Abstract
Citramalic acid (citramalate) serves as a five-carbon precursor for the chemical synthesis of methacrylic acid. We compared citramalate and acetate accumulation from glycerol using Escherichia coli strains expressing a modified citramalate synthase gene cimA from Methanococcus jannaschii. These studies revealed that gltA coding citrate synthase, leuC coding 3-isopropylmalate dehydratase, and acetate pathway genes play important roles in elevating citramalate and minimizing acetate formation. Controlled 1.0 L batch experiments confirmed that deletions in all three acetate-production genes (poxB, ackA, and pta) were necessary to reduce acetate formation to less than 1 g/L during citramalate production from 30 g/L glycerol. Fed-batch processes using MEC568/pZE12-cimA (gltA leuC ackA-pta poxB) generated over 31 g/L citramalate and less than 2 g/L acetate from either purified or crude glycerol at yields exceeding 0.50 g citramalate/g glycerol in 132 h. These results hold promise for the viable formation of citramalate from unrefined glycerol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The commercial manufacture and use of biodiesel has been rapidly emerging in Europe and US during the last 2 decades. As an alternative to petrochemical fuels, biodiesel is superior in its health and environmental impact, including low sulfur content, lower emission of harmful off-gases, and a better lifecycle of CO2 [9]. One key challenge in the development and adoption of biodiesel is the low value by-product glycerol, which is generated at about 10% mass ratio from the esterification or transesterification of vegetable oil and animal fats [29]. Fortunately, many microorganisms can naturally utilize glycerol as the sole carbon and energy source, and glycerol is a potential substitute for the traditional carbohydrates such as sucrose or starch in industrial fermentation processes [7, 8, 18]. Glycerol has been evaluated as a raw material for the production of many microbial products, including hydrogen [36], 1,3-propanediol [10], 2,3-butanediol [44], and succinic acid [20].
Methacrylic acid (MAA) is a commodity chemical with an estimated annual global market of about 2.2 million tons, and it is used primarily for the synthesis of poly(methylmethacrylate) [45]. This polyester is widely used as a transparent thermoplastic in construction, furniture, medical material, and display technologies. The most common route for MAA synthesis converts acetone cyanohydrin to methacrylamide sulfate using sulfuric acid [6, 32, 37]. Sulfuric acid regeneration and hazards associated with volatile cyanides are concerns for industrial MAA production, and companies have sought other routes from isobutene, isobutyric acid, and ethylene [6, 32]. Direct microbial production of MAA and acrylate with its reduced hazards has been proposed, but acrylates are extremely toxic to microorganisms such as Escherichia coli [2, 39].
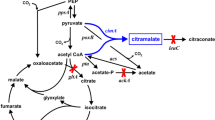
Recently, we reported a microbial approach to produce citramalic acid [citramalate, (R)-2-methylmalic acid, (2R)-2-hydroxy-2-methylbutanedioate] from renewable carbohydrates. Citramalate can be directly converted to MAA by base-catalyzed decarboxylation and dehydration [24]. In a fed-batch fermentation, 46.5 g/L citramalate was formed with a yield of 0.63 g/g from glucose using an engineered Escherichia coli expressing a modified cimA gene derived from Methanococcus jannaschii coding citramalate synthase [42]. Despite the deletion of citrate synthase (coded by gltA) and acetate kinase (ackA), about 10 g/L acetate were still formed as an undesirable by-product from glucose. The maximum theoretical yield of citramalate from glycerol in E. coli is 0.80 g/g (Fig. 1), and the stoichiometric equation for the biochemical conversion is as follows:
Biosynthesis of citramalate in Escherichia coli expressing the cimA gene coding citramalate synthase. Key genes (and coded enzymes) are: leuC and leuD (3-isopropylmalate dehydratase), gltA (citrate synthase), glcB and aceB (malate synthase), pta (phosphotransacetylase), ackA (acetate kinase), poxB (pyruvate oxidase), and ppsA (phosphoenolpyruvate synthetase)
The goal of this study was to examine citramalate formation from glycerol by Escherichia coli. In addition to studying whether 5-carbon citramalate can be generated directly from both purified and crude 3-carbon glycerol at high yield, we examined strategies to reduce the formation of acetate as a by-product (see Fig. 1).
Materials and methods
Strain construction
Strains used in this study are shown in Table 1. Gene mutations were transduced into E. coli MG1655 from their respective strains in the KEIO collection [5] by the P1 phage method. The ∆pta knockout was constructed using the λ Red recombination [12]. After completing the ∆pta knockout, the ackA-pta operon was sequenced confirming that ∆ackA-pta was deleted (Eurofins Scientific, Louisville, KY, USA). To knockout multiple genes in single strain, the Kan antibiotic marker was removed using pCP20 [12]. In knockout strains, forward primers external to the target gene and reverse primers within the kanamycin resistance cassette were used to check for proper chromosomal integration. In cured strains, the removal of the markers was verified by PCR. Plasmid pZE12-cimA was transformed into all strains for citramalate production [42]. This cimA gene from M. jannaschii has been evolved to enhance activity and to reduce isoleucine inhibition [3, 23]. For this study, the E. coli codon-optimized gene was used (GenScript, Piscataway, USA).
Growth medium
XP medium was composed of (per L): 3.00 g glycerol, 1.00 g/L peptone, 1.44 g KH2PO4, 2.11 g K2HPO4, 2.00 g K2SO4, 3.50 g NH4Cl, 20.00 mg Na2(EDTA)·2H2O, 0.15 g MgSO4·7H2O, 20 mg thiamine·HCl, 0.25 mg ZnSO4, 0.125 mg CuCl2·2H2O, 1.25 mg MnSO4·H2O, 0.875 mg CoCl2·6H2O, 0.06 mg H3BO3, 0.25 mg Na2MoO4·2H2O, 5.50 mg FeSO4·7H2O, and 20 mg citric acid. For the growth of strains having leuC or leuD knockouts, the medium was supplemented with 0.20 g/L l-leucine. For the growth of strains having gltA knockouts, the medium was supplemented with 1.00 g/L l-glutamate, since E. coli is unable to utilize citrate under aerobic conditions [25]. In addition, 50 mg/L ampicillin and/or 100 mg/L kanamycin were added for plasmid-containing strains or strains having antibiotic resistance. The crude glycerol from biodiesel process was generously provided by a local biodiesel producer (Down To Earth Energy, LLC, Monroe, GA, USA) and was composed of 58.6% w/w glycerol and 0.3% w/w methanol.
Shake flask, batch, and fed-batch processes
To compare various strains for citramalate production in shake flasks, cells were first grown in 3 mL Lysogeny Broth (LB) at 37 °C and 250 rpm (19 mm pitch). After 10–14 h, 0.5 mL was used to inoculate 50 mL of XP medium supplemented with 0.2 mM IPTG in 500 mL baffled shake flasks (in triplicate). After growth at 37 °C and 250 rpm (19 mm pitch) for 24 h, these shake flask cultures were analyzed for citramalate synthase activity, citramalate, and intracellular acetyl-CoA concentration.
To examine citramalate production under controlled bioreactor conditions, cells were first grown as described above in 3 mL LB and then 50 mL XP medium. After 18 h, the shake flask contents were used to inoculate a 2.5 L bioreactor (Bioflo 2000, New Brunswick Scientific Co., New Brunswick, NJ, USA) containing 1.0 L modified XP medium with 30 g/L glycerol, 5 g/L peptone, 3 g/L glutamate, and 1 g/L leucine (but otherwise as described above) and 0.2 mM IPTG initially. For duplicate batch and fed-batch processes, the agitation was 400 rpm, and air was sparged at 1.0 L/min, which maintained the dissolved oxygen above 40% of saturation. The pH was controlled at 7.0 using 20% (w/v) NaOH, and the temperature was controlled at 37 °C. For the fed-batch process, an additional 30 g glycerol and 5 g peptone dissolved in 60 mL of water were added when the glycerol concentration decreased below 5 g/L. The calculation of yield is based on the mass of the compound generated divided by the mass of glycerol consumed. Statistical analyses were completed using Student’s t test (two-tailed, equal variance), and p < 0.05 was considered the criterion for significance.
Analytical methods
The optical density at 600 nm (OD) (UV-650 spectrophotometer, Beckman Instruments, San Jose, CA, USA) was used to monitor cell growth. Extracellular organic acids and glycerol were analyzed by HPLC using a Refractive Index detector as previously described [15]. Glutamate concentration was measured using a glutamate assay kit (Sigma-Aldrich Co., St. Louis, MO, USA). Intracellular acetyl-CoA was analyzed by the previously established method [19].
Cell-free extracts were also used to measure citramalate synthase activity by the generation of free CoA and its reaction product with 5,5′-dithiobis(2-nitrobenzoic acid) detected at a wavelength of 412 nm [23]. One unit of activity is the amount of enzyme that generates 1 μmol of CoA in 1 min at 37 °C.
Results and discussion
Comparison of citramalate and acetate formation by various strains
In E. coli expressing citramalate synthase coded by the cimA gene, citramalate accumulates as the reaction product of the condensation of pyruvate and acetyl-CoA. In wild-type E. coli expressing citramalate synthase (MG1655/pZE12-cimA), just over 1 g/L citramalate formed from 3 g/L glycerol, resulting in a citramalate yield of 0.36 g/g (Fig. 2). This wild-type strain expressing citramalate synthase generated substantial acetate in shake flasks, resulting in a yield of 0.033 g acetate/g glycerol (Fig. 2). Since acetyl-CoA and pyruvate are involved in numerous enzyme reactions, we compared citramalate formation from glycerol using several strains having knockouts in genes associated with these metabolites.
Comparison of citramalate yield and acetate yield from 3 g/L glycerol in triplicate shake flasks using various knockout strains of E. coli expressing the cimA gene. The medium used for leuC or leuD strains was supplemented with 0.2 g/L leucine, while the medium used for gltA strains was supplemented with 1 g/L glutamate. The error bars represent standard deviations
Acetyl-CoA is converted to malate via malate synthase coded in E. coli by the glcB and aceB genes [31, 35]. We, therefore, constructed MEC481 (MG1655 aceB), MEC482 (MG1655 glcB), and MEC485 (MG1655 aceB glcB). Compared to MG1655/pZE12-cimA, MEC481/pZE12-cimA and MEC482/pZE12-cimA showed about 28 and 35% higher citramalate accumulation, respectively (Fig. 2). The strain having knockouts in both malate synthase genes, MEC485/pZE12-cimA, resulted in only 22% greater citramalate compared to the wild type. Acetyl-CoA is also converted to citrate via citrate synthase coded by the gltA gene [14], and we, therefore, examined citramalate production in MEC480 (MG1655 gltA) expressing citramalate synthase. MEC480/pZE12-cimA grew poorly on XP medium, but growth was restored when the medium was supplemented with 1 g/L glutamate. MEC480/pZE12-cimA grown with supplemented glutamate accumulated 0.58 g citramalate/g glycerol, 63% more than MG1655/pZE12-cimA. Since MG1655/pZE12-cimA grown in XP medium supplemented with 1 g/L glutamate also generated the same yield of citramalate as the same strain without added glutamate (data not shown), we attribute the 63% increase in citramalate formation in MEC480/pZE12-cimA to the gltA knockout and not to the presence of glutamate. Therefore, media for strains having the gltA knockout were henceforth supplemented with 1 g/L glutamate. These strains having knockouts of enzymes associated with the glyoxylate shunt or the TCA cycle (i.e., aceB, glcB, and gltA) accumulated no detectable acetate.
Citramalate could be potentially metabolized in E. coli by 3-isopropylmalate dehydratase coded by the leuC (large subunit) and leuD (small subunit) genes [16, 17]. The two subunits are both required for the activity of isopropylmalate isomerase, an enzyme which is necessary for leucine biosynthesis in E. coli [43], and each of these individual deletions was examined by comparing MEC490 (MG1655 gltA leuC) and MEC491 (MG1655 gltA leuD). With the deletion of either leuC or leuD, E. coli did not grow in XP medium with glycerol as the sole carbon source, despite the presence of peptone (data not shown). Growth was restored by the addition of 0.2 g/L leucine, and MEC490/pZE12-cimA accumulated 0.68 g citramalate/g glycerol, 13% greater than MEC480/pZE12-cimA (significant at p < 0.05), while MEC491/pZE12-cimA accumulated 0.65 g citramalate/g glycerol (Fig. 2). MEC490/pZE12-cimA and MEC491/pZE12-cimA both accumulated a similar concentration of acetate as MG1655/pZE12-cimA.
Although MEC490 (gltA leuC knockouts) formed significantly more citramalate than MEC480 with only the gltA knockout, the additional leuC knockout also led to acetate formation from glycerol. To reduce acetate formation in the E. coli gltA leuC expressing citramalate synthase, we examined several pathways related to the acetate and pyruvate metabolism. Four enzymes exist in E. coli related to acetate and acetyl-CoA. Acetate kinase coded by ackA and phosphotransacetylase coded by pta are typically considered the primary routes for the conversion of acetyl-CoA to acetyl-phosphate (acetyl-P) and to acetate [26, 30]. Acetyl-P can form acetate via other routes, also, since it can serve as a phosphate donor in gene regulation and protein-dependent transport systems [22, 41]. On the other hand, acetyl-CoA synthetase coded by acs functions as an anabolic route and scavenges acetate to acetyl-CoA [28]. Finally, pyruvate oxidase coded by poxB can play a role in aerobic growth of E. coli and in acetate formation from pyruvate [1]. We also examined phosphoenolpyruvate synthetase coded by ppsA, which could affect the intracellular pyruvate and acetyl-CoA pools [33]. We constructed several strains having these knockouts, expressed citramalate synthase, and determined the citramalate and acetate formation in shake flasks (Fig. 2).
The additional deletion in the ackA gene or the combination of ackA and pta genes increased citramalate yield slightly (p < 0.05) to 0.71 and 0.69 g/g, respectively. However, both MEC499/pZE12-cimA and MEC562/pZE12-cimA still formed acetate with yields of about 0.018–0.020 g/g (Fig. 2). Compared to E. coli gltA leuC ackA-pta expressing citramalate synthase, an additional ppsA deletion did not affect citramalate or acetate formation significantly, while an additional acs knockout actually elevated acetate yield to 0.030 g/g. Inexplicably, one previous investigation of an acs deletion strain resulted in lower specific acetate formation from glucose [11], while in another study, overexpression of acs significantly reduced acetate formation [28]. In our study using strains with additional gene deletions, the increase in acetate formation when acs is deleted (in the ackA-pta background) suggests that some acetate is formed via pyruvate oxidase, and that acetyl-CoA synthase provides the cells with a means to metabolize that acetate partially. In support of this conclusion, the poxB knockout (in the ackA-pta background) eliminated acetate formation in the shake flask culture, and increased citramalate yield from glycerol significantly (p < 0.05) to 0.74 g/g. To determine whether poxB or the combination of pta poxB was important to eliminate acetate formation, we also examined MEC596/pZE12-cimA, which generated 0.73 g citramalate/g glycerol and no detectable acetate. These results conclusively show that pyruvate oxidase is a key enzyme in the accumulation of acetate during citramalate production in E. coli. The deletion of poxB has similarly shown reduced acetate formation in an ackA-pta strain during the aerobic production of succinate by E. coli [27].
Acetyl-CoA is an important substrate for citramalate synthase, and we measured intracellular acetyl-CoA concentration in all triplicate shake flask experiments. These results were used to determine whether any correlation exists between intracellular acetyl-CoA and citramalate yield in the 13 different strains (Fig. 3). The results show that increased citramalate yield correlates with increased acetyl-CoA concentration with a linear correlation of R 2 = 0.64 (though any correlation needs not to be linear). The values observed for acetyl-CoA concentration are about 10–20 times greater than those reported during growth on glucose in defined medium, though they are in line with values during stationary phase [38, 40]. In our study, glycerol was exhausted at the time of the 24 h sample during all shake flasks experiments.
Relationship between citramalate yield and intracellular acetyl-CoA concentration in shake flasks using various knockout strains of E. coli expressing the cimA gene (shown in Fig. 2)
Controlled batch citramalate production from glycerol
To determine whether shake flask results were transferable to larger scale, we next examined citramalate production at the 1.0 L scale in controlled bioreactors. In duplicate, we compared six strains expressing citramalate synthase: MG1655, MEC490, MEC499, MEC562, MEC568, or MEC596. To accommodate greater cell growth, the medium contained 30 g/L glycerol supplemented with 5 g/L peptone, as well as 3 g/L glutamate and 1 g/L leucine (for strains with gltA leuC knockouts). The results of these batch processes are shown in Table 2.
MG1655/pZE12-cimA reached an OD of over 20 in 24 h and accumulated 4.3 g/L citramalate (yield of 0.143 g/g) and 0.05 g/L acetate in 30 h (yield of 0.002 g/g). All other strains examined had the gltA and leuC knockouts which significantly slowed growth despite the presence of glutamate and leucine in the medium, and they generally reached an OD of 10 in 24–30 h. The gltA leuC knockouts alone (MEC490/pZE12-cimA) resulted in only 5.2 g/L citramalate (yield of 0.175 g/g) and 11.4 g/L acetate (yield of 0.380 g/g). In comparison, the addition of an ackA deletion increased citramalate and diminished acetate formation. Nevertheless, the ackA deletion was insufficient to prevent acetate formation. The addition of a poxB deletion to the gltA leuC ackA strain further decreased acetate formation. The lowest accumulation of acetate was observed under controlled batch conditions using the strain with all three acetate pathway knockouts (gltA leuC ackA-pta poxB), and MEC568/pZE12-cimA also led to the greatest citramalate production (about 17.5 g/L). Typically, the phosphotransacetylase and acetate kinase activities are significant during cell growth, while pyruvate oxidase appears to become important during the stationary phase [13]. Pyruvate oxidase, moreover, bypasses acetyl-CoA formation altogether. The controlled batch experiments contrast with previous shake flask results and demonstrate that shake flask results are weak predictors of larger scale processes. In particular, MEC490, MEC499, MEC596, and MEC568 showed insignificant acetate formation in shake flasks, whereas in the controlled and prolonged batch processes, acetate accumulation was observed for all these strains. In general, the difference between citramalate yields and acetate yields among the six strains were significantly different (p < 0.05). The exception to this rule is MEC499/pZE12-cimA and MEC562/pZE12-cimA for which neither citramalate nor acetate formation could be distinguished, demonstrating that an additional pta knockout has much less impact in the ackA strain MEC499 on citramalate and acetate formation compared to the additional poxB knockout.
During the growth of these strains, succinate, lactate, ethanol, and pyruvate were not detected, and citramalate synthase activity was not affected by the E. coli strain genotype (data not shown). The combination of gltA leuC ackA-pta and poxB knockouts was important to achieve a high yield of citramalate and minimal acetate, and therefore, MEC568 was used for further studies.
Fed-batch production of citramalate
The final concentration of a fermentation product can often be maximized by continuous feeding of the carbon source. We, therefore, next completed duplicate experiments using a fed-batch process in which 30 g glycerol and 5 g peptone were again added to the fermenter once when the glycerol concentration decreased below 5 g/L. MEC568/pZE12-cimA was selected for this study, because this strain achieved the greatest citramalate yield in batch processes (Table 2). Like the batch process described above, for these fed-batch processes, the OD reached 10.0 within 36 h at which time the citramalate concentration was 12.5 g/L (Fig. 4). After 132 h, the final citramalate concentration reached an average of 31.4 g/L, corresponding to a yield of 0.52 citramalate g/g glycerol. In addition, only 1.8 g/L acetate was formed as by-product.
Citramalate production using crude glycerol
The rapid growth of the biodiesel industry has resulted in surplus availability of crude glycerol production, which has a purity of 60–80% based on the type of oil used as feedstock [4]. Crude glycerol also often contains 10–15% methanol, 1.5–2.5% ash, and 3.0–5.0% soap as impurities [4]. To determine if E. coli could be used to generate citramalate from crude glycerol, we next examined the fed-batch process using unrefined glycerol obtained directly from a local biodiesel manufacturer in place of purified glycerol. In this fed-batch process, about 31 g/L citramalate (0.51 g/g yield) and 1.9 g/L acetate were obtained using MEC568/pZE12-cimA (Fig. 5). This result is virtually identical to the fed-batch process using purified glycerol, and demonstrates that refining glycerol is not necessary for citramalate production by E. coli. Interestingly, the final OD was 22% greater when crude glycerol was used (10.3 vs. 8.4), possibly because of the presence of other unidentified carbon sources in the crude material. Crude glycerol has been used in other studies of biological conversions to value-added chemicals. For example, ethanol formation was similar for purified and unrefined glycerol by a Klebsiella pneumoniae mutant [34], and the same 1,3-propanediol concentration was achieved using purified or crude glycerol in a fed-batch fermentation, although the productivity was lower using crude glycerol [21].
Gene knockouts and fermentation optimization improve citramalate production from glycerol and also reduce acetate accumulation. Near elimination of acetate formation necessitates deletions in genes for both pathways associated with acetate formation: ackA coding acetate kinase, pta coding phosphotransacetylase, and poxB coding pyruvate oxidase. Fed-batch fermentations demonstrated that identical citramalate over 30 g/L can be generated from pure or crude glycerol at yield greater than 0.50 g citramalate/g glycerol. This result holds promise that crude glycerol could be used as for citramalate production and ultimately as a source of methacrylate.
References
Abdel-Hamid AM, Attwood MM, Guest JR (2001) Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483–1498
Arya AS, Lee SA, Eiteman MA (2013) Differential sensitivities of the growth of Escherichia coli to acrylate under aerobic and anaerobic conditions and its effect on product formation. Biotechnol Lett 35:1839–1843
Atsumi S, Liao JC (2008) Directed evolution of Methanococcus jannaschii citramalate synthase for biosynthesis of 1-propanol and 1-butanol by Escherichia coli. Appl Environ Microbiol 74:7802–7808
Ayoub M, Abdullah AZ (2012) Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew Sust Energy Rev 16:2671–2686
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2(2006):0008
Bauer W Jr (2000) Methacrylic acid and derivatives. Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim
Bauer F, Hulteberg C (2012) Is there a future in glycerol as a feedstock in the production of biofuels and biochemicals? Biofuels Bioprod Bioref 7:43–51
Behr A, Eilting J, Irawadi K, Leschinski J, Lindner F (2008) Improved utilisation of renewable resources: new important derivatives of glycerol. Green Chem 10:13–30
Bournay L, Casanave D, Delfort B, Hillion G, Chodorge JA (2005) New heterogeneous process for biodiesel production: a way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal Today 106:190–192
Chatzifragkou A, Papanikolaou S, Dietz D, Doulgeraki AI, Nychas GE, Zeng A (2011) Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl Microbiol Biotechnol 91:101–112
Contiero J, Beatty CM, Kumari S, DeSanti CL, Strohl WR, Wolfe AJ (2000) Effects of mutations in acetate metabolism in high-cell-density growth of Escherichia coli. J Ind Microbiol Biotechnol 24:421–430
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Dittrich CR, Bennett GN, San KY (2005) Characterization of the acetate-producing pathways in Escherichia coli. Biotechnol Prog 21:1062–1067
Eikmanns B, Thum-Schmitz N, Eggeling L, Lüdtke K, Sahm H (1994) Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140:1817–1828
Eiteman MA, Chastain MJ (1997) Optimization of the ion-exchange analysis of organic acids from fermentation. Anal Chim Acta 338:69–75
Fultz PN, Kemper J (1981) Wild-type isopropylmalate isomerase in Salmonella typhimurium is composed of two different subunits. J Bacteriol 148:210–219
Fultz PN, Kwoh DY, Kemper J (1979) Salmonella typhimurium newD and Escherichia coli leuC genes code for a functional isopropylmalate isomerase in Salmonella typhimurium-Escherichia coli hybrids. J Bacteriol 137:1253–1262
Ganesh I, Ravikumar S, Hong SH (2012) Metabolically engineered Escherichia coli as a tool for the production of bioenergy and biochemicals from glycerol. Biotechnol Bioproc Eng 17:671–678
Gao L, Chiou W, Tang H, Cheng X, Camp HS, Burns DJ (2007) Simultaneous quantification of malonyl-CoA and several other short-chain acyl-CoAs in animal tissues by ion-pairing reversed-phase HPLC/MS. J Chromatogr B 853:303–313
Gao C, Yang X, Wang H, Rivero CP, Li C, Cui Z, Qi Q, Lin CSK (2016) Robust succinic acid production from crude glycerol using engineered Yarrowia lipolytica. Biotechnol Biofuels 9:179
Hiremath A, Kannabiran M, Rangaswamy V (2011) 1,3-Propanediol production from crude glycerol from jatropha biodiesel process. New Biotechnol 28:19–23
Hong JS, Hunt AG, Masters PS, Lieberman MA (1979) Requirement of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci USA 76:1213–1217
Howell DM, Xu H, White RH (1999) (R)-Citramalate synthase in methanogenic archaea. J Bacteriol 181:331–333
Johnson DW, Eastham GR, Poliakoff M, Huddle TA (2015) Method of producing acrylic and methacrylic acid. US Patent 8,933,179 B2
Koser SA (1924) Correlation of citrate utilization by members of the colon-aerogenes group with other differential characteristics and with habitat. J Bacteriol 9:59–77
Lee TY, Makino K, Shinagawa H, Nakata A (1990) Overproduction of acetate kinase activates the phosphate regulon in the absence of the phoR and phoM functions in Escherichia coli. J Bacteriol 172:2245–2249
Lin H, Bennett GN, San KY (2005) Genetic reconstruction of the aerobic central metabolism in Escherichia coli for the absolute aerobic production of succinate. Biotechnol Bioeng 89:148–156
Lin H, Castro NM, Bennett GN, San KY (2006) Acetyl-CoA synthetase overexpression in Escherichia coli demonstrates more efficient acetate assimilation and lower acetate accumulation: a potential tool in metabolic engineering. Appl Microbiol Biotechnol 71:870–874
Ma F, Hanna MA (1999) Biodiesel production: a review. Biores Technol 70:1–15
Matsuyama A, Yamamoto-Otake H, Hewitt J, MacGillivray RTA, Nakano E (1994) Nucleotide sequence of the phosphotransacetylase gene of Escherichia coli strain K12. Biochim Biophys Acta 1219:559–562
Molina I, Pellicer MT, Badia J, Aguilar J, Baldoma L (1994) nMolecular characterization of Escherichia coli malate synthase G. Differentiation with the malate synthase A isoenzyme. Eur J Biochem 224:541–548
Nagai K (2001) New developments in the production of methyl methacrylate. Appl Catal A-Gen 221:367–377
Niersbach M, Kreuzaler F, Geerse RH, Postma PW, Hirsch HJ (1992) Cloning and nucleotide sequence of the Escherichia coli K-12 ppsA gene, encoding PEP synthase. Molec Gen Genet 231:332–336
Oh BR, Seo JW, Heo SY, Hong WK, Luo LH, Joe M, Park DH, Kim CH (2011) Efficient production of ethanol from crude glycerol by a Klebsiella pneumonia mutant strain. Biores Technol 102:3918–3922
Ornston LN, Ornston MK (1969) Regulation of glyoxylate metabolism in Escherichia coli K-12. J Bacteriol 98:1098–1108
Sabourin-Provost G, Hallenbeck PC (2009) High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Biores Technol 100:3513–3517
Salkind M, Riddle EH, Keefer RW (1959) Acrylates and methacrylates—raw materials, intermediates, and plant integration. Ind Eng Chem 51:1232–1238
Takamura Y, Nomura G (1988) Changes in the intracellular concentration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol 134:2249–2253
Todd JD, Curson ARJ, Sullivan MJ, Kirkwood M, Johnston AWB (2012) The Ruegeria pomeroyi acuI gene has a role in DMSP catabolism and resembles yhdH of E. coli and other bacteria in conferring resistance to acrylate. PLoS One 7:e35947
Vadali RV, Bennet GN, San KY (2004) Cofactor engineering of intracellular coA/acetyl-CoA and its effect on metabolic flux redistribution in Escherichia coli. Metab Eng 6:133–139
Wanner BL, Wilmes-Riesenberg MR (1992) Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J Bacteriol 174:2124–2130
Wu X, Eiteman MA (2016) Production of citramalate by metabolically engineered Escherichia coli. Biotechnol Bioeng 113:2670–2675
Yang HL, Kessler DP (1974) Genetic analysis of the leucine region in Escherichia coli: gene-enzyme assignments. J Bacteriol 117:63–72
Yang T, Rao Z, Zhang X, Xu M, Xu Z, Yang S (2015) Enhanced 2,3-butanediol production from biodiesel-derived glycerol by engineering of cofactor regeneration and manipulating carbon flux in Bacillus amyloliquefaciens. Microb Cell Fact 14:122
Zhang K, Woodruff AP, Xiong M, Zhou J, Dhande YK (2011) A synthetic metabolic pathway for production of the platform chemical isobutyric acid. Chemsuschem 4:1068–1070
Acknowledgements
The authors thank Sarah A. Lee and Daniel Geller for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Eiteman, M.A. Synthesis of citramalic acid from glycerol by metabolically engineered Escherichia coli . J Ind Microbiol Biotechnol 44, 1483–1490 (2017). https://doi.org/10.1007/s10295-017-1971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1971-7