Abstract
A packed bed bioreactor (PBBR) was developed for rapid establishment of nitrification in brackish water hatchery systems in the tropics. The reactors were activated by immobilizing ammonia-oxidizing (AMONPCU-1) and nitrite-oxidizing (NIONPCU-1) bacterial consortia on polystyrene and low-density polyethylene beads, respectively. Fluorescence in situ hybridization demonstrated the presence of autotrophic nitrifiers belong to Nitrosococcus mobilis, lineage of β ammonia oxidizers and nitrite oxidizer Nitrobacter sp. in the consortia. The activated reactors upon integration to the hatchery system resulted in significant ammonia removal (P < 0.01) culminating to its undetectable levels. Consequently, a significantly higher percent survival of larvae was observed in the larval production systems. With spent water the reactors could establish nitrification with high percentage removal of ammonia (78%), nitrite (79%) and BOD (56%) within 7 days of initiation of the process. PBBR is configured in such a way to minimize the energy requirements for continuous operation by limiting the energy inputs to a single stage pumping of water and aeration to the aeration cells. The PBBR shall enable hatchery systems to operate under closed recirculating mode and pave the way for better water management in the aquaculture industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On assuming the dimensions of an industry, aquaculture systems are bound to operate under strict environmental safety standards. With high land and water costs, the systems are destined to maintain high biological carrying capacity in relatively little space with minimal water exchange. These requirements led to the advent of recirculating aquaculture systems (RASs) which allowed companies to (1) be competitive in both domestic and world commodity markets by locating production closer to markets, (2) improve environmental control, (3) reduce catastrophic losses due to diseases, (4) avoid violation of environmental regulations on effluent discharge, (5) reduce management and labor costs, and (6) improve product quality and consistency [29].
Driven by the above demands, several attempts have been made to develop and optimize RAS focusing on total ammonia nitrogen (TAN) as the key limiting water quality parameter [18, 31, 33]. The toxic effects of ammonia have been demonstrated for several cultured crustaceans [26, 27, 72] and found more pronounced in early developmental stages. Nitrite is also harmful to larvae as it causes reduction of hemolymph oxyhemocyanin (in Penaeus monodon) with concomitant increase in the partial pressure of oxygen (pO2) in hemolymph and reduced oxygen affinity (P 50) [9]. However, it is less toxic than ammonia [3], and only under conditions of long-term exposure the toxicity is manifested [69] in the reared animals. Likewise, ammonia and nitrite toxicity in Macrobrachium rosenbergii adults and larvae have been investigated by various researchers [7, 8, 43, 65] and felt the need for their regulation for successful larval production. However, nitrate is relatively harmless to the cultured aquatic organisms [62].
In biological ammonia removal systems nitrifying activity of bacteria suspended in seawater has been reported to be extremely low primarily due to their slow growth rate and inhibition of nitrification by free ammonia and nitrite [19]. However, immobilization techniques have been useful to overcome the situation [61] and accordingly, fixed film nitrification biofilters are commonly used for TAN removal in RAS [56, 57, 70]. In such installations attached growth as biofilm offers several advantages over suspended culture-based systems, such as handling convenience, increased process stability to shock loading and prevention of the bacterial population from being washed off [17, 44]. In the light of the emergence of various types of biofilters, a performance rating strategy as well as standards for reporting the performance have been brought out to benefit the customers to choose the most appropriate one [10, 14, 38]. In spite of following such protocols, at least in a few cases, the immobilized nitrifiers in RAS have exhibited low performance, besides demanding too long a start-up period imposing operational difficulties [23, 61]. Therefore, instead of selecting a nitrification system from market it became imperative for the tropics to develop a user-friendly and economically viable technology having the advantages of short start-up time and easiness to integrate to the existing hatchery designs without modifications. Accordingly, a specialized nitrifying packed bed bioreactor (PBBR) (Patent application no. 828/DEL/2000 of 13 September 2000) was developed with indigenous nitrifying bacterial consortia (NBC) and tested for its potential for nitrification in a M. rosenbergii seed production system in support of the industry.
Materials and methods
Fabrication of the PBBR
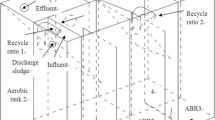
Cross-sectional view of the nitrifying bioreactors (ammonia oxidizing and nitrite oxidizing) connected serially is given in Fig. 1. Both the reactors have the same configuration consisting of shell made of fiberglass with a base of 30 cm2 and an overall height of 45 cm. A perforated base plate made of Perspex, carrying 30 cm long and 2 cm diameter 9 PVC pipes (airlift pumps) fixed at 10 cm equidistance, is positioned at the base of the reactor. When air gets passed through, the 10 cm3 area filled with the support medium surrounding each airlift pump acts as an aeration cell. The base plate is elevated by 5 cm from the bottom supported by 5 cm long PVC pipes having 3 cm diameter. An inlet pipe is fixed at a water discharge height of 35 cm up from the base of the reactor. The outlet pipe, which emerges from the base of the reactor, bends upward at water discharge height of 35 cm from the base to the next reactor.
Based on a previous study [1], polystyrene (PS) and low-density polyethylene (LDPE) were selected as suitable support materials for immobilizing ammonia-oxidizing and nitrite-oxidizing consortia, respectively. This selection was based on percentage consumption of NH4–N/NO2–N and production of NO2–N/NO3–N by the immobilized nitrifiers on the beads, cost of the raw material and easiness to mold into beads. The beads were having 5 mm diameter and a surface area of 0.785 cm2 with spikes on the surface. The reactors have been packed with the respective support material; the characteristics are described in Table 1.
Nitrifying bacterial consortia
Two types of NBC, ammonia-oxidizing non-penaeid culture-1 (AMONPCU-1) and nitrite-oxidizing non-penaeid culture-1 (NIONPCU-1), developed by enrichment technique from brackish water systems under perpetual salinity regimes around 15 g/L were used after getting optimized growth and culture conditions [2]. This consisted of simple seawater-based medium having 15 g/L salinity supplemented with 10 mg/L substrate ((NH4)2·SO4/NaNO2), 2 mg/L KH2PO4 at an optimum temperature of 28°C and pH 8.5 for ammonia and 7.5 for nitrite oxidizers. After harvesting, the cultures were maintained at 4°C with periodic addition of the substrate ((NH4)2·SO4/NaNO2) and adjustment of pH (using 1% Na2CO3) to the optimum. For generating sufficient biomass in order to facilitate their immobilization in the reactors, both the consortia were acclimated to room temperature (27 ± 0.5°C) in 250 mL conical flasks on a shaker for 7 days, amplified in a 2 L baby fermentor for 1 month and subsequently mass produced in an indigenous NBC production unit (NBCPU) under optimum pH, temperature and salinity. The NBCPU consists of a 200 L fermentor vessel made of polyethylene, fixed with a central 0.5 HP AC/DC agitator (500 W, 0–500 rpm). Provisions have been given for (a) temperature regulation employing a thermo-circulator, (b) pH probe insertion, (c) addition of medium, (d) supply of filter sterilized air and (e) harvesting matured consortium [28].
Fluorescence in situ hybridization (FISH) of the consortia
As a preliminary characterization, FISH analysis of the consortia was carried out using seven different nitrifier-specific 16S rRNA-targeted oligonucleotide probes labeled with Cy3, Cy5 or fluorescein (Table 2). The fluorescent oligonucleotide probes were purchased from Thermo Electron Corp. (Germany). The specificity and the hybridization conditions were confirmed with ‘Probebase’ [32]. Actively growing consortia, harvested by centrifugation, were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) containing KH2PO4 and NaCl, prepared in diluted seawater having salinity 15 g/L. The samples were stored at −20°C in a 1:1 mixture of PBS:ethanol until further processing. Hybridizations were performed on six-well Teflon-coated slides (Electron Microscopy Sciences, USA). Prior to the hybridization, the slides were coated with poly-l-lysin, 10 μL of the fixed consortia were spread on to the well, dried at 46°C for 10 min, and dehydrated by successive passage through 50, 80 and 98% ethanol (3 min each). Working solutions of the probes were prepared to obtain a final concentration 5 pmol/μL for CY3/5 and 8.3 pmol/μL for fluorescein-labeled probes. Hybridization buffer (2 mL) containing 360 μL 5 M NaCl, 40 μL 1 M Tris–HCl (pH 8.0), 4 μL 10% SDS and formamide were added according to the probe used (Table 2). For hybridization, 10 μL hybridization buffer was dispensed into the wells, and then 1 μL probe stock solution was added. A hybridization tube was prepared by folding a tissue paper into a 50 mL falcon tube into which the remainder of the hybridization buffer was dispensed. After the addition of probes, the slides were immediately transferred into the hybridization tube and incubated for 1.5 h at 46°C in a hybridization oven (Thermo Electron Corp.). Washing buffer containing 1 M Tris/HCl, 5 M NaCl and 0.5 M EDTA at pH 8 was prepared as per the formamide concentration in the hybridization buffer in a separate 50 mL Falcon tube and made up to 50 mL by adding MilliQ. Finally, 50 μL of 10% (w/v) SDS was added and the washing buffer was preheated at 48°C in a water bath. On elapse of the incubation period, the hybridization slides were taken out and rinsed and transferred to the washing buffer, where the slides were incubated for 10–20 min at 48°C. After the incubation the slides were rinsed with MilliQ water and dried. The cells were counter stained with DAPI having the final concentration of 0.2 μg/mL for 1 min, washed, dried and added an anti-fading mounting fluid (Vectashield, Vector Laboratories Inc., Burlingame, CA). The slides were observed under Olympus BX 51 epifluorescent microscope equipped with a monochromatic camera (Evolution VF, Media Cybernetics Inc., MD, USA). Images were processed using the “Image pro-express” software (Media Cybernetics Inc., MD, USA).
Activation of the reactors with NBC
The beads (substratum) were immersed in 0.1 N HCl for 3 h, washed with 10% Extran (Enviroeuip, Sydney, Australia), rinsed with tap water followed by distilled water and air dried. The reactor 1 was filled with 60,000 PS beads and the reactor 2 with the same number of LDPE. The ammonia-oxidizing and the nitrite-oxidizing consortia (20 L each) were introduced into the reactors 1 and 2, respectively, and airlift pumps operated by supplying 1 L/min to effect adequate circulation of the culture through the beads and to assure supply of O2 and CO2 for activation. Optimum culture conditions, as described under “Nitrifying bacterial consortia”, were maintained in each reactor during the activation period. The substrate concentrations (NH4–N/NO2–N) in both the reactors were made up to 10 mg/L daily by the addition of aqueous ammonium sulfate or sodium nitrite. Evaporation loss was made up by adding distilled water daily.
Integration of the bioreactors into M. rosenbergii seed production system
The facility used consisted of two larval rearing tanks of 5,000 L capacity, one integrated with the activated reactors and the other without any, used as control. Chlorinated–dechlorinated seawater (salinity 15 g/L) was used for all the experiments. The tanks were initially filled with 2,000 L seawater, freshly hatched mysis of M. rosenbergii, dipped in 0.025 mg/L formalin (SRL, Mumbai, India) for 20 s, 0.03 mg/L iodophore (Growel Formulations, Hyderabad, India) for 20 s and washed in running seawater, were introduced into the tanks at a stocking density of 0.2 million per tank.
The process flow diagram of the experimental system is given in Fig. 2. The ammonia-oxidizing and nitrite-oxidizing reactors were connected serially. The influent from the rearing tank was pumped into an overhead tank (282 L) from where water flowed through the two reactors serially by gravitation and got collected in a 140 L collection tank, from where the treated water got into the larval rearing tank. Pumping of the influent from the larval rearing tank was controlled by an automated water level controller (V-guard, Kerala, India) fitted inside the overhead tank. A regulator valve was connected to the overhead tank to maintain a flow rate of 4 L/min to the system attaining a total circulation of 5,760 L/day.
During the experiment, the rearing water was supplemented with 1 mg/L EDTA (Matrix Formulations, Hyderabad, India), 5 mg/L sulfated vitamin C (Matrix Formulations, Hyderabad, India) and 1 mg/L treflan (Growel Formulations, Hyderabad, India). The larvae were fed with freshly hatched Artemia nauplii up to stage 9 (when pleopods with setae appear) and with both Artemia nauplii and egg custard subsequently. The experiment was continued for 17 days till the larvae metamorphosed to post-larvae, and repeated three times for concurrent results. At the end of the experiment the survival was estimated by counting the larvae manually and the relative percentage survival (R.P.S.) was estimated as the following equation [21]:
In another experiment, the reactor was tested for its nitrification potential in spent water after the larval culture. Water from the larval rearing tanks, subsequent to harvest of post-larvae, was collected and stored in a 5,000 L capacity storage tank. This was subsequently circulated through the bioreactor assembly at a rate of 2 L/min. Meanwhile, another system without integration of the reactor was kept as the control. The experiment was repeated three times.
Analyses
When the reactors were in the activation mode, substrate/product levels were determined daily by estimating ammonia (TAN) [59], nitrite (NO2–N) [5] and nitrate (NO3–N) [60]. The nitrifying biomass was determined gravimetrically by passing 10 mL bacterial suspension from the reactors through pre-weighed cellulose acetate syringe filters of 0.22 μm porosity with a diameter of 13 mm. Water samples from the larval rearing tanks were analyzed once in 3 days for alkalinity, hardness [4], ammonia, nitrite and nitrate as above. The heterotrophic bacterial community of the rearing water was determined once in a week by standard spread plate method employing ZoBell’s Marine Agar 2216 E prepared in diluted seawater of salinity 15 g/L.
In spent water nitrification experiments, water quality parameters such as phosphate, sulfate, iron, chloride, dissolved oxygen, BOD [4], ammonia, nitrite and nitrate as above were estimated for 8 days.
Statistical analyses
The relationship between removal of ammonia and nitrite and the biomass in suspension during the activation mode was estimated by simple correlation coefficient analysis. The nitrification efficiency and significant percent survival of larvae in the control and reactor integrated tanks were estimated by one-way analysis of variance. Least significant difference (LSD) at 0.1% level was calculated for delineation of the two treatments.
Results
FISH of the consortia
Fluorescence in situ hybridization analysis of the two NBC with seven nitrifying bacterial specific probes confirmed the presence of autotrophic nitrifiers (Fig. 3). Most of the nitrifiers observed were in the form of aggregates. FISH of AMONPCU-1 revealed presence of the autotrophic ammonia oxidizer, Nitrosococcus mobilis, lineage of β ammonia oxidizers, and that of NIONPCU-1 the autotrophic nitrite oxidizer, Nitrobacter sp. However, Nitrosomonas, Nitrosospira, anaerobic ammonia oxidizers and nitrite oxidizers belonging to phylum Nitrospira were not detected in both the consortia.
Fluorescence in situ hybridization of the nitrifying bacterial consortia (a epifluorescent image of AMONPCU-1 with CY3-labeled probe NSO190 targeting β ammonia oxidizers; b epifluorescent image of AMONPCU-1 with fluorescein-labeled probe NmV targeting Nitrosococcus mobilis lineage; c epifluorescent image of NIONPCU-1 with CY5-labeled probe NIT2 targeting Nitrobacter sp.)
Activation of the reactors
Activation kinetics of PBBR during the period of immobilization of the consortia, AMONPCU-1 and NIONPCU-1, are presented in Fig. 4. In both the reactors nitrification could be established within 24 h of initiation of the process and there was progressive reduction in the suspended biomass and increase in NO2–N and NO3–N, respectively. The system was monitored for 7 days, during which there was reduction of more than 90% of the bacterial biomass from the activation medium with 78% TAN and 75.3% NO2–N removal. There was negative correlation between the percentage removal of TAN (r = − 0.96, P < 0.01), NO2–N (r = − 0.93, P < 0.01) and the suspended biomass. Average ammonia and nitrite removal rates in the reactor at the end of the activation period were 46.82 mg TAN/(m2 day) and 45.14 mg NO2–N/(m2 day).
Integration of the reactors into the hatchery system
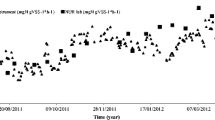
The minimum and maximum values of pH, temperature, salinity, alkalinity, hardness and total bacterial count in the rearing water of the experimental and control tanks during each treatment are summarized in Table 3. Heterotrophic bacterial community expressed as colony forming units (CFUs) in ZoBell’s Marine Agar in the control tank increased substantially and there was no remarkable difference in the other water quality parameters between the tanks. The extent of nitrification during the period is presented in Fig. 5. In the control tanks TAN exhibited progressive increase with its subsequent decline and concomitant increase of NO2–N after 14 days; however, NO3–N was never found built up in the system. Meanwhile, there was significant TAN removal (P < 0.01) in the experimental tanks with significant (P < 0.05) NO2–N removal. Within 8 days both TAN and NO2–N concentrations were below detectable levels. NO3–N exhibited progressive increase to 7.6 mg/L within 17 days of the experiment.
The overall percent survival of larvae in the control and test tanks was estimated and presented in Table 4. The tank with the reactor exhibited significantly higher (P < 0.001) percentage survival (LSD at 0.1% = 15.19) with an average R.P.S. of 22.86%.
The average water quality parameters of the spent water are given in Table 5. TAN, NO2–N and NO3–N were lower in the experimental tanks than in those of the controls (fourth day) indicating higher percentage removal of TAN (78%), NO2–N (79%) and BOD (56%).
Discussion
Proper selection and sizing of biofilters are critical to the technical and economic viability of RAS [38]. In saltwater systems RAS plays an important role in the production of healthy and properly sized fingerlings [16] and has significant implications in maintaining the required water quality as the system demands operations under oligotrophic conditions. The PBBR described here are packed with plastic media having specific surface area of 205 m2/m3. This is comparable to those in trickling filters used in aquaculture [25]. The plastic beads with spikes on the surface provide high void ratios that avoid clogging [15] increase the aeration within the system; poor aeration reduces nitrification capacity of the biofilter [71]. As a matter of fact most of the biofilters on recirculation systems have been focusing on aerobic fixed films [30, 52, 58] and in several systems plastic media used to be the substrata for immobilization [22, 52, 56]. In the present case plastic beads have been used due to its reusability and inertness besides the cost factor and preferential acceptability by the NBC for attachment and growth.
The NBC used here originated from a brackish water environment by enrichment with confirmed nitrification potential and designated as AMONPCU-1 and NIONPCU-1 [2]. Transmission electron microscopic observations demonstrated characteristic cyst formation and intracytoplasmic membranes similar to autotrophic nitrifiers [28]. Using FISH the consortia could be partially characterized, demonstrating the presence of N. mobilis, lineage of β ammonia oxidizers and Nitrobacter sp. in AMONPCU-1 and NIONPCU-1, respectively. Where as, other nitrifiers such as Nitrosomonas, Nitrosospira, phylum Nitrospira, and anaerobic ammonia oxidizers were not observed. In literature oligonucleotide probe-based FISH, targeting signature regions of the 16S rRNA of ammonia and nitrite-oxidizing bacteria, has been successfully applied for phylogenetic identification in environmental and engineered systems [24, 41, 45, 55, 68]. Rowan et al. [51] studied the composition and diversity of ammonia-oxidizing bacterial communities in a biological aerated filter (BAF) and a trickling filter and all the samples analyzed appeared to be dominated by AOB most closely related to N. mobilis.
As a general principle low concentration of nutrients in aquaculture systems [48] results in slow growth of nitrifiers and low bacterial yield to form effective biofilm by natural process. This necessitates activated bioreactors with high attached bacterial density for optimal performance. Under such situations the time required for activating the reactors becomes a crucial factor for their successful and timely starting up and operation. To satisfy this requirement, NBC were used for activating the reactors by which nitrification could be established within 24 h of initiation and attained 78% ammonia and 75.3% nitrite removal by the seventh day. Attachment of the NBC and formation of biofilm were irreversible, which demonstrated the soundness of the technology. Wherever such activations had not been carried out, 2–3 months were reported for the establishment of nitrification in marine [39] and 2–3 weeks in freshwater systems [40].
On integrating the PBBR to the larval rearing system, ammonia oxidation was established within a day and it took 8 days for nitrite oxidation. Meanwhile, in the control larval rearing systems 14 days were required for the initiation of nitrification. The delay in establishing active nitrite oxidation in the reactor integrated system suggests a consequence of lower multiplication rate of nitrite oxidizers compared to that of ammonia oxidizers [47]. Under the ‘nitrifying bioreactor integrated mode’ the maximum average TAN and NO2–N concentrations in the larval rearing tanks were 0.18 and 0.25 mg/L, respectively, the values typical of any marine system. It has to be emphasized that marine larval rearing systems demand TAN and NO2–N levels below 0.1 mg/L well below the maximum limit (0.3 mg N/L) under the oligotrophic category [37]. During the progression of the experiment the NO3–N concentrations increased progressively up to 7.6 mg/L, however, it remained well below the toxic levels for M. rosenbergii larval culture [34]. Management of ammonia in the larval rearing systems of M. rosenbergii is important as significantly lower survival rates (0–20%) of larvae were noticed at total ammonia concentrations ranging from 1 to 8 mg/L with 0.43–3.41 mg/L non-ionic ammonia at pH 9 [35]. The higher relative percent survival (22.86%) obtained in the reactor integrated experimental system proved the impact of the technology in enhancing the larval survival.
Under oligotrophic conditions ammonia diffuses into a relatively thin vertically homogenous biofilm that is dominated by autotrophs, principally due to low BOD (<5 g/m3) of the culture water [36]. Such a situation could be observed here where organic loading to the system was as low as 0.31 mg/L BOD. On the basis of the above BOD–nitrification relationship, it may be inferred that there has been minimal heterotrophic inhibition of nitrification [53, 73] in the reactors as also evidenced by the progressive increase in the rate of nitrification from the day of initiation. Since nitrification reactions occur in the biofilm and not in the bulk fluid [42], the substrate utilization rate depends on local substrate concentrations within the biofilm. At such local reaction sites, reactant concentrations are depressed and products elevated [6]. This warranted circulation of water through the cartridge with the biofilm, and the rate of TAN removal could be theoretically proportional to the rate of circulation. That was why the reactors were designed to have both vertical and horizontal flow of water through the aeration cells for providing oxic conditions. Zhu and Chen [74] established that the turbulence caused by diffused air substantially improved the nitrification efficiency of fixed film biofilters. Tschui et al. [63] ascertained that nitrification rates could be increased with increased water velocities along with increased airflow. This sort of stronger turbulence in the reactor cartridge decreases the laminar boundary layer and simultaneously enhances diffusion. Such highly aerated systems reduce the chances for the generation of anaerobic pockets with in the reactor.
RAS adoption for larvae, fry and fingerling production is driven by bio-security issues [46, 50] and water recirculation dramatically reduces the possibility of pathogen introduction [12, 20]. In this context integration of PBBR for nitrification of hatchery spent water with high percentage removal of ammonia (78%), and nitrite (79%) by fourth day strengthens the possibility of reuse of water with limited discharge and reduced intake paving the way for bio-security.
Packed bed biofilter systems have been utilized in a variety of formats for recirculating shrimp production systems because of their economic feasibility [13, 64]. The PBBR designed and evaluated here was configured in such a way that the flow of water could be maintained by gravitational force and the energy needed could be restricted to pumping water to the reservoir tank and to operate an air pump to effect aeration. If nitrification is not completed during a single circulation, there is provision to recirculate it through the treatment system over and again. However, such requirements might be overcome by increasing the biomass of the nitrifying consortia used for activation of the reactors or by enhancing the hydraulic retention time. Another specialty of the package is upgradeability of the system with different types of filters for removal of particulate matter and UV disinfection equipment for elimination of pathogens which might enter the system accidentally.
The PBBR evaluated here shall enable hatchery systems to operate as closed recirculation systems, maintaining water quality during the operation and minimizing discharge of spent water. Collectively the technology shall pave way for better water management in the aquaculture industry. Besides, by integrating the reactors during larval production significantly high percentage larval survival also could be obtained. The PBBR designed here is flexible as it is interchangeable between prawn (salinity 15 g/L) and shrimp (salinity 30 g/L) larval rearing systems by replacing the NBC depending on the salinity [2]. A modification of the system can be used for shrimp maturation facility too as recirculation is one of its prime requirements for maturation in the perspectives of water quality and bio-security.
References
Achuthan C (2000) Development of bioreactors for nitrifying water in closed system hatcheries of penaeid and non-penaeid prawns. PhD Thesis, Cochin University of Science and Technology, Kochi, India, 116 pp
Achuthan C, Rejish Kumar VJ, Manju NJ, Philip R, Bright Singh IS (2006) Development of nitrifying bacterial consortia for immobilizing in nitrifying bioreactors designed for penaeid and non-penaeid larval rearing systems in the tropics. Indian J Mar Sci 35:240–248
Alcaraz G, Chiappa-Carrara X, Espinoza V, Vanegas C (1999) Acute toxicity of ammonia and nitrite to white shrimp Penaeus setiferus postlarvae. J World Aquacult Soc 30:90–97. doi:10.1111/j.1749-7345.1999.tb00321.x
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Bendschneider K, Robinson RJ (1952) A spectrophotometric method for the determination of nitrite in seawater. J Mar Res 11:87–96
Boller M, Gujer W, Tschui M (1994) Parameters affecting nitrifying biofilm reactors. Water Sci Technol 29:1–11
Cavalli RO, Berghe EV, Lavens P, Thuy TTN, Wille M, Sorgeloos P (2000) Ammonia toxicity as a criterion for the evaluation of larval quality in the prawn Macrobrachium rosenbergii. Comp Biochem Physiol C125:333–343
Chen JC, Lee Y (1997) Effects of nitrite on mortality, ion regulation and acid–base balance of Macrobrachium rosenbergii at different external chloride concentrations. Aquat Toxicol 39:291–305. doi:10.1016/S0166-445X(97)00029-5
Cheng SY, Chen JC (1995) Hemolymph oxygen content, oxyhemocyanin, protein levels and ammonia excretion in the shrimp Penaeus monodon exposed to ambient nitrite. Comp Physiol B 164:530–535
Colt J, Lamoureux J, Patterson R, Rogers G (2006) Reporting standards for biofilter performance studies. Aquacult Eng 34:377–388. doi:10.1016/j.aquaeng.2005.09.002
Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M (2001) In situ characterization of Nitrospira like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol 67:5273–5284. doi:10.1128/AEM.67.11.5273-5284.2001
Davis JT (1990) Red drum brood stock and hatchery production. SRAC Publication, No. 323, Mississippi, 4 pp
Davis DA, Arnold CR (1998) The design, management and production of recirculating raceway system for the production of marine shrimp. Aquacult Eng 17:193–211. doi:10.1016/S0144-8609(98)00015-6
Drennen DCII, Hosle KC, Francis M, Weaver D, Aneshansley E, Beckman G, Johnson CH, Cristina CM (2006) Standardized evaluation and rating of biofilters. II. Manufacturer’s and user’s perspective. Aquacult Eng 34:403–416. doi:10.1016/j.aquaeng.2005.07.001
Eding EH, Kamstra A, Verreth JAJ, Huisman EA, Klapwijk A (2006) Design and operation of nitrifying trickling filters in recirculating aquaculture: a review. Aquacult Eng 34:234–260. doi:10.1016/j.aquaeng.2005.09.007
Fielder S, Allan GL (1997) Inland production of marine fish. In: Hyde K (ed) The new rural industries: a hand book for farmers and investors. Rural Industries Research and Development Corporation, Australian Government, pp 108–113
Fitch MW, Pearson N, Richards G, Burken JG (1998) Biological fixed film systems. Water Environ Res 70:495–518. doi:10.2175/106143098X134226
Fontenot Q, Bonvillain C, Kilgen M, Boopathy R (2007) Effects of temperature, salinity, and carbon:nitrogen ratio on sequencing batch reactor treating shrimp aquaculture wastewater. Bioresour Technol 98:1700–1703. doi:10.1016/j.biortech.2006.07.031
Furukawa K, Ike A, Ryu S, Fujita M (1993) Nitrification of NH4–N polluted seawater by immobilized acclimated marine nitrifying sludge (AMNS). J Ferment Bioeng 76:515–520. doi:10.1016/0922-338X(93)90251-3
Goldburg RJ, Elliot MS, Naylor MA (2001) Marine aquaculture in the United States: environmental impacts and policy options. Pew Oceans Commission, Arlington, p 44
Gram L, Melchiorsen J, Spanggaard B, Huber I, Nielsen TF (1999) Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl Environ Microbiol 65:969–973
Gross A, Nemirovsky A, Zilberg D, Khaimov A, Brenner A, Snir E, Ronen Z, Nejidat A (2003) Soil nitrifying enrichments as biofilter starters in intensive recirculating saline water aquaculture. Aquaculture 223:51–62. doi:10.1016/S0044-8486(03)00067-X
Jae-Koan S, Jung H, Kim MR, Kim BJ, Nam SW, Kim SK (2001) Nitrification performance of nitrifiers immobilized in PVA (polyvinyl alcohol) for a marine recirculating aquarium system. Aquacult Eng 24:181–194. doi:10.1016/S0144-8609(01)00063-2
Juretschko S, Timmermann G, Schmid M, Schleifer KH, Pommerening-Röser A, Koops HP, Wagner M (1998) Combined molecular and conventional analysis of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol 64:3042–3051
Kamstra A, van der Heul JW, Nijhof M (1998) Performance and optimization of trickling filters on eel farms. Aquacult Eng 17:175–192. doi:10.1016/S0144-8609(98)00014-4
Kır M, Kumlu M, Eroldo OT (2004) Effects of temperature on acute toxicity of ammonia to Penaeus semisulcatus juveniles. Aquaculture 241:479–489. doi:10.1016/j.aquaculture.2004.05.003
Koo JG, Kim SG, Jee JH, Kim JM, Bai SC, Kang JC (2005) Effects of ammonia and nitrite on survival, growth and moulting in juvenile tiger crab, Orithyia sinica (Linnaeus). Aquacult Res 36:79–85. doi:10.1111/j.1365-2109.2004.01187.x
Kumar VJR, Achuthan C, Manju NJ, Philip R, Bright Singh IS (2008) Mass production of nitrifying bacterial consortia for the rapid establishment of nitrification in saline recirculating aquaculture systems. World J Microbiol Biotechnol (in press)
Lee PG (1995) A review of automated control systems for aquaculture and design criteria for their implementation. Aquacult Eng 14:205–227. doi:10.1016/0144-8609(94)00002-I
Lekang OI, Kleppe H (2000) Efficiency of nitrification in trickling filters using different filter media. Aquacult Eng 21:181–199. doi:10.1016/S0144-8609(99)00032-1
Losordo TM, Westers H (1994) System carrying capacity and flow estimation. In: Timmons MB, Losordo TM (eds) Aquaculture water reuse systems: engineering design and management. Elsevier, pp 9–60
Loy A, Maixner F, Wagner M, Horn M (2007) probeBase—an online resource for rRNA-targeted oligonucleotide probes: new features. Nucleic Acids Res 35:D800–D804. doi:10.1093/nar/gkl856
Lyssenko C, Wheaton F (2005) Impact of positive ramp short-term operating disturbances on ammonia removal by trickling and submerged-upflow biofilters for intensive recirculating aquaculture. Aquacult Eng 35:26–37. doi:10.1016/j.aquaeng.2005.08.002
Mallasen M, Valenti WC, Ismael D (2004) Effects of nitrate concentration on larval development of the giant river prawn, Macrobrachium rosenbergii. J Appl Aquacult 14:55–69. doi:10.1300/J028v14n03_05
Mallasen M, Valenti WC (2005) Larval development of the giant river prawn Macrobrachium rosenbergii at different ammonia concentrations and pH values. J World Aquacult Soc 36:32–41
Malone RF, DeLosReyes AA (1997) Categories of recirculating aquaculture systems. In: Timmons MB, Losordo T (Eds) Advances in aquacultural engineering: proceedings from the Aquacultural Engineering Society (AES) technical sessions at the fourth international symposium on tilapia in aquaculture, Coronado Springs Resort, Walt Disney World, Orlando, 9–12 November 1997, pp 197–208
Malone RF, Beecher LE (2000) Use of floating bead filters to recondition recirculating waters in warmwater aquaculture production systems. Aquacult Eng 22:57–74. doi:10.1016/S0144-8609(00)00032-7
Malone RF, Pfeiffer JP (2006) Rating fixed film nitrifying biofilters used in recirculating aquaculture systems. Aquacult Eng 34:389–402. doi:10.1016/j.aquaeng.2005.08.007
Manthe DP, Malone RF (1987) Chemical addition for accelerated biological filter acclimation in closed bluecrab shedding systems. Aquacult Eng 6:227–236. doi:10.1016/0144-8609(87)90006-9
Masser MP, Rakocy J, Losordo TM (1999) Recirculating aquaculture tank production systems. Management of recirculating systems. SRAC Publication No. 452, United States Department of Agriculture, 12 pp
Mobarry BK, Wagner M, Urbain V, Rittmann BE, Stahl DA (1996) Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol 62:2156–2162
Moreau M, Liu Y, Capdeville B, Audic JM, Calvez L (1994) Kinetic behaviour of heterotrophic and autotrophic biofilms in waste water treatment process. Water Sci Technol 29:385–391
Naqvi AA, Adhikari S, Pillai BR, Sarangi N (2007) Effect of ammonia–N on growth and feeding Macrobrachium rosenbergii (De-Man). Aquacult Res 38:847–851. doi:10.1111/j.1365-2109.2007.01736.x
Nogueira R, Lazarova V, Manem J, Melo LF (1998) Influence of dissolved oxygen on the nitrification kinetics in a circulating bed biofilm reactor. Bioprocess Eng 19:441–449. doi:10.1007/s004490050546
Okabe S, Satoh H, Watanabe Y (1999) In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 65:3182–3191
Otoshi CA, Arce SM, Moss SM (2003) Growth and reproductive performance of broodstock shrimp reared in a biosecure recirculating aquaculture system versus a flow-through pond. Aquacult Eng 29:93–107. doi:10.1016/S0144-8609(03)00048-7
Paller MH (1992) An analytical model for predicting the carrying capacity of submerged biofilters used in aquaculture. J Appl Aquacult 1:1–25. doi:10.1300/J028v01n03_01
Piedrahita RH (2003) Reducing the potential environmental impacts of tank aquaculture effluents through intensification and recirculation. Aquaculture 226:35–44. doi:10.1016/S0044-8486(03)00465-4
Pommerening-Röser A, Rath G, Koops HP (1996) Phylogenetic diversity within the genus Nitrosomonas. Syst Appl Microbiol 19:344–351
Pruder GD (2004) Biosecurity: application in aquaculture. Aquacult Eng 32:3–10. doi:10.1016/j.aquaeng.2004.05.002
Rowan AK, Snape JR, Fearnside D, Barer MR, Curtis TP, Head IM (2003) Composition and diversity of ammonia-oxidising bacterial communities in wastewater treatment reactors of different design treating identical wastewater. FEMS Microbiol Ecol 43:195–206. doi:10.1111/j.1574-6941.2003.tb01059.x
Sandu SI, Boardman GD, Watten BJ, Brazil BL (2002) Factors influencing the nitrification efficiency of fluidized bed filter with a plastic bead medium. Aquacult Eng 26:41–59. doi:10.1016/S0144-8609(02)00003-1
Satoh H, Okabe S, Norimatsu N, Watanabe Y (2000) Significance of substrate C/N ratio on structure and activity of nitrifying biofilms determined by in situ hybridization and use of microelectrodes. Water Sci Technol 41:317–321
Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten MSM, Metzger JW, Schleifer KH, Wagner M (2000) Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 23:93–106
Schramm A, De Beer D, Van Den Heuvel JC, Ottengraf S, Amann R (1999) Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol 65:3690–3696
Seo JK, Jung IH, Kim MR, Kim BJ, Nam SW, Kim SK (2001) Nitrification performance of nitrifiers immobilized in PVA (polyvinyl alchohol) for a marine recirculating aquarium system. Aquacult Eng 24:181–194. doi:10.1016/S0144-8609(01)00063-2
Shnel N, Barak Y, Ezer T, Dafni Z, Van Rijn J (2002) Design and performance of a zero discharge tilapia recirculating system. Aquacult Eng 24:181–194
Singh S, Ebeling J, Wheaton F (1999) Water quality trails in four recirculating aquacultural systems configurations. Aquacult Eng 20:75–84. doi:10.1016/S0144-8609(99)00003-5
Solorzano L (1969) Determination of ammonia in natural waters by the phenol hypochlorite method. Limnol Oceanogr 14:799–801
Strickland JDH, Parsons TR (1968) A practical handbook of seawater analysis, 2nd edn. Fish Res Board, Canada, p 310
Sung-Koo K, Kong I, Lee BH, Kang L, Lee MG, Suh KH (2000) Removal of ammonium–N from a recirculation aquaculture system using an immobilized nitrifier. Aquacult Eng 21:139–150. doi:10.1016/S0144-8609(99)00026-6
Tomasso JR (1994) Toxicity of nitrogenous wastes to aquaculture animals. Rev Fish Sci 2:291–314
Tschui M, Boller M, Gujer W, Eugster J, Mader C, Stengel C (1994) Tertiary nitrification in aerated pilot biofilters. Water Sci Technol 29:53–60
Tseng KF, Su HM, Su MS (1998) Culture of Penaeus monodon in a recirculating system. Aquacult Eng 17:138–147. doi:10.1016/S0144-8609(98)00011-9
Wang WN, Wang AL, Zhang YJ, Li ZH, Wang JX, Sun RY (2004) Effects of nitrite on lethal and immune response of Macrobrachium nipponense. Aquaculture 232:679–686. doi:10.1016/j.aquaculture.2003.08.018
Wagner M, Rath G, Amann R, Koops HP, Schleifer KH (1995) In situ identification of ammonia-oxidizing bacteria. Syst Appl Microbiol 18:251–264
Wagner M, Rath G, Koops HP, Flood J, Amann R (1996) In situ analysis of nitrifying bacteria in sewage treatment plants. Water Sci Technol 34:237–244. doi:10.1016/0273-1223(96)00514-8
Wagner M, Noguera DR, Juretschko S, Rath G, Koops HP, Schleifer KH (1998) Combining fluorescent in situ hybridization (FISH) with cultivation and mathematical modeling to study population structure and function of ammonia oxidizing bacteria in activated sludge. Water Sci Technol 37:441–449. doi:10.1016/S0273-1223(98)00143-7
Wheaton F, Hochheimer J, Kaiser GE (1991) Fixed film nitrification filters for aquaculture. In: Brune DE, Tomasso JR (eds) Aquaculture and water quality. World Aquaculture Society, Baton Rouge, pp 272–303
Wheaton FW, Hochheimer JN, Kaiser GE, Krones MJ, Libey GS, Easter CC (1994) Nitrification filters principles. In: Timmons MB, Losordo TM (eds) Aquaculture water reuse systems: engineering design and management. Elsevier, Amsterdam, pp 101–126
Wik T (2003) Trickling filter and biofilm reactor modeling. Rev Environ Sci Biotechnol 2:193–212. doi:10.1023/B:RESB.0000040470.48460.bb
Young-Lai WW, Daures CM, Charmantier G (1991) Effect of ammonia on survival and osmoregulation in different life stages of the lobster Homarus americanus. Mar Biol (Berl) 110:293–300. doi:10.1007/BF01313716
Zhu S, Chen S (2001) Effects of organic carbon on nitrification rate in fixed film biofilters. Aquacult Eng 25:1–13. doi:10.1016/S0144-8609(01)00071-1
Zhu S, Chen S (2003) Effects of air-diffusion turbulent flow on nitrification rate in fixed film biofilters: a comparison study. N Am J Aquaculture 65:240–247. doi:10.1577/C02-015
Acknowledgments
This work was carried out with the financial assistance from Department of Biotechnology (BT/AA/03/10/79/94 and BT/DRI 794/AAQ/03/092/99) and Department of Science and Technology (SR/SO/AS-15/2003) Government of India. Matsyafed Shrimp Hatchery, Quilon, Kerala is thankfully acknowledged for extending the larval rearing facility for the demonstration and validation. Acknowledge Dr. Valsamma Joseph, Lecturer, National Centre for Aquatic Animal Health, Cochin University of Science and Technology for the analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rejish Kumar, V.J., Achuthan, C., Manju, N.J. et al. Activated packed bed bioreactor for rapid nitrification in brackish water hatchery systems. J Ind Microbiol Biotechnol 36, 355–365 (2009). https://doi.org/10.1007/s10295-008-0504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0504-9