Abstract
An oleaginous and psychrotrophic strain (F38-3) of Sporobolomyces roseus Kluyver & van Niel was isolated from a salt marsh environment in Nova Scotia, Canada following a screening program to select for high producers of 18-carbon unsaturated fatty acids. Fatty acid production was characterised as a function of temperature at 20 g glucose L−1, and optimal yields were obtained at 14°C, achieving 5.7 g dw biomass and 39.2% total fatty acids by dry weight, with 18:1, 18:2 and 18:3 all-cis fatty acids accounting for 49.4%, 14.3% and 6.7% of total fatty acids (TFA), respectively—the highest reported for this species. Production of 18:3 was inversely correlated to growth temperature, rising from 2% of TFA at 30°C to 8.9% at 6°C. Cultivation of isolate F38-3 on universally 13C (U-13C) labelled glucose and subsequent transesterification and isolation of the fatty acid methyl esters (FAMEs) by preparative chromatography yielded pure, highly 13C-enriched (>90%) 18:1, 18:2 and 18:3 all-cis FAMEs. The U-13C 18:1 FAME was catalytically converted to U-13C 18:1 trans-9 and purified to >99.5% purity. The U-13C 18:2 was converted by alkaline isomerisation into a 50/50 mixture of 18:2 cis-9, trans-11 and 18:2 trans-10, cis-12 isomers and purified to >95.0% purity. Overall, 10%, by weight, of labelled glucose fed to isolate F38-3 was recovered as fatty acid methyl esters and 7.5% as 18-carbon unsaturated fats, and the final isomerisation reactions resulted in yields of 80% or greater. The ultimate goal of the work is to develop methodologies to produce 13C-labelled metabolic tracers as tools to study the metabolism of trans fats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, the public has a generally negative view of dietary trans fats (primarily elaidic acid, 18:1 trans-9) derived from partial hydrogenation of vegetable oils, found in fried and baked foods as well as margarines, and associated with increased risk of adverse cardiovascular events [4]. Although it is clear humans have ingested trans fats from ruminant sources, up to 5% of total fat, as a natural part of their diets over evolutionary time [2], the advent of industrial hydrogenation at the turn of the 20th century has led to greatly increased consumption [24]. As a consequence, dietary advice from multiple sources recommends reduction in trans fat intake (e.g. American Heart Association) to <1% of total fat intake, and legislation in the USA and Canada requires labelling of trans fat content on store-bought foods. Even so, trans fat labelling regulations are numerically challenged; thus, “zero trans fat” is defined by the Food and Drug Administration (FDA) as less than 0.5 g per serving, and by Health Canada as less than 0.2 g per serving (provided saturated fats and trans fats combined are less than 2 g per serving), leading Remig et al. [24] to point out that consumption of “zero trans fat” labelled foods could result in unhealthy intakes. Even though the link between intake of 18:1 trans-9 fat and elevated low-density cholesterol and depressed high-density cholesterol in blood is established, the mechanisms remain obscure [13, 19].
In contrast to 18:1 trans-9, other trans fats, notably the geometric and positional isomers of conjugated linoleic acid (CLA), naturally found in meat and dairy products derived from ruminants, are considered to have positive health effects. Naturally occurring CLA is primarily 18:2 cis-9, trans-11 (rumenic acid), although in dietary supplement products, CLAs are produced by alkaline isomerisation of linoleic acid and primarily consist of an equal mixture of 18:2 cis-9, trans-11 and 18:2 trans-10, cis-12 isomers. Purported health benefits of CLAs include reduction of body fat and anti-atherosclerotic, bone protection, insulin sensitising, anti-inflammatory and anti-cancer effects [5, 6, 25]. Even so, much of the evidence for the health effects of CLAs is derived from animal studies while in humans the results have been equivocal [20]. One major impediment to a better understanding of CLA metabolism in humans, pointed out by these authors, is the lack of suitable, i.e. stable, isotope-labelled analogues of CLAs for use as metabolic tracers.
Universally labelled, stable isotope (U-13C) fatty acids represent an ideal means with which to probe fatty acid metabolism using mass spectrometry [14, 17]. These techniques permit comprehensive assessment of adsorption, distribution, metabolism and excretion using in vivo models with biochemically equivalent, but isotopically unique, compounds. However, U-13C-labelled 18:1 trans-9 and 18:2 conjugated fatty acids are not commercially available, and although total synthesis is possible, it would be challenging and expensive because of the large number of potential geometric and positional isomers, multi-step routes and availability of the requisite 13C-labelled synthons. In contrast, use of heterotrophic, oleaginous microorganisms isolated from salt marsh habitats, grown on 13C-labelled carbon sources, has been demonstrated to be an efficient means of producing isotopically labelled long-chain polyunsaturated fats such as docosahexaenoic acid [15]. Herein we report screening of heterotrophic microbes isolated from salt marsh environments in Nova Scotia, Canada for producers of 18-carbon unsaturated fatty acids, optimisation of growth and fatty acid production of the most promising isolate as a function of temperature using N-limited media and its application to produce U13C-labelled 18-carbon, cis, unsaturated fatty acids and their subsequent modification to U-13C-labelled 18:1 trans-9 and 18:2 cis-9, trans-11 and 18:2 trans-10, cis-12 isomers by synthetic means.
Materials and methods
Chemicals
Fatty acid methyl ester standards (23:0, 18:1 cis-9, 18:1 trans-9, 18:2 cis-9, cis-12, 18:2 cis-9, trans-11 and 18:2 trans-10, cis-12) were obtained from NuCheck Prep (Elysian, MN). Labelled, U-13C glucose (99% 13C) was obtained from Cambridge Isotope Laboratories (Andover, MA). Instant Ocean sea salts were from Aquarium Systems Inc. (Ottawa, ON, Canada). All solvents were of analytical grade and purchased from Fisher Scientific (Nepean, ON, Canada). Yeast extract, peptone, agar, glucose, selenium (100 mesh) catalyst, ethylene glycol, Nile red and potassium hydroxide were obtained from Sigma–Aldrich (Oakville, ON, Canada).
Collection and isolation
In September 2009, 21 salt marsh habitats along the eastern Atlantic shore and the Bay of Fundy, Nova Scotia, Canada were sampled, typically for a combination of Spartina alterniflora (Loisel), Zostera marina L. and sediment or floating particulates. The samples were sealed in plastic bags, wet, and stored in a cooler. On return to the laboratory, small sub-samples were either baited with pollen or directly streaked on antibiotic-containing agar media as previously described [10, 15]. Colonies typical of both thraustochytrids and yeasts were targeted for successive single-colony isolations on solid media, ultimately yielding 55 pure isolates for a subsequent two-stage screening process: first for biomass, and second for lipid yield and composition. For biomass production, cultures were grown in 100 mL liquid media in 250-mL conical, baffled, flasks at 18°C for 5 days at 180 rpm on an orbital shaker. The medium comprised 20 g sea salts, 20 g glucose, 2 g peptone and 2 g yeast extract dissolved in 1 L distilled and deionised water and sterilised by autoclave. Cultures were harvested by centrifugation, rinsed once with distilled and deionised water and freeze-dried to obtain a dry weight yield. Only isolates yielding biomass of 2.5 g L−1 or greater were subject to detailed lipid analysis. The most promising isolates were observed by differential interference contrast and fluorescence microscopy (Zeiss, A1 imager) following staining with Nile red (10 μL of 100 μg mL−1 solution of Nile red in acetone added to 1 mL of culture).
Biomass extraction and transesterification of fatty acids
Typically 20–30 mg of dry cells was directly transesterified using 3 mL 2% H2SO4 in methanol for 30 min at 90°C in a thermostated heating block in sealed 10-mL Reacti-Vials with magnetic stirring fleas (Pierce, Rockford, IL). An accurately weighed spike of 23:0 methyl ester solution was added to each vial as internal standard prior to the reaction, and the contents were thoroughly mixed by brief sonication before heating. After cooling and addition of 1 mL water, the methyl esters were recovered by partitioning into hexane (2 × 2 mL) and brought to final volume of 5 mL in a volumetric flask prior to gas chromatography (GC) analysis.
DNA extraction and PCR of isolate F38-3
DNA was extracted by suspending approximately 200 mg of cells in 1 mL Puregene DNA lysis buffer (Gentra). Cells were disrupted in a Fast Prep (MP Biomedicals), and after centrifugation, the supernatant was extracted with phenol/chloroform and then chloroform. DNA was precipitated with ethanol and dissolved in 0.5 mL water. Amplification of the 18S ribosomal RNA (rRNA) gene used the following primers: 18S-4F (CTGGTTGATYCTGCCAGT) and 18S-1787R (CAGGTTCACCTACRG) [21]. Polymerase chain reactions (PCRs) were done with rTaq (Amersham) and included 50 ng template and 10 ng of each primer. PCR conditions were initial denaturation at 96°C for 2 min, followed by 30 cycles of 96°C (30 s), 53°C (30 s) and 72°C (2 min), and a final extension step of 72°C for 3 min. PCR products were purified through a Microcon (Millipore) and cloned by ligation to pCR2.1TOPO. Ligation mixtures were transformed into E. coli TOP10, and the resulting colonies were screened by PCR with the original amplification primers. Plasmid DNA was prepared from four positive colonies using the QIAprep kit (Qiagen) and were sequenced from both ends at Genewhiz. The DNA sequence from isolate F38-3 was deposited in GenBank under accession no. HQ913900.
Optimisation and 13C labelling
Optimisation with respect to growth temperature was done with four replicate cultures at each temperature point between 6°C and 34°C in 4°C increments. 13C-labelling experiments were conducted with the same media as described above, but using 100 mL media in each of 10 × 500-mL baffled, conical flasks to better facilitate gas exchange and substituting 20 g U13C glucose in place of the natural material. After autoclaving, the flasks were inoculated with 1 mL exponentially growing culture and grown for 5 days at 18°C. Extraction of bulk FAME from labelling experiments was done as described above, but with 100 mg freeze-dried biomass per Reacti-Vial in batches of eight. Cultures were tested periodically during the optimisation and labelling experiments to ensure glucose exhaustion before harvest. A 0.5-mL aliquot of culture broth was spin-filtered through a 0.45-μm membrane (Millipore Ultrafree); 10 μL of the filtrate was then diluted 500-fold with 50/50 MeOH/H2O and subject to liquid chromatography (LC)-electrospray ionisation (ESI) mass spectrometry (MS) analysis as described below.
Purification of FAMEs
Preparative separation of both cis and trans FAMEs were done isocratically with 90% acetonitrile in water on a 19 × 300 mm Symmetry Shield C-18 column (Waters, Milford, MA) at flow rate of 40 mL min−1 using a Shimadzu preparative high-performance liquid chromatography (HPLC) system (Kyoto, Japan) with ultraviolet (UV) detection at 203 nm. No more than 50 μL FAME was injected per run, and collected fractions were reduced to dryness by vacuum centrifuge. In some cases, a second preparative separation was required to achieve high purity.
GC–MS/FID analysis
Fatty acid methyl esters (FAME) were analysed on a Varian Saturn 2200 GC–MS system (Varian Inc., Palo Alto. CA). The GC was a model CP3800 equipped with both a CTC Analytics CombiPal autosampler (Zwingen, CH) and a flame ionisation detector (FID). For screening and optimisation studies, samples (1 μL) were injected at temperature of 250°C with split ratios of 1/25 (FID) or 1/100 (MS) on a 30 m × 0.25 mm ID × 0.25 μm film thickness Famewax column (Restek Corp., Bellefonte, PA). The temperature program was as follows: initial temperature 195°C, ramped at 5°C min−1 to 240°C and held for 9 min for total run time of 18 min. Ultra-high-purity helium was used as carrier gas at flow rate of 1.1 mL min−1. For analysis of trans FAMEs by FID, an HP88 60 m × 0.25 mm ID × 0.20 μm film thickness column was used (Chromatographic Specialties, Brockville, ON). Samples (1 μL) were injected at temperature of 250°C with split ratios of 1/100 (FID) or 1/50 (MS). The temperature program was as follows: initial temperature 125°C, held for 1 min, ramped at 8°C min−1 to 145°C, held for 26 min, then ramped at 2°C min−1 to 220°C for total run time of 67 min. Ultra-high-purity helium was used as carrier gas at flow rate of 1.4 mL min−1 (FID) or 1.3 mL min−1 (MS).
LC-APCI MS and LC-ESI MS analyses
LC-atmospheric pressure chemical ionisation (APCI) MS analysis of the 13C-labelled and natural-abundance 18-carbon unsaturated FAMEs to assess 13C enrichment was done isocratically at 78% acetonitrile in water on a 2.1 × 100 mm Symmetry shield column (Waters, Milford, MA) using an Agilent 1100 series chromatograph (Palo Alto, CA) at flow rate of 0.3 mL min−1, and a 2 μL injection volume of 100 μg mL−1 solution of the purified FAME. Effluent from the column was directed to a TSQ triple quadrupole mass spectrometer (Thermo Electron Corp., San Jose, CA) fitted with an APCI source. Spectra were averaged across the chromatographic peak at half height and subjected to background subtraction, and the intensities of each ion between 290 and 300 m/z were used to determine the extent of 13C enrichment and the residual pool of naturally abundant methyl ester by the method of Biemann [8]. LC-ESI MS analysis of the culture broth for glucose was done isocratically at 80% acetonitrile in water using a 1 μL injection on a 2.1 × 150 mm Zip Hilic column (Canadian Life Sciences, Peterborough, ON) at flow rate of 0.3 mL min−1 using the same instrumentation as above, but the MS was fitted with an ESI source. Glucose was detected by single-ion monitoring at m/z 179 (or 185 for 13C glucose) for the [M–H]− ion and quantified by external standards.
Synthesis of U13C-labelled trans fatty acids
Purified U13C-labelled 18:1 cis FAME (0.5 g) was reacted with a selenium catalyst (100 mesh) under nitrogen atmosphere at 200°C for 120 min to yield a 70/30 18:1 trans/cis mixture. The reaction mix was dissolved in 20 mL hexane, and subjected to repeated fractional crystallisation at −80°C and then a final purification step using preparative chromatography as described above. Purified U13C-labelled 18:2 cis FAME (0.25 g) was subjected to alkaline isomerisation in ethylene glycol and saturated potassium hydroxide under nitrogen atmosphere at 140°C for 120 min. The 18:2 cis-9, trans-11 and 18:2 trans-10, cis-12 isomer mixture was separated from other reaction products using preparative chromatography as described above.
Results
Screening for biomass and fatty acid production
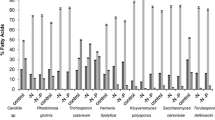
The biomass achieved by the 55 isolates is shown in Fig. 1a. At 20 g glucose L−1 many grew poorly, achieving less than 2.5 g dry weight per litre, and were eliminated from further examination. Of the 20 isolates that attained greater than 2.5 g L−1, and based on microscopic examination, the majority were identified as yeasts, with the exception of F20-1 and F24-2, which were thraustochytrids, and F15-2, a mycelial fungus. Fatty acid analysis of these isolates, showing both total lipid yield and total yield of 18-carbon unsaturated fatty acids (the sum of 18:1, 18:2 and 18:3), is provided in Fig. 1b. This second stage of screening narrowed the field to four oleaginous isolates with the ability to accumulate fatty acids in excess of 20% of their dry weight: F15-2 (21.9%), F20-1 (44.5%), F24-2 (42.7%) and F38-3 (34.8%). The fatty acids produced by the thraustochytrids F20-1 and F24-2 were high in palmitoleic (16:1) and docosahexaenoic (22:6), but produced relatively low levels of 18-carbon unsaturated fatty acids. One isolate, F38-3, was clearly identified as the best candidate for further examination, with both high biomass and lipid yield, with 63.7%, 12.0% and 2.9% of total fatty acids as 18:1, 18:2 and 18:3, respectively, and total yield of 18-carbon unsaturated fatty acids of 1.86 g L−1, nearly three times greater than the next best isolate (F15-2, 0.66 g L−1).
Macroscopically, isolate F38-3 exhibited intense orange/pink pigmentation, and on agar plates would produce numerous satellite colonies surrounding the original inoculum streak as well as a characteristic spore print on the inside of the Petri dish lid when the plates were incubated in the inverted position, indicative of ballistospore discharge. It was noted that F38-3 was isolated from a slick of particulate material, floating on the surface of a narrow saltwater channel, which formed an image of the overhanging vegetation and presumably a spore print. Indeed, S. roseus is known as the “mirror yeast” [9]. Microscopically, the cells were non-motile, oval to elongated oval in shape, 3.5–4.0 μm in width and 6.0–9.0 μm long. Under differential interference contrast, several (typically 2–4), large, circular, oil bodies up to 2 μm in diameter were clearly visible within the cells, fluorescing intensely under UV excitation following Nile red staining, confirming the presence of neutral lipids.
Genetic identification of isolate F38-3
The DNA sequence of the F38-3 18S rRNA gene (GenBank no. HQ913900) was found to be nearly identical to that of Sporobolomyces roseus AFTOL-ID 1549 (GenBank no. DQ832235) [16], with only a single nucleotide difference in 1,680 bp. Isolate F38-3, S. roseus, was deposited in the Canadian Collection of Fungal Cultures, Ottawa as accession DAOM 241997.
Optimisation of growth and lipid production as a function of temperature
Culture temperature had a pronounced effect on the biomass yield of S. roseus (Fig. 2a). At 34°C, the culture failed to grow and glucose was not consumed. However, increasing biomass yields were achieved for each 4°C decrease in incubation temperature to a maximum between 10°C and 14°C, although good growth was still found at 6°C, the lowest temperature tested. Growth temperature dictated the time required to achieve glucose exhaustion and therefore the time of harvest, being 4 days (30°C and 26°C), 5 days (22°C to 14°C), 7 days (10°C) or 11 days (6°C). Total lipid as a percentage of dry weight (Fig. 2b) varied from 32.5% at 30°C to a maximum of 39.2% at 14°C, although no strong effect of temperature on fatty acid content was noted between 6°C and 24°C. Lipid yield (Fig. 2c) reached a maximum at 14°C, largely due to increased biomass rather than increased fatty acid content.
The percentage of total fatty acids (TFA) as 18:1 was actually lowest at 14°C (49.2%), rising slightly at both lower and higher temperatures, but jumping to 60% at 26°C and 30°C (Fig. 3a), although the actual yields were highest at 14°C (Fig. 3b). The reverse pattern was seen for percentage 18:2 of TFA, which was relatively constant at 15% of TFA at 22°C and lower temperatures, but dropped to 10% at 26°C and 30°C (Fig. 3c). Similar to 18:1, however, 18:2 yields were maximal at 14–18°C (Fig. 3d). In contrast to 18:1 and 18:2, there was a clear, inverse, linear relationship between 18:3 percentage composition (Fig. 3e) and growth temperature, increasing from 1% at 30°C to 9% at 6°C, with yields maximal at 6°C (Fig. 3f). At all temperatures, the sum of 16:0, 18:0, 18:1, 18:2 and 18:3 fatty acids accounted for greater than 95% of TFA, with 16:0 remaining stable across the temperatures tested (~20% of total fatty acids) and 18:0 varying only slightly between 2% and 4% of TFA (data not shown).
C-18 unsaturated fatty acid production by Sporobolomyces roseus as a function of growth temperature. a 18:1 production as a percentage of total fatty acids (TFA); note the discontinuity at temperatures of 26°C and above. b 18:1 yield. c 18:2 production as a percentage of TFA. d 18:2 yield. e 18:3 production as a percentage of TFA. f 18:3 yield. All points are means ± SD (n = 4)
13C labelling and synthesis of U13C-labelled trans fatty acids
After harvest, freeze drying and transesterification, 5.6 g 13C-labelled biomass yielded 2.1 g FAME. Following preparative separation, 18:1 (900 mg), 18:2 (250 mg) and 18:3 (125 mg) all-cis FAMEs were recovered at greater than 99% purity (by GC-FID). The isotopic enrichment of each FAME is shown in Fig. 4, in comparison with their natural-abundance counterparts. In all cases, a high degree (>90%) of 13C enrichment was achieved; with the base peak in each labelled fatty acid representing the fully labelled ion, the minor amounts of unlabelled FAME present in the labelled material are likely derived from inoculum carryover. Ultimately, 300 mg high-purity (>99.8% by GC-FID) U-13C 18:1 trans-9 and 220 mg U-13C 18:2 as a 50/50 mixture of the cis-9, trans-11 and trans-10, cis-12 isomers (>95.0% by GC-FID) were obtained following fractional crystallisation and preparative chromatography.
APCI-MS spectra of natural-abundance and 13C-labelled 18-carbon unsaturated fatty acid methyl esters produced by Sporobolomyces roseus grown on U-13C glucose. a 12C 18:1. b U-13C 18:1. c 12C 18:2. d U-13C 18:2. e 12C 18:3. f U-13C 18:3. In all cases 13C-labelled fatty acids were enriched to greater than 90% (atom percent), with the base peak representing the fully labelled ion; however, the methyl ester carbon is natural abundance and derived from methanol during transesterification. Arrows indicate minor (<1%) but detectable pools of unlabelled FAME
Discussion
Sporobolomyces roseus is widely distributed from tropical to alpine regions, being associated with a plant leaf surface habitat (the phylloplane) and decaying vegetation [1, 11]. While generally considered to inhabit terrestrial niches, S. roseus, as shown here, is capable of abundant growth in submerged saline media and, thus, is potentially also a component of salt marsh ecosystems. Previous interest has focussed on ballistospore discharge [9], carotenoid production [12], facultative methanotrophy [27], phenol metabolism at low temperature [7] and production of yeast-specific toxins [18]. Earlier studies have reported lipid production by S. roseus [11], but with only moderate total fatty acid yields (4.2 ± 2.3% of dry weight), significantly lower than the maximal 40% reported here. This is despite similar C:N ratios employed in the media; although in our study sea salts (2%) were used, reducing these (0.5%) had no effect (data not shown). Further, Davoli et al. [11] did not report the presence of 18:3 fatty acids, although the cultivation temperature they employed (24°C) does not rule out production at lower temperatures.
Although the medium used in the experiments reported here was not fully defined, the relatively high glucose levels with respect to nitrogen sources were capable of supporting high biomass and lipid production and are routinely used in our laboratory for lipid production by thraustochytrids. While incremental gains in biomass and lipid production are likely with optimisation of the medium composition and other factors, and may be the subject of future experimentation, here the primary objectives were the effects of temperature. The strain of S. roseus isolated in this study is psychrotrophic rather than psychrophilic, as it is still capable of growth at 30°C, but with an optimal temperature for biomass yield of between 8°C and 14°C. The lower limit of growth was not determined. Further, isolate F38-3 may also be defined as oleaginous, accumulating greater than 20% of its dry weight as oil, a feature of only a minority of known yeasts [22]. It is possible that variation in lipid production occurs between different S. roseus isolates, as van Eijk et al. [26] reported that the fatty acid profiles (as %TFA) of four different strains of S. roseus, grown at 24°C, varied considerably (18:1 at 35.3–67.7%, 18:2 at 5.0–18.7% and 18:3 at 2.1–7.9%), although only profiles not actual yields were reported. In studies on a related species, S. ruberrimus, Razavi et al. [23] report 13% of dry weight as total lipid yield when grown at high glycerol concentrations, but only 3.3% as fatty acids. Therefore, to the best of our knowledge, the fatty acid yields reported here for S. roseus are the highest to date. High fatty acid yields have recently been reported for a cold-adapted yeast Rhodotorula glacialis, isolated from a glacial alpine environment, with similar yields (0.35 g biomass and 0.12 g lipid g−1 glucose versus 0.30 g biomass and 0.12 g lipid g−1 glucose for F38-3) when grown at similar glucose concentrations [3]. Higher yields of 0.16 g lipid g−1 glucose were obtained at 120 g glucose L−1 in a bioreactor [3], though such levels are prohibitive given our application with expensive 13C-labelled substrate. Nonetheless, the major fatty acid produced by R. glacialis was 18:2, and at ca. 40% of TFA, it would represent a superior source of this fatty acid compared with S. roseus.
Sporobolomyces roseus has proved to be a useful tool for production of both natural-abundance and 13C-labelled 18-carbon unsaturated lipids, and manipulation of culture temperature may be used to optimise the yields of specific fatty acids. Although the initial batch of 13C-labelled material in this study was produced at 18°C, optimal yields of 18-carbon unsaturated lipids are achieved at 14°C, and this temperature will be used for future production. At temperatures of 26°C and above, production of the 18:2 and 18:3 fatty acids is significantly curtailed, suggesting reduced activity of the desaturase enzymes. Although the production of 18:3 was inversely correlated with growth temperature, and in this study maximal at 6°C, further gains in yield at still lower temperatures would be offset by excessive culture times. Following production of natural all U-13C cis 18-carbon fatty acids using S. roseus, straightforward synthetic routes may be employed to produce both U-13C 18:1 trans-9 and U-13C 18:2 cis-9, trans-11 and 18:2 trans-10, cis-12 isomers for research applications as metabolic tracers.
References
Abranches J, Morales PB, Rosa CA, Mendonça-Hagler LC, Hagler AN (1997) The incidence of killer activity and extracellular proteases in tropical yeast communities. Can J Microbiol 43:328–336
Ackman RG (1997) Has evolution and long-term coexistence adapted us to cope with trans fatty acids? J Food Lipids 4:295–318
Amaretti A, Raimondi S, Sala M, Roncaglia De, Lucia M, Leonardi A, Rossi M (2010) Single cell oils of the cold adapted yeast Rhodotorula glacialis. Microb Cell Fact 9:73
Ascherio A, Hennekens CH, Buring JE, Master C, Stampfer MJ, Willet WC (1994) Trans fatty acids intake and risk of myocardial infarction. Circulation 89:94–101
Belury MA (2002) Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Ann Rev Nutr 22:505–531
Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G (2006) Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem 17:789–810
Bergauer P, Fonteyne P-A, Nolard N, Schinner F, Margesin R (2005) Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts. Chemosphere 59:909–918
Biemann K (1962) Mass spectrometry: organic and chemical applications. McGraw-Hill, New York
Buller AHR (1933) Researches on fungi, vol 5. Longmans, Green & Co., London
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterisation of polyunsaturated fatty acid producing thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72:1161–1169
Davoli P, Mierau V, Weber RWS (2004) Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl Biochem Microbiol 40(4):392–397
Davoli P, Weber RWS (2002) Carotenoid pigments from the red mirror yeast, Sporobolomyces roseus. Mycologist 16(3):102–108
Feldman EB, Kris-Etherton PM, Kritchevsky D, Lichtenstein AH (1996) Position paper of trans fatty acids. Am J Clin Nutr 63:663–670
Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachere JC, Begin ME, Brenna JT, Windust A, Cunnane SC (2006) Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids 75(3):213–220
Le MP, Fraser C, Gardner G, Liang W-W, Kralovec JA, Cunnane SC, Windust AJ (2007) Biosynthetic production of universally 13C-labelled polyunsaturated fatty acids as reference materials for natural health product research. Anal Bioanal Chem 389:241–249
Matheny PB, Gossmann JA, Zalar P, Arun Kumar TK, Hibbett DS (2006) Resolving the phylogenetic position of the Wallemiomycetes: an enigmatic major lineage of Basidiomycota. Can J Bot 84:1794–1805
McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC (2004) A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J Lipid Res 45:474–485
McCormack PJ, Wildman HG, Jeffries P (1994) Production of antibacterial compounds by phyllopane-inhabiting yeasts and yeastlike fungi. Appl Environ Microbiol 60:927–931
Mensink RP, Katan MB (1993) Trans monounsaturated fatty acids in nutrition and their impact on serum lipoprotein levels in man. Prog Lipid Res 32:111–122
Plourde M, Jew S, Cunnane SC, Jones PJH (2008) Conjugated linoleic acids: why the discrepancy between animal and human studies? Nutr Rev 66(7):415–421
Poitelon J-B, Joyeux M, Welte B, Duguet J-P, Peplies J, Dubow MS (2009) Identification and phylogeny of eukaryotic 18S rDNA phylotypes detected in chlorinated finished drinking water samples from three Parisian surface water treatment plants. Lett Appl Microbiol 49:589–595
Ratledge C (2002) Regulation of lipid accumulation in oleaginous micro-organisms. Biochem Soc Trans 30(6):1047–1050
Razavi SH, Mousavi SM, Yeganeh HM, Marc I (2007) Fatty acid and carotenoid production by Sporobolomyces ruberrimus when using technical glycerol and ammonium sulfate. J Microbiol Biotechnol 17(10):1591–1597
Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC (2010) Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc 110:585–592
Silveira MB, Carraro R, Monereo S, Tébar J (2007) Conjugated linoleic acid (CLA) and obesity. Public Health Nutr 10:1181–1186
van Eijk GW, Roeymans HJ, Weijman ACM (1982) Biochemical characteristics: volatiles, carotenoids, sterols and fatty acids. Stud Mycol 22:39–49
Wolf HJ, Christiansen M, Hanson RS (1980) Ultrastructure of methanotrophic yeasts. J Bacteriol 141:1340–1349
Acknowledgments
The authors would like to thank the Joint Cooperation Program of the National Research Council Canada, National Science Council of Taiwan and the Industrial Technology Research Institute Taiwan for financial support to undertake this work. The authors state they have no conflict of interest and are in agreement to publish in the Journal of Industrial Microbiology and Biotechnology. All experiments described in this manuscript were in accordance with the laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Y., Fraser, C., Gardner, G. et al. Isolation and optimisation of the oleaginous yeast Sporobolomyces roseus for biosynthesis of 13C isotopically labelled 18-carbon unsaturated fatty acids and trans 18:1 and 18:2 derivatives through synthesis. J Ind Microbiol Biotechnol 39, 153–161 (2012). https://doi.org/10.1007/s10295-011-1010-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-1010-z