Abstract
We report the conversion of glycerol to pyruvate by E. coli ALS929 containing knockouts in the genes encoding for phosphoenolpyruvate synthase, lactate dehydrogenase, pyruvate formate lyase, the pyruvate dehydrogenase complex, and pyruvate oxidase. As a result of these knockouts, ALS929 has a growth requirement of acetate for the generation of acetyl CoA. In steady-state chemostat experiments using excess glycerol and limited by acetate, lower growth rates favored the formation of pyruvate from glycerol (0.60 g/g at 0.10 h−1 versus 0.44 g/g at 0.25 h−1), while higher growth rates resulted in the maximum specific glycerol consumption rate (0.85 g/g h at 0.25 h−1 versus 0.59 g/g h at 0.10 h−1). The presence of glucose significantly improved pyruvate productivity and yield from glycerol (0.72 g/g at 0.10 h−1). In fed-batch studies using exponential acetate/glucose-limited feeding at a constant growth rate of 0.10 h−1, the final pyruvate concentration achieved was about 40 g/L in 36 h. A derivative of ALS929 which additionally knocked out methylglyoxal synthase did not further increase pyruvate productivity or yield, indicating that pyruvate formation was not limited by accumulation of methylglyoxal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is increasing interest in the use of glycerol as a substrate for bioprocesses, primarily because of the increasing availability of crude material from biodiesel production [1]. Crude glycerol might be directly suitable as a microbial feedstock, particularly for the production of low-value commodity chemicals such as succinic acid, ethanol, and propionic acid [1]. One challenge in the use of this substrate, however, lies in its lower energy value compared with glucose and other 6-carbon carbohydrates. Although glycerol generates as much adenosine triphosphate (ATP) as glucose per mole of pyruvate formed, more ATP is required for the biochemical formation from glycerol of several precursor molecules needed for biomass, including glucose 6-phosphate, fructose 6-phosphate, ribose 5-phosphate, and erythrose 4-phosphate. Therefore, compared with glucose, use of glycerol as a sole carbon source would be expected to result in a lower yield of pyruvate and pyruvate-derived biochemicals. One means to redirect glycerol to the desired product would be to supply a limiting amount of an energy-rich substrate, ideally one which would also generate some of the needed precursor molecules, such as glucose itself.
Pyruvic acid (pyruvate) is widely used in food, chemicals, and pharmaceuticals and as a starting material for several specialty chemicals [2]. After appropriate metabolic modifications, microorganisms can produce significant quantities of pyruvate from glucose and other renewable resources [2–4]; for example, pyruvate readily accumulates from glucose substrate in E. coli strains that lack activities in the pyruvate dehydrogenase complex, pyruvate formate lyase, and pyruvate oxidase [5–8]. Because multiple metabolic blocks to prevent pyruvate assimilation also restrict the synthesis of the key metabolite acetyl CoA, such strains commonly require acetate as a growth co-substrate. Providing excess glucose and controlling growth through acetate limitation is a convenient approach to maximizing the rate of glycolysis and hence pyruvate formation [9]. A fed-batch process implementing this strategy has achieved 90 g/L pyruvate with yield of 0.68 g/g glucose and productivity of 2.1 g/L h [9].
The goal of this study was to examine the use of glycerol as a substrate for the formation of pyruvate in an acetate-limited (fed-batch) bioprocess. In anticipation of reduced pyruvate yields compared with using glucose as a substrate, we also examined the addition of glucose to the medium as a means for cells to synthesize precursor molecules in glycolysis and the pentose phosphate pathway. The envisioned process uses both acetate and glucose as limiting substrates (and hence their concentrations both remain nearly zero), while maintaining excess glycerol to maximize the rate of pyruvate formation.
Materials and methods
Strains
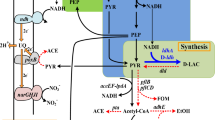
Escherichia coli ALS929 (Hfr zbi::Tn10 poxB1 ∆ (aceEF) rpsL pps-4 pfl-1ldhA::Kan) [9] and ALS1115 (ALS929 mgsA::Cam) were used for this study. As a result of knockouts in genes encoding pyruvate formate lyase, the pyruvate dehydrogenase complex, and pyruvate oxidase, these strains are unable to generate sufficient acetyl CoA from glucose or glycerol and therefore require acetate for growth. Figure 1 shows the metabolic pathways for ALS929 which lead to this acetate requirement. The yccG or mgsA gene which encodes methylglyoxal synthase [10] was knocked out using the lambda Red recombination system. Primers were designed which could amplify the chloramphenicol acetyltransferase gene and promoter from pACYC184 bracketed by the first and last 50 bases of the ppc coding sequence. The forward primer 5′-ATGTACATTATGGAACTGACGACTCGCACTTTACCTGCGCGGAAACATATTTGAGAAGCACACGGTCACA-3′ contains the first 50 bases of the mgsA coding sequence followed by bases 3,601–3,620 of pACYC184, while the reverse primer 5′-TTACTTCAGACGGTCCGCGAGATAACGCTGATAATCGGGGATCAGAATATTACCTGTGACGGAAGATCAC-3′ contains the last 50 bases of the mgsA coding sequence followed by bases 400–419 of pACYC184. The DNA from pACYC184 is underlined in the primers. The two primers were used to amplify a 1,163-bp fragment from pACYC184 DNA using the polymerase chain reaction (PCR) with Pfu polymerase. The resulting DNA was gel-isolated and electroporated into prepared DY330 electrocompetent cells [11]. Cam(R) colonies were then selected. The presence of the mgsA::Cam knockout was confirmed by performing PCR with the following two primer pairs which could amplify the mgsA coding sequence. The forward primer 5′-GGAACTGACGACTCGCACTT-3′ contains bases 12–31 of the mgsA gene, while the reverse primer 5′-TTACTTCAGACGGTCCGCGA-3′ contains bases 449–468 of the mgsA gene. PCR amplification with these two primers yields a 457-bp fragment from the wild-type mgsA gene and a 1,152-bp fragment from the mgsA::Cam knockout. The mgsA::Cam knockout was then moved into ALS929 by P1 transduction.
The metabolic pathways of E. coli ALS929 in the formation of pyruvate from glycerol and glucose. ALS929 has knockouts in genes encoding for pyruvate-formate lyase, pyruvate dehydrogenase complex, phosphoenolpyruvate (PEP) synthase, pyruvate oxidase, and lactate dehydrogenase (indicated by double bars). Because of these gene deletions, acetate is a required secondary substrate for generation of acetyl CoA. Dotted lines indicate biochemical precursors from which biomass is generated
Growth conditions
Cells were first grown in a 250-mL shake flask containing 30 mL TYA medium for about 8 h, before transferring 5 mL to a 250-mL shake flask containing 50 mL of SF medium. After 12 h of growth, the contents of this shake flask were used to inoculate a bioreactor. TYA medium contained (per L): 10.0 g tryptone, 5.0 g NaCl, 1.0 g yeast extract, and 1.36 g Na(CH3COO)·3H2O. SF medium contained (per L): 10.0 g glycerol, 2.3 g Na(CH3COO)·3H2O, 5.66 g Na2HPO4·7H2O, 1.5 g KH2PO4, 0.25 g NaCl, 0.5 g NH4Cl, 0.1 g MgSO4·7H2O, 0.013 g CaCl2·2H2O, 0.02 g thiamine·HCl, and 0.5 g l-isoleucine. For chemostat experiments, the bioreactor contained carbon sources as described plus (per L): 2.51 g K2HPO4, 1.44 g KH2PO4, 0.4 g NH4Cl, 4.0 g (NH4)2SO4, 0.15 g MgSO4·7H2O, 0.01 g CaCl2·2H2O, 0.05 g Na2EDTA·2H2O, 0.25 mg ZnSO4·7H2O, 0.125 mg CuCl2·2H2O, 1.25 mg MnSO4·H2O, 0.875 mg CoCl2·6H2O, 0.06 mg H3BO3, 0.8859 mg Al2(SO4)3, 0.25 mg Na2MoO4·2H2O, 5.50 mg FeSO4·7H2O, 0.02 g thiamine·HCl, and 0.2 g l-isoleucine. For fed-batch experiments, the bioreactor contained carbon sources as described plus (per L): 0.5 g NaH2PO4·H2O, 0.75 g KH2PO4, 0.50 g K2HPO4·3H2O, 0.2 g NH4Cl, 2.0 g (NH4)2SO4, 1.024 g MgSO4·7H2O, 0.01 g CaCl2·2H2O, 0.5 mg ZnSO4·7H2O, 0.25 mg CuCl2·2H2O, 2.5 mg MnSO4·H2O, 1.75 mg CoCl2·6H2O, 0.12 mg H3BO3, 1.772 mg Al2(SO4)3, 0.5 mg Na2MoO4·2H2O, 18.29 mg FeSO4·7H2O, 0.02 g thiamine·HCl, 0.75 g l-isoleucine, and 10 mmol glycine betaine (“betaine”). All media contained 40 mg/L kanamycin.
Flasks were incubated at 37°C and 250 rpm (19 mm pitch). In all bioreactor experiments, the temperature was 37°C with agitation at 400 rpm and an air flow rate of 1.0 L/min (mass flow controllers; Unit Instruments, Orange, CA). Pure oxygen was supplemented as necessary to ensure that the dissolved oxygen concentration remained above 40% saturation.
Chemostat
Continuous fermentations of 1.0 L volume operated as chemostats and were initiated in batch mode in a 2.5-L bioreactor (Bioflow 2000; New Brunswick Scientific Co. Inc., Edison, NJ, USA). pH was maintained at 7.0 with 20% (w/v) NaOH. A steady-state condition was assumed after five residence times, at which time the oxygen and CO2 concentrations in the effluent gas remained unchanged. Acetate-limited chemostats at several dilution rates were conducted using 30 g/L glycerol, 1 g/L acetate, and no glucose. A chemostat simultaneously limited in acetate and glucose was examined using 30 g/L glycerol, 1 g/L acetate, and 2 g/L glucose. The concentrations of selected substrates ensured that the chemostats were either acetate limited or acetate/glucose limited; that is, in all cases acetate and glucose were essentially exhausted from the medium, while glycerol was supplied in excess. Moreover, the concentrations of acetate and glucose used generated a steady-state biomass concentration of 2–3 g/L (OD = 5–6).
Fed-batch processes
Fed-batch processes were carried out in a 2.5-L bioreactor (Bioflow 2000; New Brunswick Scientific Co., Edison, NJ, USA) initially containing 1.0 L medium with 20 g/L glycerol and 1 g/L acetate. Cells grew at their maximum specific growth rate (i.e., without nutrient limitation) until the initial acetate was nearly exhausted (OD about 7). At this time, the fed-batch mode commenced with feed containing 400 g/L glycerol, 25 g/L acetate, and 50 g/L glucose or with feed containing 320 g/L glycerol and 25 g/L acetate. An exponential feed ensured that cell growth was controlled at a constant specific rate of about 0.10 h−1. As necessary, the concentration of glycerol in the feed was adjusted to ensure that this substrate was always in excess but did not accumulate above 30 g/L. The pH was controlled at 7.0 using 5% (w/v) NH4OH/25% KOH. Like the chemostat processes, the fed-batch processes were either acetate limited or acetate/glucose limited, with the concentrations of these substrates therefore generally less than the detection limit of 10 mg/L during the process.
Analyses
Optical density at 600 nm (OD) (UV-650 spectrophotometer; Beckman Instruments, San Jose, CA) was used to monitor cell growth, and this value was correlated to dry cell mass. Concentrations of soluble organic compounds were determined by liquid chromatography [12]. Concentrations of oxygen and CO2 in the off-gas were measured (Ultramat 23 gas analyzer; Siemens, Germany).
Results and discussion
Formation of pyruvate from glycerol
Escherichia coli containing the key gene mutations aceEF, ldhA, pfl, poxB, and pps accumulates pyruvate under aerobic conditions in a glucose and acetate medium [8]. By virtue of these mutations, the strain is unable to generate acetyl CoA from glycerol or glucose and has an absolute growth requirement for a secondary substrate which can generate acetyl CoA such as acetate (Fig. 1). However, acetate limitation forces a high rate of glycolysis and therefore pyruvate formation [9]. Glycerol should also serve as a biochemical source for pyruvate, and therefore we were interested in determining the rate of pyruvate formation from this substrate. Chemostats with E. coli ALS929 were used to compare dilution rates (i.e., growth rates).
We observed significant differences in process performance between the three studied growth rates of 0.10, 0.15, and 0.25 h−1 (Table 1). Higher growth rates favored increased specific rates of glycerol and acetate consumption. The distribution of products changed significantly between low and high growth rates. At high growth rates, carbon was fairly evenly distributed between pyruvate and biomass: the pyruvate yield was 0.44 g/g, while the biomass yield was 0.30 g/g. In contrast, over three times more pyruvate than biomass was formed at the lowest growth rate, at which the pyruvate yield was 0.60 g/g while the biomass yield was only 0.17 g/g. Specific CO2 evolution rate was about three times greater at the highest growth rate than at the lowest growth rate. However, even at the highest rate of production, evolved CO2 accounted for only 6.5% of total carbon consumed.
These observations indicate that lower growth rates were much more effective in directing glycerol to the desired product pyruvate, with proportionately less biomass and CO2. This conclusion is also reflected in the high ratio of glycerol-to-acetate utilization at the lowest growth rate. Cells consumed 8 mol glycerol per mole of acetate at 0.10 h−1, while at 0.25 h−1 the cells consumed less than 4 mol glycerol per mole of acetate. These results can be explained in terms of the cells’ maintenance requirement. In addition to generating a portion of the biomass, glycerol is converted to pyruvate to generate ATP. However, pyruvate is a biochemical “dead end” in these strains, so this biochemical accumulates. The lower the cell growth rate, the larger the fraction of total energy demands that must be expended to fulfill the maintenance requirements. With increasing nongrowth energy requirements, the cells must consume proportionately more glycerol as a means to generate that energy, leading to higher pyruvate yield at lower growth rates. Growth rates lower than 0.10 h−1 might further increase yield but would be less practical to control and would come at the expense of volumetric productivity. Indeed, among the growth rates studied, the maximum volumetric pyruvate production rate of 0.73 g/L h was observed at 0.15 h−1, while the other two growth rates generated pyruvate at lower rates of 0.68 g/L h (for 0.10 h−1) and 0.61 g/L h (for 0.25 h−1). For these chemostat experiments, carbon recovery was consistently 78–80%.
Use of two limiting substrates simultaneously: acetate and glucose
As noted, the lowest growth rate studied (0.10 h−1) resulted in the highest pyruvate yield of 0.60 g/g, although this result was still significantly lower than the maximum reported (0.78 g/g) in a similar acetate-limited process with excess glucose [9]. We speculated that this lower yield was due to the demand for 4–6 carbon precursor molecules in glycolysis and the pentose phosphate pathway. We therefore conducted one additional chemostat at 0.10 h−1 in which 2 g/L glucose was also included in the feed. Although all of the glucose could be consumed, because biomass formation was still limited by the availability of acetate, glycerol was in excess.
Several surprising results were observed in the steady-state experiment using glucose (in addition to glycerol and acetate) in the medium (Table 1). The specific rate of glycerol consumption was actually higher in the presence of glucose, and the specific rate of pyruvate production was 25% greater. Importantly, in the presence of glucose, the pyruvate yield was 20% greater at 0.72 g/g. This increased yield occurred primarily because more glycerol was converted to pyruvate and at a faster rate, leading to volumetric productivity of 1.08 g/L h, about 60% greater than that observed in the absence of glucose. Note that the increase in pyruvate generation was twice as much as the glucose that was provided: glucose addition facilitated glycerol utilization. Also, less CO2 was generated in the presence of glucose, probably reflecting the diminished need for complete oxidation of substrates to meet energy requirements. Finally, over 10 mol glycerol was consumed per mole of acetate. Not only can both glycerol and glucose be consumed simultaneously, but these observations suggest that a relatively small quantity of glucose in the presence of glycerol can meet precursor demands for much more effective consumption of glycerol. For this chemostat, the effluent contained no detectable acetate and about 70 mg/L glucose, and the carbon balance was 82%. We note that catabolite repression would not occur under steady-state limiting conditions because the glucose concentration remains very low [13, 14]. Indeed, our experiments demonstrate that the presence of glucose in the feed did not reduce the specific consumption of glycerol, but in fact actually led to a slight increase in specific glycerol consumption rate. Moreover, in the absence of glucose, pyruvate was generated at a rate of 4.07 mmol/g h, while in the presence of glucose (which was consumed at 0.44 mmol/g h) pyruvate was generated at a rate of 5.06 mmol/g h, a slightly higher rate than would have been obtained if all the glucose were converted to pyruvate. The inclusion of glucose also improved the overall elemental carbon yield: in the absence of glucose, 58% of (all) carbon consumed became pyruvate, whereas in the presence of glucose, 63% of carbon consumed became pyruvate. Thus, the inclusion of glucose under limiting conditions improves the rate and yield of pyruvate production.
Although the yield of pyruvate from glycerol (0.72 g/g) compares favorably with the maximum yield from glucose previously reported in chemostat operation (0.78 g/g) [9], the specific rate of pyruvate production from glycerol (0.44 g/g h) is less than one-quarter the maximum value from glucose (2.01 g/g h) [9]. These results highlight the slower utilization of glycerol compared with glucose as an energy-carbon source.
Fed-batch processes
Chemostat experiments provide steady-state information to assist in the design of fed-batch processes, an operational mode which allows a product to accumulate to a higher final concentration than in continuous mode. We therefore next compared two fed-batch processes using an exponential feed to maintain a specific growth rate of about 0.10 h−1, one without glucose and a second with glucose. Similar to the chemostat experiments, growth was limited by acetate (except for a short initial time interval). Because pyruvate accumulation would simultaneously require the accumulation of K+ (or another counterion) to maintain the pH, we also added the osmoprotectant betaine to the medium [15, 16]. Figure 2 shows that the results for the acetate-limited process were similar to those for the acetate/glucose-limited process. In both cases, cell growth slowed when the OD reached about 20 and the pyruvate concentration reached 35–40 g/L. The acetate-limited process achieved slightly higher final concentrations, with average yield of 0.62 g/g and average productivity of 0.88 g/L h. The acetate/glucose-limited process consistently showed slightly lower final concentrations and average productivity was also 0.88 g/L h, but the yield from glycerol averaged 0.95 g/g. Similar to the observation in the chemostat studies, although a portion of the glucose could yield pyruvate, the presence of glucose improved glycerol utilization. Note that the concentrations of acetate and glucose were nearly zero throughout these nutrient-limited processes.
Formation of pyruvate from glycerol in a fed-batch process at growth rate of 0.10 h−1 using E. coli ALS929 with acetate (open symbols) and with acetate and glucose (solid symbols) as limiting substrates: OD, (white circles, black circles); pyruvate, (white triangles, black triangles). Note that the acetate and glucose concentrations were close to zero throughout the fed-batch portions of these processes. Moreover, glycerol was added as described in the text to ensure that it was always present in (nonzero) excess
Although the volumetric rates and yields observed in the fed-batch process were consistent with chemostat results, we were not able to achieve pyruvate concentrations greater than 40 g/L, less than the approximately 90 g/L previously achieved from glucose [9]. A significant difference between a chemostat and a fed-batch process is the greater potential for accumulation of inhibitors in the latter operational mode. We therefore speculated that an intracellular metabolite generated from glycerol might be accumulating to prevent cell growth at OD of about 20. One candidate inhibitor is methylglyoxal, a known potent inhibitor of E. coli and other cells [17]. To test whether methylglyoxal accumulation was involved in cessation of pyruvate formation in ALS929, we constructed an ALS929 derivative (denoted ALS1115) with deleted mgsA, the gene that encodes for methylglyoxal synthase. We conducted an identical fed-batch process using ALS1115 at 0.10 h−1 and observed no change in pyruvate formation rate or final concentration (data not shown), indicating that methylglyoxal is not the source of the apparent inhibition in pyruvate formation.
In conclusion, E. coli ALS929 is able to accumulate pyruvate using glycerol as substrate. Glycerol consumption, and pyruvate formation, is enhanced by addition of glucose, which would relieve the demand for glycerol in the production of some sugar phosphates used in anabolic reactions. In a fed-batch process, pyruvate production appears to be limited to 40 g/L, perhaps as a result of the buildup of an unknown inhibitory metabolite that is not present during the conversion of glucose to pyruvate. Although the concentrations of pyruvate generated from glycerol remain below the maximum concentrations observed using the same strain grown on glucose, this study highlights some potential benefits of supplementing glycerol-containing media with glucose under growth limitations.
References
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–219
Li Y, Chen J, Lun SY (2001) Biotechnological production of pyruvic acid. Appl Microbiol Biotechnol 57:451–459
Causey TB, Shanmugam KT, Yomano LP, Ingram LO (2004) Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci 101:2235–2240
Zelic B, Gostovic S, Vuorilehto K, Vasic-Racki B, Takors R (2004) Process strategies to enhance pyruvate production with recombinant Escherichia coli: from repetitive fed-batch to in situ product recovery with fully integrated electrodialysis. Biotechnol Bioeng 85:638–646
Yokota A, Shimizu H, Terasawa Y, Takaoka N, Tomita F (1994) Pyruvic acid production by a lipoic acid auxotroph of Escherichia coli W1485. Appl Microbiol Biotechnol 41:638–643
Yokota A, Terasawa Y, Takaoka N, Shimizu H, Tomita F (1994) Pyruvic-acid production by an F-1-atpase-defective mutant of Escherichia coli W1485lip2. Biosci Biotechnol Biochem 58:2164–2167
Tomar A, Eiteman MA, Altman E (2003) The effect of acetate pathway mutations on the production of pyruvate in Escherichia coli. Appl Microbiol Biotechnol 62:76–82
Zelic B, Gerharz T, Bott M, Vasič-Racki D, Wandrey C, Takors R (2003) Fed-batch process for pyruvate production by recombinant Escherichia coli YYC202 Strain. Eng Life Sci 3(7):299–305
Zhu Y, Eiteman MA, Altman R, Altman E (2008) High glycolytic flux improves pyruvate production by a metabolically engineered Escherichia coli strain. Appl Environ Microbiol 74:6649–6655
Saadat D, Harrison DH (1998) Identification of catalytic bases in the active site of Escherichia coli methylglyoxal synthase: cloning, expression, and functional characterization of conserved aspartic acid residues. Biochemistry 37:10074–10086
Yu D, Ellis HM, Lee E-C, Jenkins NA, Copeland NG, Court DL (2000) An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA 97(11):5978–5983
Eiteman MA, Chastain MJ (1997) Optimization of the ion-exchange analysis of organic acids from fermentation. Anal Chim Acta 338:69–75
Egli T, Lendenmann U, Snozzi M (1993) Kinetics of microbial growth with mixtures of carbon sources. Antonie Van Leeuwenhoek 63:289–298
Lendenmann U, Egli T (1995) Is Escherichai coli growing in glucose-limited chemostat culture able to utilize other sugars without lag? Microbiology 141:71–78
Underwood SA, Buszko ML, Shanmugam KT, Ingram LO (2004) Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl Environ Microbiol 70:2734–2740
Zhou S, Grabar TB, Shanmugam KT, Ingram LO (2006) Betaine tripled the volumetric productivity of d(-)-lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol Lett 28:671–676
MacLean MJ, Ness LS, Ferguson GP, Booth IR (1998) The role of glyoxalase I in the detoxification of methylglyoxal and in the activation of the KefB K+ efflux system in Escherichia coli. Mol Microbiol 27:563–571
Acknowledgments
The authors acknowledge the financial support of the Georgia Experiment Station. We thank Ronni Altman and Rupal Prabhu for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Eiteman, M.A., Lee, S.A. et al. Conversion of glycerol to pyruvate by Escherichia coli using acetate- and acetate/glucose-limited fed-batch processes. J Ind Microbiol Biotechnol 37, 307–312 (2010). https://doi.org/10.1007/s10295-009-0675-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0675-z