Abstract
Fermentation of liquid hot water (LHW) pretreated Miscanthus giganteus (MG) by Clostridium beijerinckii NCIMB 8052 was investigated towards understanding the toxicity of lignocellulose-derived inhibitors to solventogenic Clostridium species vis-à-vis butanol production. While C. beijerinckii NCIMB 8052 did not grow in undiluted MG hydrolysate-based fermentation medium, supplementation of this medium with Calcium carbonate enabled the growth of C. beijerinckii NCIMB 8052 and production of butanol. Using high-performance liquid chromatography (HPLC) and spectrophotometric assays, LHW-pretreated MG was found to contain lignocellulose-derived microbial inhibitory compounds; some of which were transformed by exponentially growing C. beijerinckii to less inhibitory compounds during fermentation. Contrary to all expectations, the reduction product of furfural, furfuryl alcohol, inhibited butanol production by C. beijerinckii by more than 16 %. Collectively, these results provide new insights into why lignocellulosic biomass hydrolysates are recalcitrant to fermentation to biofuels and chemicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Researches on the production of biofuel may help reduce the use of fossil fuels, alleviate global energy crises, and ultimately reduce greenhouse gas emissions. Conventional biofuels from corn and sugar canes have limitations such as low energy content and high substrate cost. Butanol production from lignocellulosic biomass has shown promise to overcome these challenges [8, 10]. Use of lignocellulosic biomass for biofuel production offers many benefits such as substrate abundance, relatively high net energy yield, low cost, and potential to alleviate market stress, which many have attributed to the use of food crops for the production of biofuels [13]. To release sugars from lignocellulosic biomass, an appropriate pretreatment process is required to disrupt the lignin matrix, to depolymerize hemicelluloses, and to reduce the crystallinity of cellulose. However, numerous microbial inhibitors are generated during the pretreatment and hydrolysis of lignocellulosic biomass to monomeric sugars [20]. These compounds may have large negative effects on butanol production mainly due to inhibition of cell growth or metabolite production by cell membrane disruption, nucleic acid damage, and enzyme inhibition [22]. Pre-fermentation detoxification steps have been intended to remove such inhibitory compounds and consequently enhance the growth of fermenting microorganisms, but they significantly increase overall fuel and chemical production costs due to costs associated with the process and potential loss of fermentable sugars during detoxification [1, 8]. Significant improvements in the fermentation of lignocellulosic biomass hydrolysates will be achieved if sugars and biomass-derived microbial inhibitors can be simultaneously metabolized by fermenting microorganisms during fermentation.
Furfural and hydroxymethyl furfural (HMF) are common furan aldehydes derived from dehydration of pentoses and hexoses during pretreatment and hydrolysis of lignocellulosic biomass [21]. Depending on the source of the biomass and the type of pretreatment employed, the concentration of furan aldehydes in lignocellulosic hydrolysates can reach 5.9 and 3.5 g/L for furfural and HMF, respectively [19]. The effects of furan aldehydes such as furfural and HMF on biofuel production by Saccharomyces cerevisiae [29], Escherichia coli [21], and solventogenic Clostridium species [8, 35] have been investigated. These furans are thought to damage cell membranes, obstruct RNA synthesis in microorganisms, and inhibit activities of glycolytic enzymes and proteins [21, 32]. Clostridium acetobutylicum ATCC 824 [35] and other microorganisms [4, 11] have demonstrated the ability to reduce furfural to less toxic furfuryl alcohol; however, the effect of furfuryl alcohol on acetone butanol ethanol (ABE) fermentation by solventogenic Clostridium species is still unclear. Besides furan aldehydes, lignocellulose-derived phenolic compounds (generated from lignin decomposition), especially low molecular weight phenolics such as 4-hydroxybenzaldehyde and p-coumaric acid, also show inhibitory effects on cell growth and alcohol fermentation [16, 19]. Further, little information is available on impacts of lignocellulose-derived phenolic compounds on microorganisms used for alcoholic fermentation due to insufficient availability of accurate qualitative and quantitative analyses [25]. Since the biotransformation of these phenolic compounds by solventogenic Clostridium species has not been investigated, this gap in knowledge needs to be filled to enable development of superior strategies for constructing inhibitor-tolerant solventogenic Clostridium strains.
The overarching objective of the present study, therefore, was to examine the feasibility of utilizing MG hydrolysates as the carbon source for growth of and butanol production by C. beijerinckii NCIMB 8052. Given that calcium carbonate (CaCO3) has been used successfully as an additive to stimulate sugar utilization [18] and to boost butanol tolerance [26] in solventogenic Clostridium species, the effect of CaCO3 on alleviation of MG hydrolysate’s toxicity to C. beijerinckii NCIMB 8052 during butanol production was examined. Moreover, CaCO3 was recently proposed to stabilize biosynthesis and growth machinery of C. beijerinckii NCIMB 8052 leading to robust growth and cell tolerance to environmental stress [14].
Materials and methods
Microorganism and culture conditions
Clostridium beijerinckii NCIMB 8052 (hereafter referred to as C. beijerinckii) was obtained from the American Type Culture Collection, Manassas, VA. C. beijerinckii stocks were maintained as spore suspensions in sterile double-distilled water at 4 °C. Two hundred microliters of C. beijerinckii spores was heat shocked at 75 °C for 10 min, cooled on ice, then inoculated into 10-mL anoxic tryptone–glucose–yeast extract (TGY) medium [35]. The culture was incubated anaerobically at 35 ± 1 °C for 12–14 h until OD600 0.9–1.1 was attained. To increase the volume of the pre-culture, actively growing C. beijerinckii culture (8 %, v/v) was transferred into 100 mL TGY medium and grown for 3–5 h until OD600 0.9–1.1 was attained. This was used as the pre-culture.
Pretreatment of Miscanthus giganteus (MG)
Prior to pretreatment, MG was ground using a Thomas-Wiley mill and 1 mm sieve (Thomas Scientific, Swedesboro, NJ, USA). The moisture content was measured by an infrared moisture analyzer (Mettler Toledo, Columbus, OH, USA) according to the NREL Laboratory Analytical Procedure (LAP) [27], which is necessary to calculate the amount of water to be added to the MG to generate different percentages of solid MG loading. Approximately 119.8 g of MG biomass with a moisture content of 9.87 % was mixed with 600 mL distilled or de-ionized water to obtain 15 % solids loading, calculated on weight/volume basis (\(\% {\text{Solid}} = \frac{{119.8 \,{\text{g }}\,{\text{MG }}\,{\text{biomass}} \times (100-9.87)\,\% }}{{600 \,{\text{mL }}\,{\text{water + 119}} . 8 \,{\text{g }}\,{\text{MG }}\,{\text{biomass}}}}\)). The liquid hot water (LHW) pretreatment was carried out in a Parr Reactor (Parr, Moline, IL, USA) at 190 °C for 20 min. After cooling to 25 °C, the MG slurry was neutralized to pH 6.0 with ammonium hydroxide. Enzymatic hydrolysis of the pretreated MG was initiated by adding cellulase (15 FPU/g cellulose), β-glucosidase (Novozyme 188) (40 U/g cellulose), and xylanase (0.25 g/100 mL) as described previously [8]. The enzymatic hydrolysis was carried out at 50 °C for 72 h after which the MG hydrolysates were stored at −20 °C until analysis or use for fermentation experiments.
Production of acetone butanol ethanol by C. beijerinckii using MG hydrolysates as the carbon source
Batch ABE fermentations by C. beijerinckii were performed in 150-mL Pyrex screw-capped media bottles in triplicate. About 100 mL P2 medium containing 46 g/L glucose and 14 g/L xylose as the carbon source, similar to MG hydrolysates, and P2 stock solutions including 1 g/L yeast extract [35] was used as the control medium. For the treatment, different volumes of MG hydrolysates (10, 25, or 40 %, v/v) and 1 g/L yeast were used for the ABE fermentation as depicted in the experimental design (Fig. 1S). The MG hydrolysate-based medium was supplemented with glucose and xylose to ensure the same amount of substrate concentration as the control. The media were autoclaved at 121 °C for 15 min after which they were cooled to 40 °C prior to transfer into the anaerobic chamber (Coy, Ann Arbor, MI, USA) with a modified atmosphere of 82 % N2, 15 % CO2, 3 % H2 and storage at 35 ± 1 °C for 14–16 h to remove residual oxygen in the media. All fermentation media were supplemented with 1 % (v/v) of sterile P2 medium stock solutions (buffer, mineral, and vitamin) [35] prior to inoculation with 6 % (v/v) of C. beijerinckii pre-culture. To evaluate the effect of CaCO3 on ABE fermentation using MG hydrolysates as substrate, 4 g/L CaCO3 was added to 100 mL control medium and to 10, 25, 50, or 100 % MG hydrolysate-based P2 medium in 150 mL Pyrex screw-capped media bottles followed by autoclaving at 121 °C for 15 min as depicted in Fig. 1S. During the course of fermentation, 5-mL samples were collected every 12 h to analyze for fermentation parameters (cell OD, pH, acetone, butanol, ethanol, acetic acid, and butyric acid) and residual lignocellulose-derived inhibitory compounds.
Impact of lignocellulose-derived microbial inhibitors on the growth of and ABE production by C. beijerinckii
Batch ABE fermentations by C. beijerinckii were performed in triplicate 150 mL Pyrex screw-capped bottles containing 100 mL anoxic P2 medium (glucose 60 g/L and yeast extract 1 g/L) and P2 stock solutions as described previously [35]. To evaluate the impact of lignocellulose-derived microbial inhibitors on the growth of and ABE production by C. beijerinckii, P2 medium was supplemented with 1–2 g/L furfural, 1–2 g/L HMF, 2–10 g/L furfuryl alcohol, and 0.2–0.5 g/L 4-hydroxybenzaldehyde or p-coumaric acid followed by autoclaving at 121 °C for 15 min. After cooling to 40 °C, the media in loosely capped media bottles were transferred into the anaerobic chamber (Coy, Ann Arbor, MI, USA) at 35 °C for 24 h for anaerobiosis. Sterile P2 medium stock solutions including buffer, mineral, and vitamin were added prior to inoculation at 6 % (v/v) with C. beijerinckii pre-culture [35]. The experimental design is depicted in Fig. 2S. During the course of the fermentation, 5 mL samples were collected every 12 h to analyze for fermentation parameters and residual lignocellulose-derived inhibitory compounds (furfural, HMF, furfuryl alcohol, 4-hydroxybenzaldehyde, and p-coumaric acid).
Analytical methods
Sugar yield was estimated by the amount of glucose and xylose obtained in hydrolysates per gram of loaded biomass. Concentrations of lignocellulose-derived microbial inhibitors in MG hydrolysates and fermentation media were determined with a DU800 spectrophotometer (Beckman Coulter Inc., Brea, CA, USA) and High liquid chromatography (HPLC) with a photodiode array (PDA) detector (Waters, Milford, MA, USA) and a 3.5-μm Xbridge C18, 150 mm × 4.6 mm column (Waters, Milford, MA, USA) as previously described [35]. Notably, the maximum absorbance spectra of measured microbial inhibitors were at 276, 220, 282, 222, 277, and 284 nm for furfural, furfuryl alcohol, HMF, HMF alcohol, 4-hydroxybenzaldehyde, and p-coumaric acid, respectively. Sugars were analyzed by HPLC with an evaporative light scattering (ELSD) detector (Waters, Milford, MA, USA) and a 9-μm Aminex HPX-87P, 300 mm × 7.8 mm column with a 4.6 mm ID × 3 cm long Aminex deashing guard column (Bio-Rad, Hercules, CA, USA) maintained at 65 °C. The mobile phase was HPLC-grade water operated at a flow rate of 0.6 mL/min [35]. Samples were filtered through 0.45 μm syringe filters prior to injection into the HPLC system. Concentrations of acetone, butanol, ethanol, acetic acid, and butyric acid were quantified using a gas chromatography system (Agilent Technologies 7890A, Agilent Technologies Inc., Wilmington, DE, USA) equipped with a flame ionization detector (FID) and 30 m (length) × 320 μm (internal diameter) × 0.50 μm (HP-Innowax film) J × W 19091 N-213 capillary column as described previously [14].
Results and discussion
Pretreatment and hydrolysis of Miscanthus giganteus
Liquid hot water (LHW) pretreatment was employed in this study to disrupt the lignin structure of MG to expose its cellulose component to enzymatic attack. This pretreatment process allows biomass to undergo high pressure cooking at elevated temperatures (190 °C in this study) without addition of acids. The primary advantages of the process include savings on catalyst cost and alkali used for neutralization of the hydrolysates and, more importantly, generation of fewer lignocellulose-derived microbial inhibitors compared to pretreatment with acidic catalysts [5]. Following enzymatic hydrolysis of the MG hydrolysates, they were analyzed for monomeric sugars and biomass degradation products. The main sugars generated from the depolymerization of cellulose and hemicelluloses following pretreatment and hydrolysis of MG were glucose, xylose, and arabinose (Table 1). The glucose and xylose yield obtained with the LHW process was 0.22 g and 0.08 g, respectively, per gram of MG biomass (Table 1S), which is lower than that (0.44 g glucose and 0.21 g xylose per gram of biomass) obtained with a combination of dilute acid presoaking and wet explosion processing [28]. While the focus of this investigation was not on optimization of pretreatment and hydrolysis of MG to monomeric sugars, it is important to note that the LHW pretreatment at 190 °C followed by enzymatic hydrolysis is not sufficient to recover all sugars from the MG biomass. Even the mild LHW process employed was sufficient to generate enough lignocellulose-derived microbial inhibitors to completely inhibit the growth of C. beijerinckii (data not shown). The lignocellulose-derived microbial inhibitors generated include degradation products of sugars such as furfural and HMF [25] and lignin-hemicelluloses such as 4-hydroxybenzaldehyde, syringic acid, syringaldehyde, p-coumaric acid, and ferulic acid [16] as listed in Table 1.
Growth, pH, and ABE production study of C. beijerinckii grown in MG hydrolysates and alleviation of MG toxicity by CaCO3
To evaluate cell growth and ABE production profiles of C. beijerinckii grown in MG hydrolysates, batch fermentations were performed using a medium containing 10, 25, and 40 % (v/v) MG hydrolysates. Severe inhibition of the growth of C. beijerinckii was observed when dilute MG hydrolysates were used as the carbon source (Fig. 1). While growth media composed of mixtures of MG hydrolysates and P2 medium in volume ratios of 1:9 and 1:3 supported the growth of C. beijerinckii to approximately 40 and 30 %, respectively, of the control medium, C. beijerinckii did not grow in a medium composed of a mixture of MG hydrolysates and P2 medium in a volume ratio of 1:1.5 (40 % MG, Fig. 1a). Although the growth of C. beijerinckii was inhibited when grown in a medium with 10 % (1:9 ratio) MG hydrolysates, the amount of ABE (11.8 g/L) produced was similar to that produced when grown in the control medium (11.4 g/L) (Fig. 1b, c). However, there was a 12-h delay in the production of ABE by C. beijerinckii grown in the medium containing 10 % (1:9 ratio) MG hydrolysates (Fig. 1b, c). It is plausible that C. beijerinckii transformed some of the microbial inhibitors such as furfural, HMF, 4-hydroxybenzaldehyde, syringic acid, syringaldehyde, and p-coumaric acid (Table 2) that are contained in MG hydrolysates into less toxic compounds during this 12-h lag. Conversely, the medium containing 25 % (1:3 ratio) MG hydrolysates repressed the growth of C. beijerinckii and drastically inhibited ABE production as manifested by lack of acids uptake and accumulation of acetic and butyric acid in the fermentation medium (Fig. 1d).
Cell growth and ABE production by C. beijerinckii NCIMB 8052 using MG hydrolysates as substrate. Cell growth (a) was estimated by optical density at 600 nm λ. Batch ABE fermentation by C. beijerinckii NCIMB 8052 was conducted using control P2 medium (b) and mixtures of MG hydrolysates and P2 medium in volume ratios of c 1:9 (10 % MG hydrolysates) and d 1:3 (25 % MG hydrolysates). ABE production was measured by gas chromatography and is represented (b–d) as follows: acetone, solid circles; ethanol, empty circles; butanol, solid triangles; acetic acid, empty triangles; butyric acid, solid squares
Notably, inhibition of C. beijerinckii by MG hydrolysates was due to degradation products generated during pretreatment and hydrolysis of MG. Thus far, a relatively small number of lignocellulosic-derived inhibitory compounds have been identified in lignocellulosic biomass hydrolysates, although tens or hundreds may be generated during acid pretreatment of lignocellulosic biomass [21]. This could be the major reason why C. beijerinckii did not even grow in undiluted MG hydrolysates. Furthermore, although compositional analysis of MG hydrolysates showed low levels of identified lignocellulose-derived microbial inhibitory compounds, their combinations may present additive and synergistic toxicity effects to C. beijerinckii [8].
Calcium carbonate is widely used as an additive to improve fermentation by yeast [31], fungus [23], and bacteria [17], mainly due to its ability to maintain fermentation pH. Moreover, some benefits of CaCO3 to fermentation include elevation of buffering capacity of the medium, stabilization of cellular activities, and enhanced tolerance to fermentation products such as butanol [14, 18]. In light of these findings, it was hypothesized that addition of CaCO3 to the fermentation broth would mitigate detrimental effects of lignocellulose-derived microbial inhibitory compounds and consequently facilitate the growth of and ABE production by C. beijerinckii. To test this hypothesis, culture media whose volume ratios of MG hydrolysates and P2 medium were 1:9, 1:3, 1:1, and 1:0 and to which CaCO3 was added were used to conduct batch ABE fermentation by C. beijerinckii. Interestingly, MG hydrolysates supported the growth of C. beijerinckii when supplemented with CaCO3, and maximum OD600 ranging from 3.7 to 5.9 was attained at 24 h fermentation time (Fig. 2a). It should be noted that in the absence of CaCO3, C. beijerinckii did not grow in P2 medium supplemented with 40 % (1:1.5 ratio) MG hydrolysates (Fig. 1a) and produced predominantly acids in a culture medium with an MG hydrolysates-to-P2 medium ratio of 1:3 (Fig. 1d). ABE production using MG hydrolysates (10–100 %) supplemented with 4 g/L CaCO3 ranged from 8.3 to 17.3 g/L (Fig. 2b–f). Plausibly, CaCO3 facilitated the utilization of MG hydrolysates by C. beijerinckii due to its buffering capacity, as it can neutralize excess organic acids produced during fermentation of MG hydrolysates (Fig. 1d). Furthermore, toxic effects of lignocellulose-derived microbial inhibitory compounds on C. beijerinckii were alleviated by CaCO3 and Ca2+, as it has been demonstrated previously that Ca2+ increases the expression of heat-shock proteins, enhances sugar utilization, stabilizes cell membrane and nucleic acids, and increases activity of ABE production enzymes in C. beijerinckii [14].
Cell growth and ABE production by C. beijerinckii NCIMB 8052 using MG hydrolysates to which 4 g/L CaCO3 was added as substrate. Cell growth (a) and ABE production profiles of C. beijerinckii NCIMB 8052 grown in the control P2 medium (b) and in mixtures of MG hydrolysates and P2 medium in volume ratios of c 1:9 (10 % MG hydrolysates), d 1:3 (25 % MG hydrolysates), e 1:1 (50 % MG hydrolysates), and f 1:0 (100 % MG hydrolysates), as carbon sources. Product concentrations (b–f) are represented as follows: acetone, solid circles; ethanol, empty circles; butanol, solid triangles; acetic acid, empty triangles; butyric acid, solid squares
Biotransformation of furfural, HMF, 4-hydroxybenzaldehyde, and p-coumaric acid by C. beijerinckii
Since ABE production by C. beijerinckii grown in MG hydrolysates and P2 medium (1:9 volume ratio) was comparable to that in the control medium, it was hypothesized that at a sub-lethal concentration of lignocellulose-derived microbial inhibitors, C. beijerinckii has an inherent capacity to tolerate and possibly detoxify some of these inhibitors during ABE fermentation. To test this hypothesis, effluent from batch ABE fermentation wherein C. beijerinckii was grown in the medium containing 10 % (1:9 ratio) MG hydrolysates (Fig. 1c) was analyzed for microbial inhibitory compounds. Interestingly, out of seven compounds identified in MG hydrolysates (Table 1), about four (furfural, HMF, 4-hydroxybenzaldehyde, and p-coumaric acid) were depleted within 12 h of fermentation (Table 2).
To better understand the depletion of the four lignocellulose-derived microbial inhibitory compounds identified in MG hydrolysates, P2 medium was supplemented with different concentrations of selected microbial inhibitory compounds (furfural, HMF, 4-hydroxybenzaldehyde, and p-coumaric acid) identified in MG hydrolysates and used for ABE fermentation by C. beijerinckii. Upon analysis of the fermentation effluent, it was found that depletion of furfural and HMF (Fig. 3a, b) in the fermentation medium during ABE fermentation by C. beijerinckii coincided with the generation of furfuryl alcohol and 2,5-bis-hydroxymethylfuran (HMF alcohol) (Fig. 3a, b). The specific conversion rate of furfural to furfuryl alcohol by C. beijerinckii (0.15 g/L/h) was higher than that of HMF to 2,5-bis-hydroxymethylfuran (0.08 g/L/h), which is consistent with the previous study using C. acetobutylicum ATCC 824 [35] (Table 3). Biotransformation of furan aldehyde to its corresponding less toxic alcohol by different microorganisms has been reported previously (Table 3), and it is recognized that the hydroxymethyl group on furfuryl alcohol or 2,5-bis-hydroxymethylfuran is generated when the aldehyde group on the furan ring accepts two electrons from electron carrier molecules such as Nicotinamide adenine dinucleotide (NADH) or Nicotinamide adenine dinucleotide phosphate (NADPH) [12].
Depletion of furfural, HMF, 4-hydroxybenzaldehyde, and p-coumaric acid in the culture medium during ABE fermentation by C. beijerinckii NCIMB 8052. Figures show biotransformation of furfural (a) and HMF (b) to furfuryl alcohol and 2,5-bis-hydroxymethylfuran (HMF alcohol), respectively, and depletion of 4-hydroxybenzaldehyde and p-coumaric acid (c) by C. beijerinckii NCIMB 8052 during growth in P2 medium supplemented with these compounds
In contrast, lower concentrations of 4-hydroxybenzaldehyde (0.5 g/L) and p-coumaric acid (0.3 g/L) were used in this study because they exert more inhibitory effects on microorganisms than do furfural and HMF. Whereas C. beijerinckii depleted 0.5 g/L 4-hydroxybenzaldehyde in 18 h during ABE fermentation with a conversion rate of 0.03 g/L/h, 0.3 g/L p-coumaric acid was completely depleted in 6 h with a conversion rate of 0.05 g/L/h (Fig. 3c; Table 3). Although the reaction products of 4-hydroxybenzaldehyde and p-coumaric acid were not analyzed in this study, previous reports have shown that Clostridium formicoaceticum has the ability to constitutively oxidize 4-hydroxybenzaldehyde to 4-hydroxybenzoate using its aldehyde oxidoreductase enzyme [9]. Further, phototrophic and fermenting bacteria have demonstrated capacity to transform 4-hydroxybenzaldehyde to the central intermediate benzoyl-CoA through a series of reactions catalyzed by aldehyde reductase, CoA ligase, and 4-hydroxybenzoyl-CoA reductase, and consequently, metabolism of benzoyl-CoA to acetyl-CoA and CO2 [15]. Whereas Clostridium species have been shown to reduce p-coumaric acid to p-hydroxyhydrocinnamic acid or 4-vinylphenol followed by decarboxylation and reduction to 4-ethylphenol, no cleavage of the aromatic ring during the transformation process has been observed [6]. Since the aromatic ring in furfural, HMF, and p-coumaric acid structures is not easily attacked by reductases (albeit the side chains on the aromatic ring undergoes substitution), complete degradation and metabolism of these compounds by microorganisms become difficult. Nonetheless, it has been postulated that if a high energy bond (thioester link) can be introduced to 4-hydroxybenzaldehyde by Coenzyme A, the aromatic ring can open up and become completely metabolized by the fermenting microorganisms [15].
Comparative growth and ABE profiles of C. beijerinckii grown in P2 medium supplemented with furfural, HMF, and furfuryl alcohol
Typically, lignocellulose-derived microbial inhibitory compounds limit cell growth, and the level of reduction in the optical density of C. beijerinckii grown in the presence of these compounds is a measure of their toxicity. While C. beijerinckii challenged with 2 g/L furan aldehyde (furfural and HMF) did not experience decrease in growth with regard to maximum cell density (Fig. 4a), it did experience an extended lag phase of approximately 6 h compared to control medium with no furfural or HMF in which C. beijerinckii experienced a less than 15 min lag (Fig. 4a). The growth of furan aldehyde-challenged C. beijerinckii cultures was delayed when furfural or HMF was still present in the culture medium but quickly recovered after 12 and 24 h following depletion of furfural and HMF, respectively (Fig. 3a, b). Further, a typical biphasic fermentation process was indicated by an initial decrease in pH during the exponential growth phase due to acid production, followed by increase and fluctuation in culture pH during the stationary growth phase due to acid re-assimilation and ABE production (Fig. 4). Clearly, the presence of furfural and HMF in the culture medium did not alter the typical biphasic profile of C. beijerinckii (Fig. 4b).
Growth (a), pH (b), acetone (c), ethanol (d), and butanol (e) production profiles of C. beijerinckii NCIMB 8052 grown in P2 medium supplemented with 2 g/L furfural or HMF are shown. Symbols: solid circles represent batch ABE fermentation by C. beijerinckii NCIMB 8052 grown in P2 medium (control medium), empty circles represent P2 medium supplemented with 2 g/L of furfural, and solid triangles represent P2 medium supplemented with 2 g/L HMF
Previous studies on effects of furfural and HMF on the growth of microorganisms are summarized in Table 3. Supplementation of fermentation media with furfural ranging from 1.0 to 3.7 g/L during fermentation by microorganisms including yeast, bacteria, and archaea resulted in cell growth decrease of about 25–99 %, except for two Methanococcus strains and two solventogenic Clostridium strains that did not experience growth inhibition in the presence of 1–2 g/L furfural (Table 3). Similarly, supplementation of culture medium with 0.9–5.0 g/L HMF often leads to inhibition of cell growth (1.4–78 %), unlike C. beijerinckii and C. acetobutylicum 824 that were not inhibited by the presence of 2 g/L HMF (Table 3). Figure 4c–e compares ABE production by C. beijerinckii challenged with 2 g/L furfural or HMF. There was no marked difference in total ABE produced by furan aldehyde challenged and unchallenged cultures of C. beijerinckii.
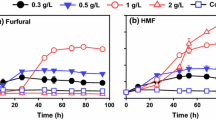
Given that the reduction product of furfural, furfuryl alcohol, has been recognized as a less toxic compound than furfural [33], the effect of furfuryl alcohol on the growth of and ABE production by C. beijerinckii was evaluated. Notably, the effect of furfuryl alcohol on solventogenic Clostridium species during ABE fermentation has never been investigated. Figure 5 illustrates the impact of furfuryl alcohol (0, 2, 3, 5, and 10 g/L) on cell growth and ABE production by C. beijerinckii. A close examination of Fig. 5 shows that while furfuryl alcohol did not inhibit the growth of C. beijerinckii at concentrations lower than 5 g/L, it inhibited butanol production even at concentrations as low as 2 g/L (Fig. 5d). While C. beijerinckii maintained the typical biphasic (acidogenic and solventogenic) pH (Fig. 5b) and acid profiles (Fig. 5f, g) during growth in the presence of furfuryl alcohol, the presence of furfuryl alcohol in the culture medium may have altered the ethanol-butanol ratio in the ABE produced by C. beijerinckii (Fig. 5c–e). The toxicity of furfuryl alcohol to C. beijerinckii may be related to its hydrophobicity, which targets the disruption of the microbial plasma membrane causing leakage of cellular content [33], unlike furfural or HMF whose primary inhibition targets are intracellular sites [22] and depletion of reductants in the bacterial cytoplasm [34]. It is plausible that the big error bars in Fig. 5c, e (ethanol and acetone production) even after several fermentation repeats, may be due to the disruption of the microbial plasma membrane; however, more studies are needed to elucidate this occurrence.
Growth and ABE production profiles of C. beijerinckii NCIMB 8052 grown in P2 medium supplemented with 2–10 g/L furfuryl alcohol. Symbols: solid circles represent C. beijerinckii NCIMB 8052 grown in P2 medium; empty circles, solid triangles, empty triangles, and solid squares represent P2 medium supplemented with 2, 3, 5, and 10 g/L furfuryl alcohol, respectively. Figures show cell growth (a), pH (b), acetone (c), ethanol (d), butanol (e), acetic acid (f), and butyric acid (g) profiles of C. beijerinckii NCIMB 8052 grown in the P2 medium supplemented with furfuryl alcohol
Impact of 4-hydroxybenzaldehyde and p-coumaric acid on C. beijerinckii growth and ABE production
Although C. beijerinckii can tolerate furan aldehydes at concentrations greater than 2 g/L, it is more vulnerable to phenolic compounds (4-hydroxybenzaldehyde and p-coumaric acid) even at concentrations lower than 0.5 g/L (Fig. 6). For example, when C. beijerinckii was challenged with 0.5 g/L 4-hydroxybenzaldehyde or 0.3 g/L p-coumaric acid during ABE fermentation, its cell growth was inhibited by 33 and 44 %, respectively (Fig. 6a). Likewise, ABE production was significantly impeded when C. beijerinckii was challenged with either 4-hydroxybenzaldehyde or p-coumaric acid (Fig. 6c–e), which resulted in lower ABE productivity when compared to that of the control (Fig. 6c, d). Nonetheless, C. beijerinckii maintained the typical biphasic (acidogenic and solventogenic) pH (Fig. 6b) and acid (Fig. 6f) profiles during growth in the presence of 4-hydroxybenzaldehyde or p-coumaric acid. Contrary to expectation, the presence of 4-hydroxybenzaldehyde in the culture medium resulted in a continuous accumulation of butyric acid after 24 h (Fig. 6g) signifying impaired re-assimilation of butyric acid, which is consistent with decreased production of butanol (Fig. 6c, d).
Growth and ABE production profiles of C. beijerinckii NCIMB 8052 grown in P2 medium supplemented with 0.5 g/L 4-hydroxybenzaldehyde or 0.3 g/L p-coumaric acid. Figures show cell growth (a), pH (b), acetone (c), ethanol (d), butanol (e), acetic acid (f), and butyric acid (g) of C. beijerinckii 8052. Symbols: solid circles represent C. beijerinckii NCIMB 8052 grown in P2 medium (control), empty circles and solid triangles represent C. beijerinckii NCIMB 8052 grown in P2 medium supplemented with 0.5 g/L 4-hydroxybenzaldehyde and 0.3 g/L p-coumaric acid, respectively
Conclusions
Pretreatment and hydrolysis of MG generated fermentable sugars and many other microbial inhibitors. Growth of and ABE production by C. beijerinckii were severely inhibited by the P2 medium whose carbon source was MG hydrolysates. C. beijerinckii was determined to have the capacity to detoxify some lignocellulose-derived microbial inhibitory compounds including furfural, HMF, 4-hydroxybenzaldehyde, and p-coumaric acid during ABE fermentation. Calcium carbonate was found to alleviate toxic effects of lignocellulose-derived microbial inhibitory compounds during fermentation by C. beijerinckii and consequently enhanced bioconversion of MG hydrolysates to ABE. While furfuryl alcohol is less inhibitory than furfural, its presence in the culture media at a relatively low concentration decreased ABE production by C. beijerinckii. Considering that bioconversion of lignocellulosic biomass hydrolysates to fuels and chemicals is limiting due to the presence of lignocellulose-derived microbial inhibitory compounds, future work will focus on the use of metabolic engineering to enhance inhibitor tolerance by fermenting microorganisms.
References
Almeida JRM, Bertilsson M, Gorwa-Grauslund MF, Gorsich S, Liden G (2009) Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl Microbiol Biotechnol 82(4):625–638
Belay N, Boopathy R, Voskuilen G (1997) Anaerobic transformation of furfural by Methanococcus deltae Delta LH. Appl Environ Microbiol 63(5):2092–2094
Boopathy R (2009) Anaerobic biotransformation of furfural to furfuryl alcohol by a methanogenic archaebacterium. Int Biodeterior Biodegrad 63(8):1070–1072
Boopathy R, Bokang H, Daniels L (1993) Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J Ind Microbiol 11(3):147–150
Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S (2011) Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res 2011:787532
Chamkha M, Garcia JL, Labat M (2001) Metabolism of cinnamic acids by some Clostridiales and emendation of the descriptions of Clostridium aerotolerans, Clostridium celerecrescens and Clostridium xylanolyticum. Int J Syst Evol Microbiol 51:2105–2111
Delgenes JP, Moletta R, Navarro JM (1996) Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb Technol 19(3):220–225
Ezeji T, Qureshi N, Blaschek HP (2007) Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng 97(6):1460–1469
Frank C, Schwarz U, Matthies C, Drake HL (1998) Metabolism of aromatic aldehydes as cosubstrates by the acetogen Clostridium formicoaceticum. Arch Microbiol 170(6):427–434
Green EM (2011) Fermentative production of butanol–the industrial perspective. Curr Opin Biotechnol 22(3):337–343
Gutiérrez T, Buszko ML, Ingram LO, Preston JF (2002) Reduction of furfural to furfuryl alcohol by ethanologenic strains of bacteria and its effect on ethanol production from xylose. Appl Biochem Biotechnol 98:327–340
Gutiérrez T, Ingram LO, Preston JF (2006) Purification and characterization of a furfural reductase (FFR) from Escherichia coli strain LYO1—an enzyme important in the detoxification of furfural during ethanol production. J Biotechnol 121(2):154–164
Hamelinck CN, Faaij APC (2006) Outlook for advanced biofuels. Energy Policy 34(17):3268–3283
Han B, Ujor V, Lai LB, Gopalan V, Ezeji TC (2013) Use of proteomic analysis to elucidate the role of calcium in acetone–butanol–ethanol fermentation by Clostridium beijerinckii NCIMB 8052. Appl Environ Microbiol 79(1):282–293
Harwood CS, Burchhardt G, Herrmann H, Fuchs G (1998) Anaerobic metabolism of aromatic compounds via the benzoyl-CoA pathway. FEMS Microbiol Rev 22(5):439–458
Hedges JI, Ertel JR (1982) Characterization of lignin by gas capillary chromatography of cupric oxide oxidation-products. Anal Chem 54(2):174–178
Jiang Y, Xu CM, Dong F, Yang YL, Jiang WH, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11(4–5):284–291
Kanouni AE, Zerdani I, Zaafa S, Znassni M, Loutfi M, Boudouma M (1998) The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate. World J Microbiol Biotechnol 14(3):431–435
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66(1):10–26
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729
Liu ZL, Blaschek HP (2010) Biomass conversion inhibitors and in situ detoxification. In: Vertès AA, Qureshi N, Blaschek HP, Yukawa H (eds) Biomass to biofuels: strategies for global industries. Wiley, Hoboken, pp 233–259
Mills TY, Sandoval NR, Gill RT (2009) Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol Biofuels 2(10):26
Ohta K, Hamada S, Nakamura T (1993) Production of high concentrations of ethanol from inulin by simultaneous saccharification and fermentation using Aspergillus niger and Saccharomyces cerevisiae. Appl Environ Microbiol 59(3):729–733
Palmqvist E, Almeida JS, Hahn-Hägerdal B (1999) Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiae in batch culture. Biotechnol Bioeng 62(4):447–454
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74(1):25–33
Richmond C, Han B, Ezeji TC (2011) Stimulatory effects of calcium carbonate on butanol production by solventogenic Clostridium species. Cont J Microbiol 5(1):18–28
Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C, Sluiter J, Templeton D, Nrel JW (2008) Determination of total solids in biomass and total dissolved solids in liquid process samples. Laboratory Analytical Procedure (LAP) National Renewable Energy Laboratory
Sørensen A, Teller PJ, Hilstrom T, Ahring BK (2008) Hydrolysis of Miscanthus for bioethanol production using dilute acid presoaking combined with wet explosion pre-treatment and enzymatic treatment. Bioresour Technol 99(14):6602–6607
Taherzadeh MJ, Gustafsson L, Niklasson C, Liden G (2000) Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl Microbiol Biotechnol 53(6):701–708
Wierckx N, Koopman F, Bandounas L, de Winde JH, Ruijssenaars HJ (2010) Isolation and characterization of Cupriavidus basilensis HMF14 for biological removal of inhibitors from lignocellulosic hydrolysate. Microb Biotechnol 3(3):336–343
Wilkins MR, Widmer WW, Grohmann K (2007) Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem 42(12):1614–1619
Zaldivar J, Martinez A, Ingram LO (1999) Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 65(1):24–33
Zaldivar J, Martinez A, Ingram LO (2000) Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 68(5):524–530
Zhang Y, Ezeji TC (2013) Transcriptional analysis of Clostridium beijerinckii NCIMB 8052 to elucidate role of furfural stress during acetone butanol ethanol fermentation. Biotechnol Biofuels 6(1):66
Zhang Y, Han B, Ezeji TC (2012) Biotransformation of furfural and 5-hydroxymethyl furfural (HMF) by Clostridium acetobutylicum ATCC 824 during butanol fermentation. N Biotechnol 29(3):345–351
Acknowledgments
Salaries and research support was provided in part by State funds appropriated to the Ohio Plant Biotechnology Consortium by The Ohio State University, Ohio Agricultural Research and Development Center (OARDC), Western Region Sungrant (Prime award No. 2010-38502-21839), and the Hatch grant (Project No. OHO01222).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Ezeji, T.C. Elucidating and alleviating impacts of lignocellulose-derived microbial inhibitors on Clostridium beijerinckii during fermentation of Miscanthus giganteus to butanol. J Ind Microbiol Biotechnol 41, 1505–1516 (2014). https://doi.org/10.1007/s10295-014-1493-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1493-5