Abstract
As one of the major microbes in the soy sauce fermentation, Candida versatilis enriches the flavor and improves the quality of soy sauce. In this study, a combination of five different GC-MS and LC-MS-based metabolome analytical approaches was used to analyze the intracellular, extracellular and whole metabolites of C. versatilis. Our results found out that a total of 132, 244 and 267 different metabolites were detectable from the intracellular, extracellular and whole part, respectively. When exposed to 0. 9 and 18 % salt, respectively, 114, 123 and 129 different intracellular metabolites, 184, 200 and 178 extracellular metabolites and 177, 188 and 186 whole metabolites were detected, respectively. Our data showed that salt enhances the metabolic capacity of C. versatilis, especially its amino acid and enhances the synthesis and secretion of some metabolites of C. versatilis, especially the aldehydes and phenols, such as vanillin, guaiacol and 5-hydroxymethylfurfural. Our data also showed that special attention has to be paid to the generation of biogenic amines when C. versatilis was treated with salt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, the combination of mathematical modeling and experimental biology has led to a new scientific field called systems biology that has extremely facilitated the quantitative analysis of the dynamic interaction between several components of a biological system [5]. As a post-genomic analysis tool, metabolomics is all about the quantitative analysis of the endogenous and exogenous components of small molecule metabolites in integrated living systems and its dynamic responses to the changes of both endogenous and exogenous factors [38]. To achieve specific fingerprints or metabolite patterns, targeted and non-targeted metabolomics analysis through focusing on either a specific group of metabolites or the detection of many separate groups of metabolites have been traditionally classified [18]. Since both approaches already provided highly valuable information in a wide variety of studies, metabolomics has emerged as an important choice to complement the existing techniques and has provided much detailed information on metabolic regulation and secondary metabolism.
Metabolites are the static end-products of gene expression and moreover form a dynamic network of part of the regulatory system in an integrated organism [24]. Metabolomics and metabolite profiling provide scientists a tool to have a global analysis on the intracellular and extracellular metabolites or on the low-molecular-mass molecules. This technique has provided a direct snapshot of the physiological status of a cell at a certain time point and under specific circumstances to understand cellular functions and unveil cellular biochemical reactions [27, 29]. It has been reported that the metabolite profiling could be affected by the presence of chemical factors such as sodium chloride, sugar, trace elements and heavy metals [49].
During food process, highly versatile microbes enter the food chain at different steps, adapt to the environment to survive and grow and contribute to the release of the useful active substances through metabolic processes. This whole process not only increases health benefits such as nutrient enrichment [15] but also accumulates potential toxic compounds including but not limited to biogenic amines, nitrates and nitrosamines. For example, biogenic amines are potential carcinogens and converted to nitrosamines when exposed to nitrites. At present, the biogenic amine levels of 1,000 mg/kg in food are considered harmful to human health [12]. Hence, metabolite analysis of microbes could contribute significantly to the progress in understanding microbes’ metabolism and other important physiological and technical aspects.
Candida versatilis used in the soy sauce fermentation could enrich flavor (such as the typical flavor component 4-EG described as smoky and sauce flavor), and improve soy product quality [35, 41]. As a salt-tolerant yeast, C. versatilis has a complex salt-tolerant system that makes it maintain normal physiological activities and metabolic fermentation under a high salt environment. Over the past two decades, various studies have been performed to investigate its adaptation mechanism to salt stress [25, 31, 32, 45], mostly by focusing on its plasma membrane H+-ATPase [44, 46] and Na+/H+ antiporter [44]. And the influence NaCl stress on yeast metabolism were also investigated, such as the change of volatile ester production, cell growth and glucose consumption in response to NaCl stress in the soy sauce production [6]. To date, none has been performed to investigate the global metabolite analysis of C. versatilis. In this study, five different GC-MS and LC-MS based metabolomics analytic methods including oximation and silylation (OS)-GC-MS, headspace solid phase micro-extraction (HPME)-GC-MS, direct solvent extraction (DSE)-GC-MS, HPLC-MS and organic acids (OAC)-LC were applied to analyze the intracellular, extracellular and whole metabolites of C. versatilis at the presence of NaCl of different concentrations (Fig. 1). We aim to investigate the influence of salt stress environment on the metabolic activity of C. versatilis and on the contribution of C. versatilis on soy sauce product flavor.
Materials and methods
Chemicals
Chemicals and solvents of analytical grade were purchased from Tianjin Chemical Reagent Research Institute (Tianjin, China). Gas chromatography grade (GC) ethoxyamine hydrochloride and N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA) were obtained from Sigma (St. Louis, MO). Chromatographic grade (HPLC) acetonitrile and methanol were bought from Merck (Darmstadt, Germany). Double distilled water for the dilution of samples was purified using a Milli-Q system (Millipore, Bedford, MA, USA).
Yeast strain and growth conditions
The C. versatilis yeast strains were kept in our laboratory and cultured in a shaking incubator at 180 rpm at 30 °C. Cells were first grown in 10 ml YPD medium for 24 h, diluted in YPD medium with a final volume of 100 ml and inoculated to their logarithmic phase (OD600 ≈ 1.0). For NaCl treatment, 15 ml of cells was transferred into a 250 ml shaking flask to reach a final volume of 150 ml medium containing 0, 90 or 180 g/l NaCl, respectively. The medium is composed of following ingredients (per liter): 20 g glucose, 10 g peptone, 5.0 g (NH4)2SO4, 0.5 g MgSO4 7H2O, 1.0 g KH2PO4, 0.1 g CaCl2. Then cells were inoculated for further analysis.
Intracellular metabolite extraction
Five milliliter culture containing 363 mg cells in free-salt medium, 342 mg cells in 9 g/l salt medium or 316 mg cells in 18 g/l salt medium, respectively were quenched with methanol–water solution (60 %, v/v, −40 °C) according to the method described previously [42].
Intracellular metabolite extraction was performed with pure methanol as reported previously [42] with slight modifications. For intracellular metabolite extraction, cells were first collected by centrifugation (5,000×g, 5 min, −20 °C). In detail, the sample was re-suspended in 2.5 ml cold pure methanol (stored at −40 °C), frozen in liquid nitrogen, thawed in an ice-bath and then centrifuged at 5,000×g for 5 min (−20 °C). The precipitate was dissolved in 2.5 ml of cold pure methanol again and the mixture was centrifuged (5,000×g, 5 min, −20 °C). The supernatant was collected and evaporated under vacuum (Labconco, US) and then stored at −80 °C for further analysis.
Extracellular metabolite extraction [3]
One milliliter of the cell culture was quickly filtered though a 0.45 μm, 25 mm diameter polytetrafluoroethylene membrane (Millipore, US) directly into a tube containing to 5 ml cold pure methanol (stored at −40 °C). The mixture was thoroughly vortexed and evaporated was performed as above.
Whole metabolite extraction [3]
One milliliter of the cell culture was done as intracellular metabolites but the quenched cell suspension was not centrifuged. Instead, it mixed with 5 ml cold pure methanol (stored at −40 °C), the mixture was thoroughly vortexed and evaporated in the aforementioned conditions.
Analytical procedures
Sugars and sugar alcohols were determined by GC-MS after the oximation and silylation derivatization as described previously [13]. Flavor components determined by direct solvent extraction (DSE)-GC-MS was performed as described previously [43] with slight modifications. In detail, 15 ml extracellular supernatant or whole cell culture was extracted with 20 ml diethyl ether for three times. The combined solvent layer was concentrated to 1 ml in a 37 °C water bath. The above step is operated rapidly to avoid the scatter of the aromatic compounds.
Flavor components determined by headspace solid phase micro-extraction (HPME)-GC-MS was carried out as described previously [7] with slight modifications. In detail, a solid phase microextraction fiber coated with 50/30 μm divinylbenzene/carboxen/polydimethylsiloxance fibre (DVB/CAR/PDMS) was used to collect high volatile compounds. Five milliliter extracellular supernatant or whole cell culture saturated with NaCl were sealed in a dedicated bottle, preheated at 45 °C and stirred at 200 rpm. The adsorption time was 30 min, and the concentrates were desorbed in the injection port of gas chromatographby holding in the splitless mode for 3 min. The solid phase microextraction fiber was cleaned by keeping it in the GC-MS injection port for additional 5 min.
Enzymes and nucleotides were determined by LC-MS as described previously [11]. The dried extracts were dissolved in 50 μl methanol and prepared for the LC-QqQ-MS system (high-performance liquid chromatography, HPLC: Agilent 1200 series, MS: Agilent 6460 with Jet Stream Technology, Agilent Technologies, Germany). HPLC column was a Thermo Hypersil C18 column (100 mm × 3 µm × 4.6 mm) and was set to 30 °C. Mobile phase was 10 mM tert-butyl alcohol/5 nm acetic acid in water (A) and methanol (B), flow rate: 0.3 ml/min, gradient curve, 20 % B at 0 min, 90 % B at 24 min, 90 % B at 34 min, 20 % B at 34.1 min, and 20 % B at 60 min.
Organic acids were determined by HPLC (OAC-LC) [30]. OAC-LC analysis was performed with a Shimadzu 20 AB (Shimadzu, Japan) with ultraviolet detector. The analytes were separated in a Spursil C18 column (250 mm × 5 µm × 4.6 mm) and was set to 30 °C for all running procedures.
All GC-MS analyses were performed with Varian 4000 GC/MS coupled to a HP 5973 quadrupole mass selective detector with electron ionization (EI) source operated at 70 eV. The capillary column used for all analysis was a VF-5MS column (30 × 0.25 µm × 0.25 mm). Each compound was identified by reference to the NIST05a library. Relative contents of the compounds were determined by the area normalisation method.
Statistical analysis
For the chromatographic data obtained from (OS)-GC-MS, (HPME)-GC-MS and (DSE)-GC-MS, the principal component analysis (PCA) was performed with package SPSS Statistics 18.0 software. For the principal components, PC1 was the axis that contains the largest possible amount of information and PC2 was perpendicular to PC1. The two main aim of PCA were reduction the number of variables and elimination of redundancy.
Results and discussion
Intracellular metabolite analysis of C. versatilis treated with salt of different concentrations
By using OS-GC-MS and HPLC-MS, 114, 123 and 129 intracellular metabolites were detected when cells were cultivated in medium containing no-salt, 9 % salt and 18 % salt, respectively (Fig. 2a). Since some intracellular metabolites were detectable in only one salt condition but not in the others a total of 132 intracellular metabolites of C. versatilis were detected (Supplementary Table 1). Our data showed that sugars, nucleotides, enzymes and acids were the main components of C. versatilis intracellular metabolites. The quantity of intracellular metabolites, especially alcohols and acids, increased along with the increased salt concentration in medium.
The higher alcohol flavor components such as isobutyl alcohol (alcoholic), 1-butanol and isoamyl alcohol (fruity, sweet) were detectable only when salt was present (Table 1). The fatty acid flavor components including lauric acid and other acids like glyoxylic acid, phenylpyruvic acid were also detectable only in the presence of salt (Table 1). These flavor components are biosynthesized by using amino acids as substrate, from fatty acid metabolism or from citric acid cycle [14, 20, 40]. Meanwhile, these organic acids can interact with other substances like alcohols and aldehydes to lead to the generation of additional flavor compounds during fermentation process [15]. As a result, it was concluded that many flavor components were produced only when C. versatilis was cultivated in salt-containing medium. Salt enhances the metabolic capacity of C. versatilis, especially its amino acid metabolisms.
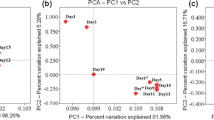
PCA analysis of intracellular metabolites of C. versatilis treated with salt of different concentrations
The biplot of intracellular metabolites under investigation on the first two PCs and the effectiveness of this chemometric approach to GC-MS data were illustrated in Fig. 2b. As shown in PC1 biplot (Fig. 2b; Supplementary Table 1), the aroma components that determine the scores on PC1 were alcohols (ethanol, 3-(methylthio)-propanol, isopropanol, isobutyl alcohol, glycerol, n-butanol, isoamyl alcohol), acids (pyruvate, benzoic acid, alpha-ketoglutarate, malic acid, citric acid, lauric acid, stearic acid, lactic acid, tartaric acid), carbohydrate (glucose, 6-phosphate glucose, 6-phosphate fructose, trehalose, fructose, xylitol, arabinose, arabitol, mannitol), amino acids (alanine, valine, serine, threonine, aspartic acid, phenylalanine). Nucleotides and enzymes (NADPH, NADH, FMN, UMP) were detected by LC-MS. It is noted that LC-MS detected prephenate which could formed tyrosine through prephenate dehydrogenases or generated phenylalanine through prephenate dehydratase.
Extracellular metabolite analysis of C. versatilis treated with salt of different concentrations
By using a combination of OS-GC-MS, SPME-GC-MS, DSE-GC-MS, HPLC-MS and OAC-LC, a total of 244 metabolites were detected from the extracellular metabolites of C. versatilis (Supplementary Table 2). 184, 200 and 178 extracellular metabolites were detected when no-salt, 9 or 18 % NaCl presented in medium (Fig. 3a). Our results showed that alcohols, acids, esters and sugars were the main flavour components of the extracellular metabolites of C. versatilis.
The effect of salt stress on extracellular metabolites of Candida versatilis. a Comparison of the quantity of extracellular metabolites of Candida versatilis, b The score plot of OS-GC-MS-PCA for extracellular metabolites of Candida versatilis, c The score plot of SPME-GC-MS-PCA for extracellular metabolites of Candida versatilis, d The score plot of DSE-GC-MS-PCA for extracellular metabolites of Candida versatilis
Several flavor components were detected only in salt-containing medium (Table 2), including 9-Octadecen-1-ol (flower odor), phenylacetaldehyde, phenylacetic acid and phenethyl alcohol and fatty acid ethyl esters. These flavor components were biosynthesized from either phenylalanine metabolism or the esterification of alcohols with fatty acid [22]. Our data indicated that the ability of C. versatilis to secret the extracellular metabolites was influenced by salt, especially the aldehyde and phenol components including 5-hydroxymethylfurfural and 5-isopropyl-2-methylphenol. These components increased along with the increased salt concentration.
5-Hydroxymethylfurfural (HMF), a well-known Maillard reaction product, was also detectable only in salt-containing medium. HMF plays a physiological role in protecting human hepatocytes and showed antioxidant and anti-tyrosinase activity [21]. Some studies suggested that HMF does not pose a serious health risk, even at a high level in specific food that not exceeds the biologically effective concentration range in cell systems [9]. However, controversial results were also reported to show that HMF can be poisonous to the nervous system and causing damages in the muscles and viscera. It has been shown that HMF at high concentrations is cytotoxic, irritating to eyes, upper respiratory tract, skin and mucous membranes [4]. HMF is converted in vitro and in vivo bysulfotransferases into sulfoxymethylfurfural (SMF), a compound which can react with DNA and other macromolecules to form adducts and thereby result in toxic and mutagenic effects [36]. Moreover, histopathological analyses revealed that SMF induced moderate damage in liver and most impressive damage in the kidneys, particularly in the proximal tubules [8]. From a safety perspective and for food quality assurance, HMF legal limits were already issued for some foodstuffs. EC Regulation No. 1493/99 sets up a limit of 25 ppm in concentrated rectified grape must [23], EC directive 1974 (74/409/EEC) sets up maximum HMF level 40 mg/kg in honey. From our data, it was conclusive that the production of all these flavor components is salt-related in C. versatilis.
PCA analysis of extracellular metabolites of C. versatilis treated with salt of different concentrations
The biplot of extracellular metabolites under investigation on the first two PCs and the effectiveness of this chemometric approach to GC-MS data were illustrated in Fig. 3. As shown in PC1 biplot (Fig. 3; Supplementary Table 2), the aroma components that determine the scores on PC1 were alcohols (3-(methylthio)-propanol, 2,3-butanediol, the hydroxy alcohol, phenethyl alcohol, tryptophol, inositol), acids (formic acid, benzoic acid, maleic acid, lactic acid, erucic acid, isovaleric acid, butyric acid, 3-mercaptopropionic acid), esters (a series of high fatty acid ethyl esters (C8, C12, C16, C17, C18), acetic acid benzyl ester, methyl palmitate), phenols (phenol, 2,4-di-tert-butylphenol, 2,6-dimethoxy-phenol), ketones and aldehydes (4-phenyl butyric aldehyde, 5-methyl furfural, 5-hydroxymethylfurfural 3-hydroxy-4,5-two methyl-2(5H) furanone), amino acids (18 amino acids), carbohydrates (a series monosaccharides, disaccharides, sugar alcohols, sugar esters). Nucleotides and enzymes (flavin mononucleotide, uracil nucleotides, didanosine, deoxyadenosine and a series of nucleotides, pantothenic acid and other enzymes) were detected by LC-MS.
It was noticed that cadaverine was detectable in all extracellular samples. As low molecular organic nitrogenous compounds of aliphatic [16], cadaverine (biogenic amines) at appropriate amount could help the body’s normal physiological function [39]. However, high level of biogenic amines constitutes a potential public health concern due to toxicological effects such as headaches, nausea, and palpitations. Furthermore, cadaverine can react with nitrite and produce volatile nitrosamines that are defined as carcinogenic compounds [10, 19].
Whole metabolite analysis of C. versatilis treated with salt of different concentrations
The whole metabolites of C. versatilis were analyzed by OS-GC-MS, SPME-GC-MS, DSE-GC-MS, HPLC-MS and OAC-LC. A total of 267 whole metabolites were detected (Supplementary Table 3). 177, 188 and 186 whole metabolites were detectable at the presence of no-salt, 9 and 18 % salt, respectively (Fig. 4a). These results showed that alcohols, acids and esters were the main flavor components of whole metabolites of C. versatilis. The quantity of whole metabolites including acids, ketones, aldehydes, phenols and amino acids increased along with the increased salt concentration in medium. However, esters decreased when medium salt concentration increased.
The effect of salt stress on whole metabolites of Candida versatilis. a Comparison of the quantity of whole metabolites of Candida versatilis. b The score plot of OS-GC-MS-PCA for whole metabolites of Candida versatilis. c The score plot of SPME-GC-MS-PCA for whole metabolites of Candida versatilis. d The score plot of DSE-GC-MS-PCA for whole metabolites of Candida versatilis
The common metabolic intermediates and volatile aroma components of whole metabolites of C. versatilis were detectable in all samples. However, the flavor components including 2-furan methanol, 2-furoic acid, 2-furaldehyde, furfural and 5-hydroxymethylfurfural, guaiacol, 4-methylphenol, 5-hydroxy-vanillin and vanillin (4-hydroxy-3-methoxybenzaldehyde) were only detectable in salt-containing medium (Table 3). 2-Furan methanol and 2-furoic acid were derived from 2-furaldehyde deoxidization suggested that salt may enhance the enzyme secretion of C. versatilis in this catalytic reaction. As being considered as safe food flavor enhancers by the expert panel of the Flavor and Extract Manufacturers’ Association (FEMA), furfural and 5-hydroxymethylfurfural have been serving as the assessing indicators of the extent of non-enzymatic browning reactions, namely Maillard type reactions (MR) [2, 23]. Our data supported that they continue to be used as a flavor ingredient [1] and their production in C. versatilis is salt-related.
As one of the most popular food ingredients or flavor components, vanillin exhibits antimutagenic, antiangiogenetic, anticolitis, antisickling, and antianalgesic effects and has much stronger antioxidant activity than ascorbic acid [37]. Together with its derivatives including guaiacol [47], these components were likely biosynthesized from phenylalanine metabolism [48] and their production were salt-related too in C. versatilis. Our data showed that amino acid components accounted for a large proportion of the whole metabolites of C. versatilis and the quantity and type of amino acids of the whole metabolites increased when salt concentration increased in medium. Amino acids were important flavor components and also the precursor substrate for biosynthesis of many other flavors substances, suggesting that the amino acid metabolism of C. versatilis was might enhanced at the presence of salt.
As a low molecular organic nitrogenous compound of aromatic [16], tyramine was only detectable at the presence of salt. As the dominating biogenic amine in soy sauce, tyramine, when higher than 100 mg/kg in food, can cause adverse effects to human health, such as dietary-induced migraine, hypertensive crisis and increase blood pressure that can lead to heart failure or brain hemorrhage [17, 28, 34]. Our data showed that generation of tyramine in C. versatilis was likely enhanced when salt was present.
PCA analysis of whole metabolites of C. versatilis treated with salt of different concentrations
The biplot of whole metabolites under investigation on the first two PCs and the effectiveness of this chemometric approach to GC-MS data were illustrated in Fig. 4. As shown in the PC1 biplot (Fig. 4 and Supplementary Table 3), the aroma components that determine the scores on PC1 were alcohols (2,3-butylene glycol, propylene glycol, butanol, glycerol, isopropanol, phenethyl alcohol, amyl alcohol, inositol, P-hydroxyphenyl ethanol), acids (isobutyric acid, benzoic acid, isovaleric acid, lactic acid, succinic acid, oxalic acid, citric acid, malic acid, n-butyric acid, 3- mercaptopropionic acid, linoleic acid), esters (short-chain fatty acid ethyl esters (C4, C8), ethyl acetate and a long-chain fatty acids ester (C12, C16, C17, C18), phenyl ethyl acetate, phenyl ethyl), phenols (2,6-dimethoxyphenyl, 2,4-di-tert-butylphenol), ketones and aldehydes (benzaldehyde, 2-methyl-heptyl aldehyde, 4-phenyl butyraldehyde, 3-hydroxy-4,5-dimethyl-2(5H) furanone, 2,5-dimethyl-benzaldehyde), amino acids (valine), carbohydrates (a series monosaccharides, disaccharides, sugar alcohols, sugar esters). Nucleotides and enzymes, flavin mononucleotide, uracil nucleotides, deoxyadenosine and a series of nucleotides, pantothenic acid and other enzymes, were detected by LC-MS
Conclusion
In this study, MS-based metabolome analytical platform consisting of five different analytical methods was applied to analyze the intracellular, extracellular and whole metabolites of C. versatilis when cultivated under salt stress. The metabolites can be grouped into alcohols, acids, esters, aldehydes, ketones, phenols, heterocycles, sugars, amino acids, vitamins, nucleotides and enzymes in accordance with the properties. Our results showed that 132, 244, 267 different intracellular, extracellular and whole metabolites were detected. At the absence of salt and at the presence of 9 and 18 % salt, respectively, 114, 123 and 129 intracellular metabolites, 184, 200 and 178 extracellular metabolites and 177, 188 and 186 whole metabolites were detected, respectively. Our data clearly showed that the quantity and kinds of metabolites were affected by salt stress. The metabolic capability of C. versatilis such as amino acid metabolism and synthesis and secretion of some metabolites, especially aldehydes and phenols, were enhanced when salt exists.
Our data also showed that special attentions have to be paid to the generation of biogenic amines when C. versatilis was treated with salt. The total biogenic amine level at 1,000 mg/kg in food is also considered to be harmful to human health. Factors that affect the content of biogenic amines in soy sauce include the manufacturing process [33]. Some factors increase the concentration of precursor amino acids of biogenic amines in the medium, while others favor the development of microorganisms with the ability to form amines. The effect of C. versatilis on the production of biogenic amines in soy sauce should be carefully monitored, and our data on biogenic amine produced by C. versatilis during high salt (18 %, w/v) liquid soy sauce fermentation was evaluated and reported in another paper [26].
References
Adams TB, Doull J, Goodman JI, Munro IC, Newberne P, Portoghese PS, Smith RL, Wagner BM, Weil CS, Woods LA, Ford RA (1997) The FEMA GRAS assessment of furfural used as a flavour ingredient. Flavor and Extract Manufacturers’ Association. Food Chem Toxicol 35(8):739–751. doi:10.1016/s0278-6915(97)00056-2
Anese M, Suman M (2013) Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Res Int 51(1):257–264. doi:10.1016/j.foodres.2012.12.024
Canelas AB, Ras C, ten Pierick A, van Dam JC, Heijnen JJ, Van Gulik WM (2008) Leakage-free rapid quenching technique for yeast metabolomics. Metabolomics 4(3):226–239. doi:10.1007/s11306-008-0116-4
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci Technol 44(4):793–810. doi:10.1016/j.lwt.2010.11.002
Carraro S, Giordano G, Reniero F, Perilongo G, Baraldi E (2009) Metabolomics: a new frontier for research in pediatrics. J Pediatr 154(5):638–644. doi:10.1016/j.jpeds.2009.01.014
Feng J, Zhan X-B, Wang D, Zhang L-M, Lin C-C (2012) An unstructured kinetic model to study NaCl effect on volatile ester fermentation by Candida etchellsii for soy sauce production. Biotechnol Bioprocess Eng 17(2):242–249. doi:10.1007/s12257-011-0416-9
Gao XL, Cui C, Zhao HF, Zhao MM, Yang L, Ren JY (2010) Changes in volatile aroma compounds of traditional Chinese-type soy sauce during moromi fermentation and heat treatment. Food Sci Biotechnol 19(4):889–898. doi:10.1007/s10068-010-0126-7
Glatt H, Sommer Y (2006) Health risks of 5-hydroxymethylfurfural (HMF) and related compounds. Acryl Other Hazard Compd Heat Treat Foods. doi:10.1533/9781845692018.2.328
Janzowski C, Glaab V, Samimi E, Schlatter J, Eisenbrand G (2000) 5-hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem Toxicol 38(9):801–809. doi:10.1016/s0278-6915(00)00070-3
Kalac P (2009) Recent advances in the research on biological roles of dietary polyamines in man. J Appl Biomed 7(2):65–74
Kato H, Izumi Y, Hasunuma T, Matsuda F, Kondo A (2012) Widely targeted metabolic profiling analysis of yeast central metabolites. J Biosci Bioeng 113(5):665–673. doi:10.1016/j.jbiosc.2011.12.013
Kim B, Byun BY, Mah J-H (2012) Biogenic amine formation and bacterial contribution in Natto products. Food Chem 135(3):2005–2011. doi:10.1016/j.foodchem.2012.06.091
Koek MM, Muilwijk B, van der Werf MJ, Hankemeier T (2006) Microbial metabolomics with gas chromatography/mass spectrometry. Anal Chem 78(4):1272–1281. doi:10.1021/ac051683+
Lerner AB (2009) Metabolism of phenylalanine and tyrosine. Adv Enzymol 14:73–128
Liu SN, Han Y, Zhou ZJ (2011) Lactic acid bacteria in traditional fermented Chinese foods. Food Res Int 44(3):643–651. doi:10.1016/j.foodres.2010.12.034
Lorenzo JM, Martinez S, Franco I, Carballo J (2007) Biogenic amine content during the manufacture of dry-cured lacon, a Spanish traditional meat product: effect of some additives. Meat Sci 77(2):287–293. doi:10.1016/j.meatsci.2007.03.020
Lu Y, Chen X, Jiang M, Lv X, Rahman N, Dong M, Gujun Y (2009) Biogenic amines in Chinese soy sauce. Food Control 20(6):593–597. doi:10.1016/j.foodcont.2008.08.020
Medina S, Ferreres F, Garcia-Viguera C, Horcajada MN, Orduna J, Saviron M, Zurek G, Martinez-Sanz JM, Gil JI, Gil-Izquierdo A (2013) Non-targeted metabolomic approach reveals urinary metabolites linked to steroid biosynthesis pathway after ingestion of citrus juice. Food Chem 136(2):938–946. doi:10.1016/j.foodchem.2012.09.004
Mohan CO, Ravishankara CN, Gopal TKS, Kumar KA, Lalitha KV (2009) Biogenic amines formation in seer fish (Scomberomorus commerson) steaks packed with O-2 scavenger during chilled storage. Food Res Int 42(3):411–416. doi:10.1016/j.foodres.2009.01.015
Oikawa T (2007) Alanine, aspartate, and asparagine metabolism in microorganisms. In: Wendisch V (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering, vol 5., Microbiology monographsSpringer, Berlin, pp 273–288. doi:10.1007/7171_2006_062
Okutsu K, Yoshizaki Y, Takamine K, Tamaki H, Ito K, Sameshima Y (2012) Development of a heat-processing method for koji to enhance its antioxidant activity. J Biosci Bioeng 113(3):349–354. doi:10.1016/j.jbiosc.2011.10.024
Parrott S, Jones S, Cooper RA (1987) 2-Phenylethylamine catabolism by Escherichia coli K12. J Gen Microbiol 133(2):347–351
Pereira V, Albuquerque FM, Ferreira AC, Cacho J, Marques JC (2011) Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Res Int 44(1):71–76. doi:10.1016/j.foodres.2010.11.011
Putri SP, Yamamoto S, Tsugawa H, Fukusaki E (2013) Current metabolomics: technological advances. J Biosci Bioeng 116(1):9–16. doi:10.1016/j.jbiosc.2013.01.004
Qi W, Fan Z-C, Wang C-L, Hou L-H, Wang X-H, Liu J-F, Cao X-H (2014) Comparative study of physiological adaptation to salt stress in the genome shuffled Candida versatilis and a wild-type salt-tolerant yeast strain. Eur Food Res Technol 238(4):675–682. doi:10.1007/s00217-013-2115-6
Qi W, Hou LH, Guo HL, Wang CL, Fan ZC, Liu JF, Cao XH (2013) Effect of salt-tolerant yeast of Candida versatilis and Zygosaccharomyces rouxii on the production of biogenic amines during soy sauce fermentation. J Sci Food Agric. doi:10.1002/jsfa.6454
Quoc-Thai N, Merlo ME, Medema MH, Jankevics A, Breitling R, Takano E (2012) Metabolomics methods for the synthetic biology of secondary metabolism. FEBS Lett 586(15):2177–2183. doi:10.1016/j.febslet.2012.02.008
Rauscher-Gabernig E, Grossgut R, Bauer F, Paulsen P (2009) Assessment of alimentary histamine exposure of consumers in Austria and development of tolerable levels in typical foods. Food Control 20(4):423–429. doi:10.1016/j.foodcont.2008.07.011
Saito N, Ohashi Y, Soga T, Tomita M (2010) Unveiling cellular biochemical reactions via metabolomics-driven approaches. Curr Opin Microbiol 13(3):358–362. doi:10.1016/j.mib.2010.04.006
Sanchez-Machado DI, Lopez-Cervantes J, Martinez-Cruz O (2008) Quantification of organic acids in fermented shrimp waste by HPLC. Food Technol Biotechnol 46(4):456–460
Silva-Garca M, Lucas C (2003) Physiological studies on long-term adaptation to salt stress in the extremely halotolerant yeast Candida versatilis CBS 4019 (syn. C halophila). FEMS Yeast Res 3(3):247–260. doi:10.1111/j.1567-1364.2003.tb00167.x
Silva-Graca M, Neves L, Lucas U (2003) Outlines for the definition of halotolerance/halophily in yeasts: Candida versatilis (halophila) CBS4019 as the archetype? FEMS Yeast Res 3(4):347–362. doi:10.1016/s1567-1356(02)00200-3
Spano G, Russo P, Lonvaud-Funel A, Lucas P, Alexandre H, Grandvalet C, Coton E, Coton M, Barnavon L, Bach B, Rattray F, Bunte A, Magni C, Ladero V, Alvarez M, Fernandez M, Lopez P, de Palencia PF, Corbi A, Trip H, Lolkema JS (2010) Biogenic amines in fermented foods. Eur J Clin Nutr 64:S95–S100. doi:10.1038/ejcn.2010.218
Stute R, Petridis K, Steinhart H, Biernoth G (2002) Biogenic amines in fish and soy sauces. Eur Food Res Technol 215(2):101–107. doi:10.1007/s00217-002-0509-y
Suezawa Y, Suzuki M (2007) Bioconversion of ferulic acid to 4-vinylguaiacol and 4-ethylguaiacol and of 4-vinylguaiacol to 4-ethylguaiacol by halotolerant yeasts belonging to the genus Candida. Biosci Biotechnol Biochem 71(4):1058–1062. doi:10.1271/bbb.60486
Svendsen C, Husoy T, Glatt H, Paulsen JE, Alexander J (2009) 5-Hydroxymethylfurfural and 5-sulfooxymethylfurfural increase adenoma and flat ACF number in the intestine of min/plus mice. Anticancer Res 29(6):1921–1926
Tai A, Sawano T, Yazama F, Ito H (2011) Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta 1810(2):170–177. doi:10.1016/j.bbagen.2010.11.004
Tang H-R, Wang Y-L (2006) Metabonomics: a revolution in progress. Prog Biochem Biophys 33(5):401–417
Til HP, Falke HE, Prinsen MK, Willems MI (1997) Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem Toxicol 35(3–4):337–348. doi:10.1016/s0278-6915(97)00121-x
Van Der Sluis C, Rahardjo YS, Smit BA, Kroon PJ, Hartmans S, Ter Schure EG, Tramper J, Wijffels R (2002) Concomitant extracellular accumulation of alpha-keto acids and higher alcohols by Zygosaccharomyces rouxii. J Biosci Bioeng 93(2):117–124
van der Sluis C, Tramper J, Wijffels RH (2001) Enhancing and accelerating flavour formation by salt-tolerant yeasts in Japanese soy-sauce processes. Trends Food Sci Technol 12(9):322–327. doi:10.1016/s0924-2244(01)00094-2
Villas-Boas SG, Hojer-Pedersen J, Akesson M, Smedsgaard J, Nielsen J (2005) Global metabolite analysis of yeast: evaluation of sample preparation methods. Yeast 22(14):1155–1169. doi:10.1002/yea.1308
Wanakhachornkrai P, Lertsiri S (2003) Comparison of determination method for volatile compounds in Thai soy sauce. Food Chem 83(4):619–629. doi:10.1016/s0308-8146(03)00256-5
Watanabe Y, Akita H, Higuchi Y, Tsujimatsu R, Kaneta T, Tamai Y (2008) Heterologous expression of Na(+)/H(+) antiporter gene (C nu NHA1) from salt-tolerant yeast Candida versatilis in Saccharomyces cerevisiae Na(+)-transporter deficient mutants. Biosci Biotechnol Biochem 72(4):1005–1014. doi:10.1271/bbb.70752
Watanabe Y, Nagayama K, Tama Y (2008) Expression of glycerol 3-phosphate dehydrogenase gene (CvGPDl) in salt-tolerant yeast Candida versatilis is stimulated by high concentrations of NaCl. Yeast 25(2):107–116. doi:10.1002/yea.1550
Watanabe Y, Yamaguchi M, Sakamoto J, Tamai Y (1993) Characterization of plasma membrane H(+)-ATPase from salt-tolerant yeast Candida versatilis. Yeast (Chichester, England) 9(3):213–220. doi:10.1002/yea.320090302
Witthuhn RC, van der Merwe E, Venter P, Cameron M (2012) Guaiacol production from ferulic acid, vanillin and vanillic acid by Alicyclobacillus acidoterrestris. Int J Food Microbiol 157(1):113–117. doi:10.1016/j.ijfoodmicro.2012.04.022
Yin L, Choon Y, Chai L, Chong C, Deris S, Illias R, Mohamad M (2013) Prediction of vanillin production in yeast using a hybrid of continuous bees algorithm and flux balance analysis (CBAFBA). In: Sidhu AS, Dhillon SK (eds) Advances in biomedical infrastructure 2013, vol 477., Studies in Computational IntelligenceSpringer, Berlin, pp 101–116. doi:10.1007/978-3-642-37137-0_11
Zain ME, El-Sheikh HH, Soliman HG, Khalil AM (2011) Effect of certain chemical compounds on secondary metabolites of Penicillium janthinellum and P. duclauxii. J Saudi Chem Soc 15(3):239–246. doi:10.1016/j.jscs.2010.09.004
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (2012AA022108, 2012GB2A100016, 2012BAD33B04, 2013AA102106, 10ZCZDSY07000, 31000768, 31171731 and IRT1166).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qi, W., Fan, ZC., Wang, CL. et al. Non-targeted metabolomic reveals the effect of salt stress on global metabolite of halotolerant yeast Candida versatilis and principal component analysis. J Ind Microbiol Biotechnol 41, 1553–1562 (2014). https://doi.org/10.1007/s10295-014-1475-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1475-7