Abstract
In this study, Corynebacterium glutamicum ATCC 13032 was engineered to produce l-citrulline through a metabolic engineering strategy. To prevent the flux away from l-citrulline and to increase the expression levels of genes involved in the citrulline biosynthesis pathway, the argininosuccinate synthase gene (argG) and the repressor gene (argR) were inactivated. The engineered C. glutamicum ATCC 13032 ∆argG ∆argR (CIT 2) produced higher amounts of l-citrulline (5.43 g/L) compared to the wildtype strain (0.15 g/L). To determine new strategies for further enhancement of l-citrulline production, the effect of l-citrulline on ornithine acetyltransferase (EC 2.3.1.35; OATase; ArgJ) was first investigated. Citrulline was determined to inhibit Ornithine acetyltransferase; for 50 % inhibition, citrulline concentration was 30 mM. The argJ gene from C. glutamicum ATCC 13032 was cloned, and the recombinant shuttle plasmid pXMJ19-argJ was constructed and expressed in C. glutamicum ATCC 13032 ∆argG ∆argR (CIT 2). Overexpression of the argJ gene exhibited increased OAT activity and resulted in a positive effect on citrulline production (8.51 g/L). These results indicate that OAT plays a vital role during l-citrulline production in C. glutamicum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corynebacterium glutamicum is a gram-positive soil bacterium with high GC content [21]. As a workhorse of industrial microbiology, C. glutamicum has been widely used for the production of amino acids, primarily l-glutamate and l-lysine [5, 16]; however, it can also be used to produce arginine, ornithine, and citrulline.
l-citrulline is an intermediate metabolite in the arginine biosynthesis pathway, and as a non-standard α-amino acid, citrulline has a wide range of potential functions in different fields. Studies have reported that citrulline is a key intermediate in the urea cycle, which is used by mammals to excrete ammonia [6]. Citrulline is also widely used in health and nutrition applications [15]. Compared to the extraction of natural products [18] and enzymatic conversion [12], microbial fermentation [8] is the most promising method for l-citrulline production.
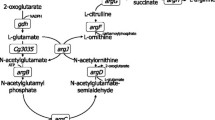
There are three routes for the biosynthesis of l-citrulline from the precursor l-glutamate in different organisms [3, 14, 24]. l-citrulline is biosynthesised through a six-step cyclic pathway (Fig. 1) that includes a series of acetylated intermediates in C. glutamicum. In this pathway, citrulline is converted to arginine by argininosuccinate synthase (argG) and arginosuccinase (argH) [3] and is regulated by the repressor ArgR shown to bind regions upstream of argC, argB, argF, and argG [19].
In the reaction, the acetyl group from acetylornithine in the citrulline biosynthetic pathway of C. glutamicum is recycled by ornithine acetyltransferase (OAT, ArgJ), which catalyses acetylornithine and glutamate into ornithine and N-acetylglutamate. ArgJ operates as a bifunctional protein and uses both substrates; this enzyme exhibits both NAGS (argA-encoded in Escherichia Coli) and OAT (argE-encoded in E. coli) activity. The earliest studies by Udaka and Kinoshita recognised that acetylated compounds form a cycle of reactions [23], whereas Sakanyan et al. [19] discovered that C. glutamicum only possesses a monofunctional ArgJ, which exhibits OAT activity but lacks NAGS activity. Recently, overexpression of the argJ gene showed increased NAGS activity by complementing the C. glutamicum arginine auxotrophic argJ strain [7]. Petri et al. [17] discovered that the monofunctional ArgJ only catalyses the fifth step of the citrulline biosynthesis pathway in C. glutamicum, and glutamate was acetylated by N-acetylglutamate synthase Cg3035. Enzyme inhibition tests showed that l-arginine had no influence on OAT activity; however, the ArgJ enzyme was inhibited when 5 mM l-ornithine was added [19].
In this study, C. glutamicum ATCC 13032 was engineered to produce citrulline by deleting the argG gene [21]; furthermore, the feedback repression by the arginine repressor (ArgR) was relieved. To investigate the inhibition of l-citrulline on OATase activity, the argJ gene from C. glutamicum ATCC 13032 was cloned and expressed to study its effects on OAT activity and l-citrulline production. Our results demonstrated that l-citrulline overproduction in the engineered strain correlated with expression levels of ArgJ, which plays a vital role in the l-citrulline biosynthesis in C. glutamicum.
Materials and methods
Strains, plasmids and primers
The strains, plasmids, and primers used in this study are listed in Tables 1 and 2.
Plasmid and strain construction
Gene argJ was amplified by PCR from the C. glutamicum ATCC 13032. After digestion with BamHI and EcoRI, the amplified product was ligated into the C. glutamicum/E. coli shuttle vector pXMJ19 to create pXMJ19-ec argJ. The recombinant plasmid was transformed into E. coli DH5α and C. glutamicum. To construct the C. glutamicum mutant strain with the argR gene deletion, crossover PCR was used to generate the argR deletion fragment. This strategy involved the replacement of a segment of the argR gene from C. glutamicum with a short synthetic fragment that maintained the translational reading frame. The 5′-upstream region of argR gene was amplified by PCR from C. glutamicum ATCC 13032 chromosomal DNA using primers argR-up-F and argR-up-R, as well as the 3′-upstream region of argR gene was amplified using primers argR-down-F and argR-down-R. Crossover PCR was carried out to generate the argR deletion fragment using primers argR-up-F and argR-down-R. The resulting argR deletion fragment was digested with EcoRI and HindIII and subsequently ligated to the suicide vector pK18 mobsacB. The recombinant plasmid pK18 mobsacB ∆argR was transformed into C. glutamicum through electroporation. Double homologous recombination was performed as described by Schafer et al. [20]. PCR and DNA sequencing verified deletion of the target genes in pK18 mobsacB derivatives and the C. glutamicum recombinants.
Bacterial strains and growth conditions
E. coli DH5α and C. glutamicum ATCC 13032 were used to construct the plasmid and the mutant strains, respectively, used in this study. E. coli and C. glutamicum were grown aerobically at 37 and 30 °C, respectively, in Luria–Bertani (LB) medium or LB medium with 10 % sucrose [20]. For l-citrulline production of C. glutamicum, the seed medium consisted of (per litre) 20 g glucose, 1.5 g K2HPO4·3H2O, 0.5 g KH2PO4, 0.4 g MgSO4·7H2O, 2.5 g urea, 0.02 g MnSO4·H2O, 0.02 g FeSO4·7H2O, 100 μg biotin, 200 μg vitamin B1, and 100 mg arginine. The culture medium consisted of (per litre) 80 g glucose, 1.0 g K2HPO4·3H2O, 1.0 g KH2PO4, 0.25 g MgSO4·7H2O, 40 g (NH4)2SO4, 0.02 g MnSO4·H2O, 0.02 g FeSO4·7H2O, 1 mg ZnCl2, 0.2 mg CuSO4, 100 μg biotin, 200 μg vitamin B1, 30 g CaCO3, and 100 mg arginine. The initial pH of all the above media was adjusted to 7.0. Shake flask cultures were prepared to test the effects of argJ on l-citrulline production. A 5-ml sample of the seed culture previously grown for 12 h was inoculated into 30 ml of the culture medium in a 500-ml flask and cultured for 72 h. All cultures were grown at 30 °C and shaken at 200 rpm on a rotary shaker. When necessary, 50 mg/L kanamycin or 20 mg/L chloramphenicol was added in the E. coli medium; 25 mg/L kanamycin or 10 mg/L chloramphenicol was added in the C. glutamicum medium.
Activity assay
Ornithine acetyltransferase (ArgJ), N-acetylglutamate kinase (ArgB), N-acetylglutamate semialdehyde dehydrogenase (ArgC), acetylornithine transaminase (ArgD), and ornithine transcarbamylase (ArgF) activities were detected according to the methods of Liu et al. [13], Udaka [22], Chun et al. [2], Friedrich et al. [4], and Kumar et al. [11], respectively.
C. glutamicum cells were grown in LB medium, harvested by centrifugation during the exponential phase, and washed in 100 mM Tris/HCl buffer (pH 7.5). Crude cell extracts were prepared by sonic disruption. All treatments were performed at 4 °C. Protein concentration was determined by the Bradford method [1]. OAT activity was measured in cell extracts by spectrophotometric determination of the formation of ornithine at 470 nm [13]. Assays were performed in a total volume of 0.5 ml containing 100 mM Tris–HCl buffer (pH 7.5), 60 mM N-Acetylornithine, 60 mM Glutamate and 200 μl cell extracts at 37 °C for 10 min. Next, the reaction was terminated by adding 500 μl volume of ninhydrin reagent (0.4 M citric acid 1 % ninhydrin in a methoxyethanol, 1:2, by vol.) and heated at 100 °C for 10 min. 500 μl volume of NaOH (4.2 M) were added and incubated for 20 min at room temperature. Subsequently, the concentration of ornithine produced was measured at 470 nm and determined by reference to a standard curve of 0–2,000 nmol omithine. One unit of OAT was defined as the amount of enzyme that catalyses the formation of 1 μmol of ornithine per min under assay conditions.
Assays of cell concentration, glucose and l-citrulline
After diluting the culture with 0.2 mol/L HCl to dissolve CaCO3, cell growth was monitored by measuring the optical density of the culture at 600 nm (OD600) using a spectrophotometer and converted to the cell dry weight (CDW), an OD600 of 1 was determined to equal 0.25 g CDW/L [10]. The glucose concentration was determined using a biosensor (Institute of Biology, Shandong Academy of Science, Shanghai, China). The Venusil-AA analytical method (Agela Technologies, Beijing) was used to determine the concentration of l-citrulline with pre-column derivatisation by phenyl isothiocyanate. A Dionex UltiMate 3000 series HPLC system was used for this study. Data collection and integration were performed using the Chromeleon Client software (version 6.80, build 2212).
Recombinant strains stability assay
To test for stability of the recombinant strains (CIT 1, CIT 2, CIT 3), the culture procedure was repeated continuously for approximately 10 generations, and citrulline concentrations were determined.
Results
Metabolic engineering of C. glutamicum for l-citrulline production
To construct a host strain capable of accumulating l-citrulline, it was first focused on inhibiting citrulline degradation of l-citrulline into arginine in C. glutamicum ATCC 13032 [21]. Thus, the argG gene encoding argininosuccinate synthase was deleted. The resulting strain of C. glutamicum ATCC 13032 ∆argG was named CIT 1. Functional verification indicated that CIT 1 is an arginine-requiring auxotrophic mutant, which used arginine for its growth. CIT 1 was cultivated in the medium and produced 2.52 g/L l-citrulline (Table 3). Compared to the wild-type C. glutamicum ATCC 13032 (0.15 g/L), the CIT 1 could accumulate a certain amount of l-citrulline.
Feedback repression is a type of metabolic regulation that usually affects amino acid metabolism. Several reports on the mechanism of ArgR repressors indicate that the expression levels of the related genes in the arginine operon are affected by the binding of these repressors to the corresponding promoter regions in C. glutamicum [25]. For the citrulline biosynthesis of C. glutamicum, an auxotrophic mutant with an additional deletion of the transcriptional regulator of citrulline biosynthesis was constructed. The argR gene was deleted to amplify citrulline biosynthetic flux. The resulting strain of C. glutamicum ∆argG ∆argR was named CIT 2. The specific activities of the citrulline biosynthesis enzymes (ArgC, ArgJ, ArgB, ArgD, and ArgF) were significantly improved by measuring the crude cell-free extracts of the recombinant strain CIT 2 (Table 4). This finding indicated that CIT 2 had higher expression levels of the citrulline biosynthesis genes when compared to CIT 1. Meanwhile, it is found that the double knockout strain produces l-citrulline concentration up to 5.43 g/L (Table 3), which is higher than that (2.52 g/L) in CIT 1. The deletion of the argG and argR genes enhanced l-citrulline production in C. glutamicum. Thus, the ArgR protein functions during the feedback repression of citrulline synthesis in C. glutamicum.
Effects of l-citrulline on OAT activity
The C. glutamicum gene (argJ) encoding the enzyme, which deacetylates acetylornithine in the citrulline biosynthetic pathway, was cloned. OAT activity was inhibited by low concentration of citrulline, and arginine had no inhibition on the activity of OAT. Figure 2 presents the effect of citrulline on OAT activity. The assays using a crude extract of CIT 2 showed that the citrulline concentration for 50 % inhibition of OAT was 30 mM.
The specific activity of OAT from C. glutamicum was determined using crude extracts of the recombinant CIT 2. The OAT enzyme from C. glutamicum was sensitive to the end product l-citrulline. From these results, the argJ gene in C. glutamicum was targeted for the production of l-citrulline.
Effect of overexpression of argJ gene on l-citrulline production
OAT was subjected to feedback inhibition by l-citrulline in C. glutamicum. OAT overexpression may overcome this inhibition, thereby increasing the production of citrulline in CIT 2. To investigate the effect of overexpression of OAT on l-citrulline production in C. glutamicum, the pXMJ19-argJ plasmid carrying the argJ gene from C. glutamicum ATCC 13032 was constructed and transformed into C. glutamicum CIT 2. The resulting strain was named CIT 3. The overexpression of the argJ gene produced a large amount of l-citrulline (8.51 g/L) (Table 3). Citrulline production in the flask cultures was significantly increased, as expected. The enhanced production of citrulline under argJ homologous overexpression suggests that it was likely due to an increase in the expression level of OAT, which is inhibited by l-citrulline. The strain CIT3 (C. glutamicum ATCC 13032 ∆argG ∆argR pXMJ19-cg argJ) could be used as a basis to further improve l-citrulline production.
Stability of recombinant strains
Citrulline yields were measured to test the stability of the recombinant strains, which were cultured continuously for 10 generations in shake flasks. The citrulline concentrations of CIT 1, CIT 2 and CIT 3 are maintained at 2.50, 5.40 and 8.50 g/L, respectively, indicating that the recombinant strains have good genetic stability.
Discussion
Based on the wild-type C. glutamicum ATCC 13032, the strains CIT 1 (C. glutamicum ATCC 13032 ∆argG), CIT 2 (C. glutamicum ATCC 13032 ∆argG ∆argR) and CIT 3 (C. glutamicum ATCC 13032 ∆argG ∆argR pXMJ19-cg argJ) were obtained.
There was a 56.7 % increase in citrulline production from CIT 2 (5.43 g/L) to CIT3 (8.51 g/L). CIT 3 produced threefold higher citrulline than CIT 1 (2.52 g/L). Overexpression of argJ gene indeed enhanced the metabolic flux of the l-citrulline biosynthetic pathway.
There were no differences in the cell growth and glucose consumption between C. glutamicum CIT 1, CIT 2 and CIT 3 (Fig. 3). OAT activity was measured in crude extracts of the constructed strains (Table 5). The highest OAT activity exceeded the activity in C. glutamicum CIT 1 8.5 times. As expected, overexpression of the argJ gene enhanced the weak expression, improved the activity, and reduced the inhibition of citrulline, and a significant increase in production could be achieved.
In summary, these results have clearly demonstrated that OAT does influence the production of citrulline in C. glutamicum. OAT is a key enzyme for enhancing the biosynthesis of citrulline from glutamate in C. glutamicum. Overexpression of the argJ gene from C. glutamicum was beneficial for achieving higher levels of citrulline production. Based on our measurements, in future studies, the argJ gene will be studied continuously to determine the mechanism of feedback inhibition.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chun JY, Lee EJ, Lee HS et al (1998) Molecular cloning and analysis of the argC gene from Corynebacterium glutamicum. Biochem Mol Biol Int 46:437–447
Cunin R, Glansdorff N, Pierard A et al (1986) Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev 50:314–352
Friedrich B, Friedrich CG, Magasanik B (1978) Catabolic N-2-acetylornithine 5-aminotransferase of Klebsiella aerogenes: control of synthesis by induction, catabolite repression, and activation by glutamine synthetase. J Bacteriol 133:686–691
Hayashi M, Ohnishi J, Mitsuhashi S et al (2006) Transcriptome analysis reveals global expression changes in an industrial l-lysine producer of Corynebacterium glutamicum. Biosci Biotechnol Biochem 70:546–550
Hecker M, Sessa WC, Harris HJ et al (1990) The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle l-citrulline to l-arginine. Proc Natl Acad Sci USA 87:8612–8616
Hwang GH, Cho JY (2010) Identification of a suppressor gene for the arginine-auxotrophic argJ mutation in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 37:1131–1136
Ikeda M, Mitsuhashi S, Tanaka K et al (2009) Reengineering of a Corynebacterium glutamicum l-arginine and l-citrulline producer. Appl Environ Microbiol 75:1635–1641
Jakoby M, Ngouoto-Nkili CE, Burkovski A (1999) Construction and application of new Corynebacterium glutamicum vectors. Biotechnol Tech 13:437–441
Kabus A, Niebisch A, Bott M (2007) Role of cytochrome bd oxidase from Corynebacterium glutamicum in growth and lysine production. Appl Environ Microbiol 73:861–868
Kumar A, Vij N, Randhawa GS (2003) Isolation and symbiotic characterization of transposon Tn5-induced arginine auxotrophs of Sinorhizobium meliloti. Indian J Exp Biol 41:1198–1204
Kwon NS, Nathan CF, Gilker C et al (1990) l-citrulline production from l-arginine by macrophage nitric oxide synthase. The ureido oxygen derives from dioxygen. J Biol Chem 265:13442–13445
Liu Y, Robyn Van Heeswijck R, Høj P et al (1995) Purification and characterization of ornithine acetyltransferase from Saccharomyces cerevisiae. Eur J Biochem 228:291–296
Lu CD (2006) Pathways and regulation of bacterial arginine metabolism and perspectives for obtaining arginine overproducing strains. Appl Microbiol Biotechnol 70:261–272
Moinard C, Cynober L (2007) Citrulline: a new player in the control of nitrogen homeostasis. J Nutr 137:1621S–1625S
Park SD, Lee JY, Sim SY et al (2007) Characteristics of methionine production by an engineered Corynebacterium glutamicum strain. Metab Eng 9:327–336
Petri K, Walter F, Persicke M et al (2013) A novel type of N-acetylglutamate synthase is involved in the first step of arginine biosynthesis in Corynebacterium glutamicum. BMC Genomics 14:713
Rimando AM, Perkins-Veazie PM (2005) Determination of citrulline in watermelon rind. J Chromatogr A 1078:196–200
Sakanyan V, Petrosyan P, Lecocq M et al (1996) Genes and enzymes of the acetyl cycle of arginine biosynthesis in Corynebacterium glutamicum: enzyme evolution in the early steps of the arginine pathway. Microbiology 142:99–108
Schäfer A, Tauch A, Jäger W et al (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73
Tang J, Hao N, Xu S et al (2013) Construction of Corynebacterium glutamicum mutant with knockout of argG gene. J Nanjing Univ Technol (Nat Sci Ed) 35:86–90
Udaka S (1966) Pathway-specific pattern of control of arginine biosynthesis in bacteria. J Bacteriol 91:617–621
Udaka S, Kinoshita S (1958) Studies on l-ornithine fermentation. I. The biosynthetic pathway of l-ornithine in Micrococcus glutamicum. J Gen Appl Microbiol 4:272–282
Xu Y, Labedan B, Glansdorff N (2007) Surprising arginine biosynthesis: a reappraisal of the enzymology and evolution of the pathway in microorganisms. Microbiol Mol Biol Rev 71:36–47
Yim SH, Jung S, Lee SK et al (2011) Purification and characterization of an arginine regulatory protein, ArgR, in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 38:1911–1920
Acknowledgments
This study was supported by the National Basic Research Program of China (973 Program) (No. 2011CBA00807), the National High Technology Research and Development Program of China (863 Program) (No. 2012AA022101), the National Natural Science Foundation of China (No. 31270162), the Natural Science Foundation of Jiangsu Province (No. BK20140932), the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (No. 13KJB530008), and the PAPD Project of Jiangsu Province, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, N., Mu, J., Hu, N. et al. Improvement of l-citrulline production in Corynebacterium glutamicum by ornithine acetyltransferase. J Ind Microbiol Biotechnol 42, 307–313 (2015). https://doi.org/10.1007/s10295-014-1561-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1561-x