Abstract
The phenolic compounds present in hydrolysates pose significant challenges for the sustainable lignocellulosic materials refining industry. Three Saccharomyces cerevisiae strains with high tolerance to lignocellulose hydrolysate were obtained through ethyl methanesulfonate mutation and adaptive evolution. Among them, strain EMV-8 exhibits specific tolerance to vanillin, a phenolic compound common in lignocellulose hydrolysate. The EMV-8 maintains a specific growth rate of 0.104 h−1 in 2 g L−1 vanillin, whereas the reference strain cannot grow. Physiological studies revealed that the vanillin reduction rate of EMV-8 is 1.92-fold higher than its parent strain, and the Trolox equivalent antioxidant capacity of EMV-8 is 15 % higher than its parent strain. Transcriptional analysis results confirmed an up-regulated oxidoreductase activity and antioxidant activity in this strain. Our results suggest that enhancing the antioxidant capacity and oxidoreductase activity could be a strategy to engineer S. cerevisiae for improved vanillin tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Encouraged by the high potential of using biofuels and biochemicals to partly substitute for petrochemical products, much effort has been dedicated to constructing powerful cell factories to produce biofuels and biochemicals from lignocellulosic materials, which are natural, abundant, and renewable [8, 18, 24]. However, during the degradation of the lignocellulosic materials, inhibitor compounds, including organic acids, furans, and phenolics, are inevitably formed with the release of sugars [8, 18, 19]. These inhibitors stunt the growth and block the metabolism of the microorganisms. Saccharomyces cerevisiae is considered to be a highly competitive cell factory for biorefining as it is robust and safe, and genetic manipulation is easy [11, 24]. A better understanding of the mechanisms that underlie the S. cerevisiae tolerance to the inhibitors is essential for the rational design and construction of a yeast cell factory.
Extensive research has revealed the harm that the weak acids and furans can exert, and how the S. cerevisiae can resist them. Undissociated acetic acid in the strain growth environment moves across the membrane through the Fps1p channel and then dissociates in the neutral cytoplasm, leading to acidification of the cytosol [16]. Cells degrade the Fps1p channel when Hog1p is activated by the increased intracellular acetate anion pool, to avoid further damage [15, 16]. The furan compounds [furfural and 5-hydroxymethylfurfural (HMF)] induce the accumulation of ROS in S. cerevisiae [1], reduce enzymatic and biological activity, break down DNA, and inhibit protein and RNA synthesis [10]. The furfural and HMF tolerance of S. cerevisiae is mainly associated with the strain’s capacity to transform furfural and HMF into the less toxic furfuryl alcohol and 2,5-bis-hydroxymethylfuran, respectively [6, 10, 13]. However, only limited studies have been carried out on yeast tolerance to low-molecular-weight phenolic compounds generated from lignin hydrolysis, which are very toxic to S. cerevisiae [19]. The type of phenolic compounds in the hydrolysate are material dependent, vanillin is a commonly used example. It was reported that the vanillin acts at a low concentration, affecting membrane components and function, and causing serious DNA damage [2–4]. The genes encoding the ergosterol biosynthetic enzymes were screened out using a set of diploid yeast deletion mutants, and were proposed to be associated with vanillin tolerance. This is because ergosterol is important for maintaining the fluidity and stability of the membrane [4]. However, knowledge about the toxicity of vanillin is still limited, and the mechanism by which the S. cerevisiae resist the intracellular vanillin is not clear.
In the present work, three S. cerevisiae strains with high tolerance to the inhibitors present in steam-exploded corn stover were obtained by ethyl methanesulfonate (EMS) mutation and adaptive evolution. Among them, strain EMV-8 showed a noticeable tolerance to vanillin. To elucidate its vanillin tolerance mechanism, the physiological and transcriptional alteration of EMV-8 was compared with its parent strain NAN-27, an industrial ethanol production S. cerevisiae strain [24]. The possible vanillin tolerance/resistance mechanism of S. cerevisiae is discussed.
Materials and methods
Strain and media
The S. cerevisiae industry strain NAN-27 [24], an ethanol-producing strain used in the Henan Tianguan Group Co. Ltd. (China), was used as the starting strain. The YPD medium contains 20 g L−1 of glucose, 20 g L−1 of tryptone, and 10 g L−1 of yeast extract, and was used as the basic yeast culture medium. The inhibitors or diluted extract of the steam-exploded corn stover were added to the YPD for screening, evolving, and tolerance testing of the strains. The YPD medium with 1 g L−1 vanillin was used to determine the vanillin conversion capacity of the strains. Fermentation was performed in shake flasks at 30 °C to determine the vanillin-converting capacity of the strains. The initial OD600 was 0.1.
Preparation of steam-exploded corn stover extract
The corn stover was pretreated with 2 % H2SO4 at 2.5 MPa for 5 min. Then, the 200-g (dry weight) steam-exploded corn stover (SECS) was incubated with 1 L of distilled water at 80 °C for 1 h. The liquid part was separated and adjusted to 1 L with distilled water and then added to another 200 g (dry weight) SECS and incubated for an additional 1 h at 80 °C to obtain the extract of SECS (ExtSECS). The ExtSECS, containing 1.71 g L−1 of formic acid, 1.20 g L−1 of acetic acid, 0.05 g L−1 of levulinic acid, 0.62 g L−1 of HMF, 0.68 g L−1 of furfural, 0.22 g L−1 of vanillin (representative of phenolics, other phenolic components were not determined), and other uncharacterized inhibitors were used as the lignocellulosic hydrolysate model.
Chemical mutagenesis
The cells of NAN-27 were cultured in YPD for 12 h and then collected and resuspended into the fresh 50 mmol L−1 phosphate-buffered solution (pH 7.0) prepared YPD medium with a cellular concentration of 107 mL−1. The 0.28 mol L−1 EMS was added into the cell re-suspension solution and after 100 min incubation at 30 °C, 5 % (w/v) Na2S2O3 was added to stop the mutagenesis. Then, the mutants were washed twice and pre-cultured in 25 % ExtSECS-YPD for 24 h at 30 °C before screening on the 50 % ExtSECS-YPD plate.
Adaptive evolution
The strain was evolved in 40–60 % ExtSECS-YPD in shake flasks. For each batch, the initial OD600 was 0.1. The OD600 and residual sugar level at 11 h for each batch were determined, and then the cell was transferred to the fresh medium. When the residual sugar level was constant in three batches, the ratio of ExtSECS in the evolving medium was increased 5 % in the next batch of evolution. After 180 batches of adaptive evolution, the high-tolerance strains were isolated based on their growth characteristics on the 60 % ExtSECS-YPD plate.
Analytical methods
The strain biomass was detected by a turbidity determination at 600 nm. The biomass of a dry weight (DCW) was calculated as biomass (g DCW L−1) = 0.227 × OD600, as previously described [24].
The glucose, ethanol, formic acid, acetic acid, levulinic acid, HMF, and furfural levels were detected by high-performance liquid chromatography (HPLC; Shimadzu, Japan) equipped with a refractive index detector (RID-10A) and an Aminex HPX-87H ion exclusion column (Bio-Rad, USA). The mobile phase was 5 mmol L−1 H2SO4, the flow rate was 0.6 mL min−1, and the temperature of the column oven was 45 °C. The vanillin and vanillyl alcohol were detected by HPLC (Shimadzu) equipped with a prominence diode array detector (SPD-M20A) and a Biosil-C18 column (Bio-Rad, USA). The detector was set at a wavelength of 210 nm. The mobile phase was 40 % aqueous methanol, and the flow rate was 0.4 mL min−1, as previously described [7].
Total antioxidant capacity assay
Overnight cultured yeast cells were transferred into fresh YPD medium with an initial OD600 of 0.5 and then harvested when the OD600 reached 12–14. The collected cells were washed twice with phosphate-buffered saline (pH 7.3) and then resuspended in phosphate-buffered saline (pH 7.3) containing 1 mmol L−1 of the protease inhibitor α-toluenesulfonyl fluoride. The resuspended cells were broken up in a FastPrep cell homogenizer (Thermo Savant, USA) as described previously [22], and then centrifuged at 10,000g and 4 °C for 20 min. The supernatant was collected and used as a crude sample for the antioxidant capacity assay.
The 3-ethylbenzthiazoline-6-sulfonic acid (ABTS) can be oxidized to green ABTS+ with an oxidant. The total antioxidant capacity assay of the strains was assayed by determining the ABTS+ scavenging activity [12] of the cell extract using the Total Antioxidant Capacity Assay Kit (Beyotime, China). The standard curve was prepared using 0.15, 0.2, 0.3, 0.45, and 0.6 mmol L−1 Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), which is an antioxidant-like vitamin E and is used in biological or biochemical applications to reduce oxidative stress or damage. Ten microliters of Trolox standard solution or samples was incubated with 200 µL fresh ABTS solution for 6 min at room temperature. The absorbance was then measured at 405 nm. The total protein concentration in the sample was measured by using a BCA protein assay reagent kit (Sangon Biotech Co., Ltd., China). The sample total antioxidant capacity was present as the Trolox equivalent antioxidant capacity (TEAC) per gram of total protein.
Transcriptome analysis
The pre-cultured strain was transferred into fresh YPD medium with an initial OD600 of 0.2 and was cultured at 30 °C for 3–3.5 h until the OD600 reached 0.8. Then, the cells were collected and washed three times with RNase-free sterile water. The total RNA was extracted by using the TRIzol total RNA extraction kit (Sangon Biotech Co., Ltd., Shanghai, China). The RNA sequencing was performed using the solexa PE method, which produces high-quality transcriptomic reads with a total size of 500 Mb, resulting in an average of 81 bp per read. The genes whose log2 (fold change) >2 or <−2, p value <0.001, were considered to be significantly regulated. The enrichment of GO terms were mapped using the Gene Ontology Term Finder tool supplied by Saccharomyces Genome Database (http://www.yeastgenome.org). The hits with p value <0.01 were displayed. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for a given gene list were calculated using a classical hypergeometric distribution statistical comparison of a query gene list against a reference gene list. The calculated p value goes through a false discovery rate (FDR) correction, taking the corrected p value <0.001 as a threshold. The KEGG pathways that fulfilled this condition are considered to be significant.
Results
Obtaining inhibitor-tolerant strains by chemical mutagenesis and adaptive evolution
To obtain a robust strain that could be used in lignocellulosic bioethanol production, the S. cerevisiae industry diploid strain NAN-27 [24] was chemically mutated by EMS. A mutant with improved tolerance to the inhibitors was screened out on a 50 % ExtSECS-YPD plate and named after EM-13. The EM-13 was further evolved in diluted ExtSECS-YPD media, and the three clones grown on the 60 % ExtSECS-YPD plate for screening were named after EMT-7, EMB-42, and EMV-8. The tolerance of strains to the specific inhibitors (acetic acid, furfural, and vanillin) was determined to study their basic features (Fig. 1). The tolerance of the mutants to these inhibitors was improved. They can grow well on the plates that contain 1 g L−1 vanillin, 1.1 g L−1 furfural, or 4 g L−1 acetic acid, while their parent strain NAN-27 could not (Fig. 1). Interestingly, among the mutants, the EMV-8 showed a higher tolerance to vanillin compared with the other strains (Fig. 1). The growth capacity of strain EMV-8 in the presence of different inhibitors was investigated further (Table 1). In inhibitor-free YPD medium, the maximum specific growth rate (µ max) of EMV-8 and its parent strain NAN-27 were similar, 0.485 ± 0.004 and 0.494 ± 0.002 h−1, respectively, However, EMV-8 exhibited excellent resistance to the vanillin. The µ max of EMV-8 in 1 g L−1 vanillin was as high as 0.311 ± 0.003 h−1, and even in the 2 g L−1 vanillin, it could grow with the µ max of 0.104 ± 0.002 h−1, while the parent strain almost stopped growing under the same conditions. The resistance of EMV-8 to the furfural was also higher than NAN-27 (Table 1).
Vanillin reduction capacity was enhanced in the high-tolerance strain
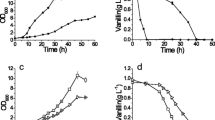
Converting inhibitors to less toxic compounds is one of the important ways that organisms avoid damage from the inhibitors. Fitzgerald et al. [5] proposed that some non-specific aryl-alcohol dehydrogenase enzyme(s) can convert vanillin to the less toxic vanillyl alcohol in S. cerevisiae. In the present work, the vanillin reduction capacity of the strains was determined in the YPD medium with 1 g L−1 vanillin. The vanillin was converted to vanillyl alcohol in both EMV-8 and NAN-27 and the yield was close to 100 %. The average specific vanillyl alcohol generation rate of EMV-8 was 0.048 ± 0.003 g g−1 DCW h−1, which is 1.92-fold higher than NAN-27 (0.025 ± 0.002 g g−1 DCW h−1) (Fig. 2). The results demonstrate that the vanillin-tolerant strain EMV-8 has significantly enhanced vanillin reduction capacity.
Basic glucose metabolism (a) and vanillin reduction (b) of the strains. The cells were cultured in the YPD medium with 1 g L−1 vanillin in 300 mL shake flasks with 100 mL medium at 30 °C. The data were the average of three biological repetitions. The square, round, triangle, diamond, and star symbols denote the concentrations of biomass, glucose, ethanol, vanillin, and vanillyl alcohol, respectively
Total antioxidant capacity was increased in the high-tolerance strain
Nguyen et al. [17] suggested that S. cerevisiae damage by vanillin is induced by oxidative stress. Therefore, the TEAC of the strains was measured by determining the ABTS+ scavenging activity of the cell extract. The EMV-8 has a 15 % higher TEAC than its parent strain NAN-27; 0.40 ± 0.01 versus 0.34 ± 0.01 mmol L−1 g−1 (Fig. 3).
Total antioxidant capacity of the strains. The cell extracts obtained from YPD cultured strains were used as the reducing agent to scavenge the ABTS+ in the reaction mixture. The total antioxidant capacity of the sample was present as the TEAC per gram of total protein. The data were the average of three biological repetitions
Transcriptional alteration of the inhibitor-tolerant strain
Transcriptional analysis of strain EMV-8 and of its parent strain NAN-27 was performed using RNA sequence technology to understand the high vanillin tolerance mechanism of EMV-8 further. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were analyzed.
The significant shared GO terms of genes with prominent changes [log2 (EMV-8 vs. NAN-27) >2] were mapped (Fig. 4) using the Gene Ontology Term Finder tool from the Saccharomyces Genome Database (http://www.yeastgenome.org). From a molecular function viewpoint, the structural constituent of ribosome (GO: 0003735) was down-regulated. The up-regulated genes were enriched in oxidoreductase activity (GO: 0016491) and transporter activity (GO: 0005215). The ubiquinol-cytochrome-c reductase genes, QCR2, QCR6-10, which related to both these two GO terms, were up-regulated. Among the sub-terms of oxidoreductase activity, the oxidoreductases acting on the CH–OH group of donors, especially the ones using NAD or NADP as an acceptor, were more significant than others (Fig. 4). The details of the genes involved are listed in Table 2. The log2 (fold change) of putative medium-chain alcohol dehydrogenase gene BDH2, glyoxylate reductase gene YPL113C, putative aryl-alcohol dehydrogenase AAD10, arabinose dehydrogenase genes ARA1 and ARA2, xylose and arabinose reductase gene YJR096W, and α-keto amide reductase gene YDL124W were 4.6, 2.6, 2.9, 2.5, 2.7, 2.3, and 2.7, respectively.
Significant shared GO terms of genes that log2-fold change >2 in EMV-8 compared with NAN-27. A p value, indicated by color in the graphic, is provided as a score of significance. Genes in the graphic are associated with the GO term(s) to which they are directly annotated. Significant hits with a p value <0.01 and terms descended from them are shown
It was reported that overexpression of aldehyde dehydrogenase 6 (ALD6) improves the furfural tolerance of S. cerevisiae [20]. While investigating the same aldehyde group, we noticed that several aldehyde dehydrogenase genes were also noticeably up-regulated in EMV-8. The log2 (fold change) of hexadecenal dehydrogenase gene HFD1 and the aldehyde dehydrogenases ALD4 and ALD6 were 2.8, 4.5, and 2.9, respectively (Table 2). Furthermore, also attributing to the enhanced antioxidant capacity, some antioxidant-related genes, including CTA1, GPX1, CTT1, SOD2, GRX2, and PRX1 were significantly up-regulated, the log2 (fold change) was 6.3, 4.3, 3.8, 2.8, 2.4, and 2.2, respectively (Table 2).
In the pathway analysis, the genes in EMV-8 that were up-regulated compared with the parent strain NAN-27 were enriched in several energy-generating pathways. The citrate cycle (TCA cycle) [PATH: ko00020], oxidative phosphorylation [PATH: ko00190], pyruvate metabolism [PATH: ko00620], fructose and mannose metabolism [PATH: ko00051], galactose metabolism [PATH: ko00052], and peroxisome [PATH: ko04146] were up-regulated. In addition, the apoptosis pathway, which is usually up-regulated under stress, was expressed at a higher level in EMV-8 than in NAN-27 (Table 3, Table S1). Compared with NAN-27, in EMV-8, almost all of the cell multiplication-related pathways, such as ribosome [PATH: ko03010], RNA transport [PATH: ko03013], pyrimidine metabolism [PATH: ko00240], purine metabolism [PATH: ko00230], RNA polymerase [PATH: ko03020], and DNA replication [PATH: ko03030] were down-regulated significantly. This might be one of the reasons why the growth rate of EMV-8 is lower than NAN-27 under stress-free conditions.
Discussion
Vanillin is one of the most potent inhibitors in lignocellulose hydrolysates. However, compared with other inhibitors such as weak acids and furans, the knowledge of vanillin tolerance in yeast is limited. More information is necessary to design a strain-optimization strategy. Three types of strategies were commonly used to study inhibitor tolerance/resistance mechanisms: (1) investigating the response of the strain when inhibitors are present under the culture conditions [1, 2, 9, 21]; (2) studying the inhibitor-sensitive mutants as selected from the gene disruption library [3, 6, 14]; and (3) comparing the difference between the inhibitor-resistant mutants and their reference strains [11, 23]. The first and second strategies mainly direct to the stress response and the survival factors, respectively. In the present work, we studied the physiological and transcriptional characteristics of a vanillin-tolerant strain following the third strategy to elucidate the factors affecting the resistance to vanillin.
Endo et al. [3, 4] demonstrated that a higher intracellular ergosterol level leads to a higher tolerance of yeast to the vanillin. However, this is not the only factor; the vanillin tolerance mechanism is more complex. The transcriptional analysis of our vanillin-tolerant strain EMV-8 indicated that the expression of genes involved ergosterol biosynthesis did not increase; in contrast, most of them were down-regulated (Table S2). This suggests that there is a different resistance mechanism in our strain. As Fitzgerald et al. [5] proposed, the vanillin can be converted to the less toxic vanillyl alcohol in S. cerevisiae strains. Our results demonstrate that the vanillin-tolerant strain EMV-8 has a higher vanillin conversion rate. The transcriptional analysis also confirmed an up-regulated oxidoreductase activity (GO: 0016491) in EMV-8, which could supply the reduced coenzyme or reduce the vanillin directly. This might contribute to the enhanced vanillin tolerance of EMV-8. Furthermore, the fermentation result also reveals that the vanillin is gradually reduced during the fermentation. In other words, the strain grows up before the vanillin concentration decreases (Fig. 2). There are some other properties to support the survival of EMV-8 in a high level of vanillin. Nguyen et al. [17] suggest that the damage of vanillin to S. cerevisiae is induced by oxidative stress. Our vanillin-tolerant strain exhibits a high antioxidant capacity. The genes encoding the proteins involved in antioxidant activity, such as superoxide dismutase and catalase, were expressed at a very high level, which has a positive effect on scavenging free radicals and keeps cells functioning under oxidative stress. This might be one of reasons that reduces the damage of vanillin and supports the initiative growth of EMV-8.
Our present work gives useful clues for further work to figure out the specific genes that are responsible for the vanillin reduction, or supply the sufficiently reduced coenzymes. Further investigation is needed to design the overall solutions to construct a robust strain for lignocellulosic hydrolysates fermentation.
References
Allen SA, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger PJ, Liu ZL, Gorsich SW (2010) Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels 3:2. doi:10.1186/1754-6834-3-2
Ding MZ, Wang X, Yang Y, Yuan YJ (2011) Metabolomic study of interactive effects of phenol, furfural, and acetic acid on Saccharomyces cerevisiae. OMICS 15(10):647–653. doi:10.1089/omi.2011.0003
Endo A, Nakamura T, Ando A, Tokuyasu K, Shima J (2008) Genome-wide screening of the genes required for tolerance to vanillin, which is a potential inhibitor of bioethanol fermentation, in Saccharomyces cerevisiae. Biotechnol Biofuels 1(1):3. doi:10.1186/1754-6834-1-3
Endo A, Nakamura T, Shima J (2009) Involvement of ergosterol in tolerance to vanillin, a potential inhibitor of bioethanol fermentation, in Saccharomyces cerevisiae. FEMS Microbiol Lett 299(1):95–99. doi:10.1111/j.1574-6968.2009.01733.x
Fitzgerald DJ, Stratford M, Narbad A (2003) Analysis of the inhibition of food spoilage yeasts by vanillin. Int J Food Microbiol 86(1–2):113–122
Gorsich SW, Dien BS, Nichols NN, Slininger PJ, Liu ZL, Skory CD (2006) Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 71(3):339–349. doi:10.1007/s00253-005-0142-3
Ji L, Shen Y, Xu L, Peng B, Xiao Y, Bao X (2011) Enhanced resistance of Saccharomyces cerevisiae to vanillin by expression of lacA from Trametes sp. AH28-2. Bioresour Technol 102(17):8105–8109. doi:10.1016/j.biortech.2011.06.057
Jonsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6(1):16. doi:10.1186/1754-6834-6-16
Li BZ, Yuan YJ (2010) Transcriptome shifts in response to furfural and acetic acid in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 86(6):1915–1924. doi:10.1007/s00253-010-2518-2
Liu ZL (2006) Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl Microbiol Biotechnol 73(1):27–36. doi:10.1007/s00253-006-0567-3
Liu ZL, Ma M, Song M (2009) Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol Genet Genomics 282(3):233–244. doi:10.1007/s00438-009-0461-7
Luo JG, Li L, Kong LY (2012) Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem 131(3):1056–1062
Ma M, Liu ZL (2010) Comparative transcriptome profiling analyses during the lag phase uncover YAP1, PDR1, PDR3, RPN4, and HSF1 as key regulatory genes in genomic adaptation to the lignocellulose derived inhibitor HMF for Saccharomyces cerevisiae. BMC Genomic 11:660. doi:10.1186/1471-2164-11-660
Mira NP, Palma M, Guerreiro JF, Sa-Correia I (2010) Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb Cell Fact 9:79. doi:10.1186/1475-2859-9-79
Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27(18):6446–6456. doi:10.1128/MCB.02205-06
Mollapour M, Shepherd A, Piper PW (2008) Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 25(3):169–177. doi:10.1002/yea.1576
Nguyen TT, Iwaki A, Ohya Y, Izawa S (2013) Vanillin causes the activation of Yap1 and mitochondrial fragmentation in Saccharomyces cerevisiae. J Biosci Bioeng. doi:10.1016/j.jbiosc.2013.06.008
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74(1):17–24
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74(1):25–33
Park SE, Koo HM, Park YK, Park SM, Park JC, Lee OK, Park YC, Seo JH (2011) Expression of aldehyde dehydrogenase 6 reduces inhibitory effect of furan derivatives on cell growth and ethanol production in Saccharomyces cerevisiae. Bioresour Technol 102(10):6033–6038. doi:10.1016/j.biortech.2011.02.101
Pereira FB, Guimaraes PM, Gomes DG, Mira NP, Teixeira MC, Sa-Correia I, Domingues L (2011) Identification of candidate genes for yeast engineering to improve bioethanol production in very high gravity and lignocellulosic biomass industrial fermentations. Biotechnol Biofuels 4(1):57. doi:10.1186/1754-6834-4-57
Shen Y, Chen X, Peng B, Chen L, Hou J, Bao X (2012) An efficient xylose-fermenting recombinant Saccharomyces cerevisiae strain obtained through adaptive evolution and its global transcription profile. Appl Microbiol Biotechnol 96(4):1079–1091. doi:10.1007/s00253-012-4418-0
Yang J, Ding MZ, Li BZ, Liu ZL, Wang X, Yuan YJ (2012) Integrated phospholipidomics and transcriptomics analysis of Saccharomyces cerevisiae with enhanced tolerance to a mixture of acetic acid, furfural, and phenol. OMICS 16(7–8):374–386. doi:10.1089/omi.2011.0127
Zhang X, Shen Y, Shi W, Bao X (2010) Ethanolic cofermentation with glucose and xylose by the recombinant industrial strain Saccharomyces cerevisiae NAN-127 and the effect of furfural on xylitol production. Bioresour Technol 101(18):7104–7110
Acknowledgments
This work was supported by the Grants of the National Basic Research Program of China (2011CB707405), the National High Technology Research and Development Program of China (2012AA022106), the National Natural Science Foundation of China (30970091), the State Key Laboratory of Motor Vehicle Biofuel Technology (No. 2013004), and the Independent Innovation Foundation of Shandong University (IIFSDU 2010TS059).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, Y., Li, H., Wang, X. et al. High vanillin tolerance of an evolved Saccharomyces cerevisiae strain owing to its enhanced vanillin reduction and antioxidative capacity. J Ind Microbiol Biotechnol 41, 1637–1645 (2014). https://doi.org/10.1007/s10295-014-1515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1515-3