Kuang, Y., & Zhou, G. (2004). Sensitivity analyses of woody species exposed to air pollution based on ecophysiological measurements. Environmental Science and Pollution Research, 11(3), 165–170.
Wittig, V. E., Ainsworth, E. A., Naidu, S. L., Karnosky, D. F., & Long, S. P. (2009). Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Global Change Biology, 15(2), 396–424. https://doi.org/10.1111/j.1365-2486.2008.01774.x
Zhang, P. Q., Liu, Y. J., Chen, X., Yang, Z., Zhu, M. H., & Li, Y. P. (2016). Pollution resistance assessment of existing landscape plants on Beijing streets based on air pollution tolerance index method. Ecotoxicology and Environmental Safety, 132, 212–223.
Acknowledgements
We thank Director, CSIR-NBRI, Lucknow, India, for providing all necessary facilities. The authors acknowledge the Council of Scientific and Industrial Research (CSIR), Govt of India, for funding this work (OLP-108). The partial financial support under International Union of Biological Sciences (IUBS), Paris project to International Society for Environmental Botanists (ISEB) and CSIR-NBRI is gratefully acknowledged. We thank Mr. Kuldeep Verma for assistance in FACE maintenance and operation.
Author information
Authors and Affiliations
Contributions
SKB and VP conceptualized the work. RJ and RK did biochemical work. SN and RD did stomata and physiology work. Sandip Behera did scanning electron microscopy work. SKB, PAS and VP analyzed the data. RR wrote the first draft. PAS, VP, and SKB improved the first draft. All authors read and approved the final draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jamal, R., Narayan, S., Dubey, R. et al. Response of tropical trees to elevated Ozone: a Free Air Ozone Enrichment study. Environ Monit Assess 195, 238 (2023). https://doi.org/10.1007/s10661-022-10713-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10713-5