Abstract
In this study, monthly variations in biomass of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) were analysed over a 1-year period by fluorescence in situ hybridization (FISH) at the full-scale Fusina WWTP. The nitrification capacity of the plant was also monitored using periodic respirometric batch tests and by an automated on-line titrimetric instrument (TITrimetric Automated ANalyser). The percentage of nitrifying bacteria in the plant was the highest in summer and was in the range of 10–15 % of the active biomass. The maximum nitrosation rate varied in the range 2.0–4.0 mg NH4 g−1 VSS h−1 (0.048–0.096 kg TKN kg−1 VSS day−1): values obtained by laboratory measurements and the on-line instrument were similar and significantly correlated. The activity measurements provided a valuable tool for estimating the maximum total Kjeldahl nitrogen (TKN) loading possible at the plant and provided an early warning of whether the TKN was approaching its limiting value. The FISH analysis permitted determination of the nitrifying biomass present. The main operational parameter affecting both the population dynamics and the maximum nitrosation activity was mixed liquor volatile suspended solids (MLVSS) concentration and was negatively correlated with ammonia-oxidizing bacteria (AOB) (p = 0.029) and (NOB) (p = 0.01) abundances and positively correlated with maximum nitrosation rates (p = 0.035). Increases in concentrations led to decreases in nitrifying bacteria abundance, but their nitrosation activity was higher. These results demonstrate the importance of MLVSS concentration as key factor in the development and activity of nitrifying communities in wastewater treatment plants (WWTPs). Operational data on VSS and sludge volume index (SVI) values are also presented on 11-year basis observations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of the EU WFD in Europe requires that wastewater treatment activated sludge plants located in “sensitive areas” have high performance efficiencies and achieve low final effluent pollutant limits: there are stringent limits for chemical oxygen demand (COD), biochemical oxygen demand (BOD), total nitrogen forms, total phosphorus, metals, aromatic and aliphatic hydrocarbons, etc. Properly designed and equipped WWTPs are normally able to efficiently remove COD, BOD and phosphates (by enhanced biological pfshosphorus removal and/or chemical precipitation for phosphates). Nitrification processes often are vulnerable for reasons such as low temperature (Zou et al. 2014), nitrogen loading fluctuations (Wanner 1994), the presence of inhibitory compounds (free ammonia and nitrite) and low dissolved oxygen concentrations and pH (Zeng et al. 2014). Fluctuations in flow and solids retention time (SRT) also affect the nitrification process (Hallin et al. 2005). Temperature is among the operating parameters that is not possible to control, such as sewage composition. Temperature effect has been shown to favour filamentous bacteria proliferation and, particularly the filamentous bacterium Microthrix parvicella, has been able to proliferate mainly in winter and causing severe problems of settling and foaming worldwide (Mamais et al. 1996; Wang et al. 2014).
Two phylogenetically different groups of lithoautotrophic bacteria are involved in nitrification: ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). While nitrifying bacteria have been thoroughly investigated in both bench-scale systems and wastewater treatment plants under stable operational conditions, there is a lack of information on annual trends of these communities in full-scale systems and on their dependence on operational parameters.
Only a few studies on AOB and NOB quantification over long periods in full-scale activated sludge systems have been reported (Hallin et al. 2005; Siripong and Rittmann 2007), and almost all of these were performed with PCR-based methods (Dionisi et al. 2002; Harms et al. 2003). These tools are useful for identifying novel species but have quantitative limitations due to DNA extraction and purification biases. Fluorescence in situ hybridization (FISH) combined with imaging software has been commonly and successfully used in environmental studies of microbial community structure (Wagner et al. 1995; Hallin et al. 2005; Dytczak et al. 2008; Benakova and Wanner 2013; Eyice et al. 2015), including communities containing AOBs and NOBs. This research used FISH to quantify nitrifying bacteria in a full-scale wastewater treatment plant over a 1-year period and to evaluate the effect of operational conditions on the population dynamics and on maximum nitrification capacity of the sludge. The objective of the work was to determine whether these relationships could be used to reliably establish an optimum condition for nitrifying bacteria and thus improve the nitrification efficiency of the system.
Another aspect of this work was related to the nitrification capacity of the plant. Respirometers, commonly used in WWTPs, allow the measurement of heterotrophic and autotrophic population kinetic parameters by processing oxygen concentration data, through addition of specific inhibitors. This type of method requires many manual respirometric measurements (Ficara et al. 2000) taken during a period over which the pH must be kept constant even though the nitrification reaction produces strong acid. To accomplish this, it is necessary to use an automated titrimetric system in which the alkalinity is adjusted throughout the experiment so as to maintain the pH constant. The application of this pH-stat titration to nitrification activity assessment was first proposed by Ramadori et al. (1980), and the first on-line application of titrimetric sensors for this purpose was reported by Massone and Rozzi (1997). While the applicability of set-point titration for monitoring biological processes has been demonstrated (Ficara et al. 2000; Fiocchi et al. 2008), the on-line automated procedure is still at an early stage of industrial development. In this work, the nitrification capacity of the plant was monitored over a 1-year period using respirometric batch tests and an on-line automated titrater TITAAN (TITrimetric Automated ANalyser), the prototype of which has been successfully tested in SBRs (Fiocchi et al. 2008).
Material and methods
The full-scale Fusina WWTP
The Fusina WWTP receives 400,000 population equivalents of a mixed domestic + industrial wastewater from Venice (Italy) containing 3200 kg TKN day−1. It consists of mechanical treatment, equalization, biological treatment (with nitrification and denitrification), secondary clarification and UV disinfection. Sludge treatment is by anaerobic digestion.
Chemical analyses and nitrification activity monitoring
The nitrification capacity of the plant was monitored by respirometric batch tests (Strathtox™, ISO, 8192, 2007) and by an automated on-line instrument based on titrimetric biosensors. In the respirometric tests, the oxygen linked to nitrification was measured by adding ammonium chloride, in the presence and absence of thiourea (which inhibits nitrification). Nitrosation, nitratation and heterotrophic activities were also determined using an automated on line instrument (TITrimetric Automated ANalyser), that four times per day measured the following microbial activities: oxygen uptake rate (endogenous and maximum), nitrosation, nitratation and alkalinity requirements for maintaining constant pH. Because the stoichiometry of the reaction: NH4+ + 3/2 O2 → 2H+ + NO2− + H2O indicates that the biological activity of ammonia oxidizers produces acidity and consumes oxygen, it is possible to derive the kinetic constants for AOB populations by titration (Ficara et al. 2000) using the on-line TITAAN instrument.
Chemical Analyses (COD, BOD, TKN, ammonia, nitrite, nitrate) were made according to APAT/IRSA-CNR methods (2006).
Fluorescence in situ hybridization analysis, microscopy and image analysis
FISH with 16S rRNA targeted probes was performed on 20 samples (three replicates each) collected between May 2011 to May 2012 using the procedure of Amann et al. (1995). The ammonia-oxidizing (AOB) and nitrite-oxidizing bacteria (NOB) were identified using the following fluorescent-labelled probes: NSO1225 for the ammonia-oxidizing β-proteobacteria (β-AOB) and Ntspa662 for most of the members of the phylum Nitrospirae and genus Nitrospira. Equimolar mixtures of the probes EUB338 (specific for most of the members of the domain bacteria), EUB338II (specific for Planctomycetales) and EUB338III (specific for Verrucomicrobiales) were used for the detection of almost all the members of the domain bacteria. The specific sequences for each of the probes and the formamide percentage were taken from the probeBase database (Loy et al. 2007). The NSO1225 and Ntspa662 probes were synthesized with Cy3 label and the EUB338 mixture probe with fluorescein isothiocyanate (FITC) label and purchased from MWG AG Biotech (Germany). All the hybridizations with AOB- and NOB-specific probes were also performed with DAPI staining for detecting the total biomass, directly to the hybridization buffer at a final concentration of l μg mL−1. Replicates were observed by using epifluorescence microscopy (Olympus BX51) at ×1000 magnification using filters for FITC (excitation, 470–490 nm; emission, 520 nm) and for CY3 (excitation, 546 nm; emission, 590 nm). Images were taken with a digital camera (Olympus XM-10). The ImageJ software package (version 1.37v, Wayne Rasband, National Institute of Health, Bethesda, MD, USA; available in the public domain at http://rsb.info.nih.gov/ij/index.html) was used for image analyses. The areas covered by hybridized cells and by cells stained with DAPI were calculated separately for each field. At least 15 field images were considered for each replicate. Based on the quantification of area of 3600 images, the AOB and NOB populations were expressed as a percentage contribution to the total biomass.
Since FISH analysis showed a high background fluorescence signal, a pre-hybridization step (6 h, 46 °C) with a calf thymus DNA solution (10 mg mL−1, Sigma-Aldrich) was needed before performing FISH procedure. In order to visualize nitrifier clusters within the 3D structure of flocs, FISH analysis was combined with confocal laser scanning microscopic (CLSM, OLYMPUS FV1000, IX81) observations. Samples were observed in a multichannel mode, using lasers with the following excitation wavelengths: 405 nm (emission 461 nm, blue channel); Ar, 488 nm (emission 519 nm, green channel); Ar/HeNe 543 nm (red channel). 3D images were constructed from a series of 2D cross-sectional images (x-y plane) that were captured at 0.5-μm intervals along the z-axis using Imaris 6.2.0 software (Bitplane AG Zurich, Switzerland). The 3D reconstruction (volume rendering) and 3D volume rendering in blend mode of CLSM images were visualized in maximum intensity projection (MIP) by using Imaris 6.2 software (Bitplane AG Zurich, Switzerland).
Data analysis
Associations between measured variables were quantified by the Pearson product-moment correlation coefficient (significance levels p < 0.05) by using the Sigma Stat (version 3.5) program. Principal component analysis (PCA) was used to determine the association between operating parameters and AOB and NOB populations. This was accomplished by diagonalization of the correlation matrix of the data. The eigenvalues of the PCs are a measure of their associated variance, and the participation of the original variables in the PCs is given by the loadings. Multiple regression analysis was performed to further identify significant effects of environmental or operational factors on AOB and NOB population abundances and maximum nitrification capacities. The Past (version 2.17) program was used.
Results and discussion
Plant description
The Fusina WWTP is a low F/M plant (0.07–0.15 kg BOD kg−1 VSS day−1), low TKN loaded (0.011–0.025 kg TKN kg−1 VSS day−1) treating unsettled raw wastewater at temperatures that range between 10 and 25 °C. Average annual operating parameters are organic loading, 0.16 kg COD kg−1 VSS day−1; sludge age, 19 days, MLSS, 4700 mg L−1, VSS/SS ratio, 65 %. Removal efficiencies are normally high: COD = 91 %, BOD = 97 % N-NH4+ = 99%, TKN = 96 %, P-PO4 3− = 73 % and of total p = 80 %. Over an 11-year period operational data show that sludge volume index (SVI) varies annually in the range 50–200 mL g−1 and is negatively correlated with temperature (CC = −0.508; p < 0.001; n = 3000) in the range 10–25 °C. SVI and MLVSS/MLSS ratio are positively correlated (CC = 0.559; p < 0.001; n = 3027) (Fig. 1b, supplementary material). After the control of the filamentous bacterium M. parvicella that caused severe foaming problems in the plant (Miana et al. 2002), the overall activated sludge filamentous organism level has remained quite stable and at low levels throughout the year (based on light microscope observations) with Type 0041 being dominant and residing mainly inside the flocs. The reason for the annual SVI variation was not a variation in filamentous bacteria levels but the variation in VSS content of the MLSS with lower SVIs to higher VSS contents. In the summer, the VSS content is about 55 while in the winter, it is about 75 %. This variation occurs because the higher summer temperatures allow more mineralization of the MLSS than during the cooler winter temperatures. This effect is pronounced at the Fusina plant because it treats unsettled wastewater.
Nitrification rates (steady state and maximum)
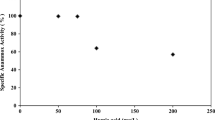
The maximum nitrosation rate (KNmax), measured both by laboratory and on-line respirometry (Strathtox™), varied in the range 2.0–4.0 mg NH4 g−1 VSS h−1 (Fig. 1). A significant positive correlation was found between the two data sets (CC = 0.735; p = 0.0155) indicating that it would be possible to use the on-line instrument in place of the time-consuming laboratory analysis. The nitrosation rate (KNmax) was 0.048–0.096 kg TKN kg−1 VSS day−1 and four times higher than the TKN loading applied (Fig. 2, supplementary material) meaning that the activated sludge could handle significantly higher TKN loadings for at least some period of the day. This indicates that the plant should have the capacity to nitrify peak TKN loads and thereby avoid ammonia breakthrough into the effluent. An attempt to understand the factors affecting the nitrosation activity of the sludge was made by using PCA and multiple regression analysis. PCA was carried out by a diagonalization of the correlation matrix (normalized var-covar) to avoid problems arising from different measurement scales and numerical ranges of the original variables. The first principal component (PC1) explains 68.73 % of the variance with the highest loading factors was associated with MLVSS (0.78) and maximum nitrosation activity (0.62) (Table 1, supplementary material). A bivariate plot of the PC1 and PC2 (23.21 % of the variance) scores is shown in Fig. 2, in which the analytical values have been differentiated according to the sampling season. The data distribution appears to be driven by MLVSS with temperature possibly also having an effect on KNmax. With a few exceptions, the data are distributed along the first component according to a temperature gradient. Samples corresponding to the winter months appear on the positive side of PC1 and samples corresponding to the summer are more clustered on the negative side of the axis. The rest of the samples (spring and autumn) are at the central part of the axis in partially overlapping clusters. These observations suggest that temperature (negative effect) and MLVSS (positive effect) might be the key factors responsible for maximum nitrosation activity. Additional multiple regression analysis shows that KNmax can be predicted from a linear combination of the considered variables being MLVSS (p = 0.035) and temperature (p = 0.006) significantly related to the maximum nitrosation rate.
AOB and NOB estimation by FISH
Because direct application of FISH probes to the activated sludge mixed liquor produced a strong diffused fluorescence, it was necessary to apply the pre-hybridization with calf thyme DNA to produce a clear signal.
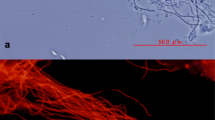
β-AOB (Fig. 3a–f) and Nitrospira spp. cells (Fig. 3g–h) observed with CLSM appear as rod-shaped bacteria associated in aggregates with variable dimensions and patchy distribution throughout the flocs. Consistent with previous studies (Benakova and Wanner 2013), image analyses revealed high variability in the AOB and NOB density among different microscopic fields. β-AOB cells were generally larger (5–15 μm), more tightly packed aggregates than Nitrospira spp. cells (<6 μm). Superimposition of confocal optical sections to form 2D and 3D images showed that the NOB populations were aggregated in more irregular morphologies (Fig. 4g–h) within the flocs and characterized by the presence of voids inside the clusters that likely facilitate nutrient and gas exchange between the surface and the deep regions of the clusters. Size and morphology of the AOB and NOB populations were in accordance with those reported in literature (Hallin et al. 2005; Benakova and Wanner 2013) although AOB clusters were also present in larger aggregates (Daims et al. 2001; Hallin et al. 2005).
In situ CLSM micrographs of β-AOB (a–f) and NOB (g, h) communities in the Fusina WWTP detected with FISH. a, d Rod-shaped bacteria associated in aggregates in the flocs, targeted with probe NSO1225 (red signal); b, e total cells stained with DAPI (blue signal); c, f the pink signal is due to the overlapping of the DAPI and probe NSO1225 signals; g irregular clusters within the flocs targeted with probe NTSPA662 (red signal); h total cells stained with DAPI (blue signal); the pink signal is due to the overlapping of the DAPI and probe NTSPA662 signals (scale bars 5 μm)
The AOB populations made up 3.54 ± 0.56% (October 2011) and 14.18 ± 0.92% (July 2011) of the total microbial community in the Fusina activated sludge. AOB values ranging from 0.034 % (Dionisi et al. 2002) in nitrifying activated sludge to 30 % (Dytczak et al. 2008) in nitrifying activated sludge/biofilm have been reported. NOB populations generally are present in lower percentages than AOB (Gerardi 2002; Dytczak et al. 2008). In our case, the NOB fractions were between 1.83 ± 0.27 % (June 2011) and 7.28 ± 0.24 % (July 2011), placing them in the ranges reported in other studies (Dytczack et al. 2008). The variation of AOB and NOB percentages relative to DAPI during the 1-year experiment is shown in Fig. 4. The AOB and NOB populations peaked in the summer when the temperatures were the highest. Since the percentages of nitrifying bacteria relative to EUB were positively correlated with those relative to DAPI (AOB/EUB vs AOB/DAPI: CC 0.877; p < 0.005; NOB/EUB vs NOB/DAPI: CC 0.896; p < 0.005), the results of the PCA analysis have been expressed relative to DAPI. PC1 explains 58.9 % of the variance (Table 2, supplementary material) and it is mainly contributed by MLVSS (−0.82). With a few exceptions (S11, S16, S19), the data appear to be distributed along the PC1 according to a temperature gradient (Fig. 5). Winter samples form a cluster at the negative side of PC1, while summer samples are more clustered at the positive side of the axis. As speculated in previous studies of community composition of AOBs in full-scale wastewater treatment processes (Hallin et al. 2005) and in nitrifying sequencing batch reactors (Dytczak et al. 2008), the positive effect of temperature on AOB and NOB communities was expected; however, in this study, the correlation between temperature and nitrifying populations was not found to be significant by multiple linear analysis (p > 0.05). Rather, multiple regression analysis revealed an inverse linear correlation between AOB (p = 0.029) and NOB (p = 0.01) and MLVSS concentrations.
Conclusions
Monitoring the nitrification activity is crucial in WWTPs. The direct estimation of nitrifying activities can be reliably accomplished by manual laboratory analyses or automatically by on-line instrumentation. The activity measurements provide a valuable tool for estimating the maximum possible TKN loading and can function as an early warning of when the TKN is approaching this maximum value. FISH is a simple technique of estimating the in situ specific nitrifying bacteria populations of activated sludges and provides for easy, long-term monitoring of nitrifying populations. This approach identified MLVSS and temperature as key factors in the development and activity of nitrifying communities in WWTPs. Results suggest that an increase in MLVSS concentration leads to a decrease in nitrifying population abundance with a higher maximum nitrification activity.
These techniques will allow one to rapidly detect nitrification activity and population variations caused by anomalous events such as toxicity and organic and TKN shock loadings. Because of this, the approach utilized in this paper will be particularly useful for industrial activated sludge plants in which considerable influent load and toxicity variations occur.
References
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Mol Biol Rev 59:143–169
APAT/IRSA-CNR. (2006) Metodi Analitici per le Acque: Parametri Chimico-Fisici 1:1-260
Benakova A, Wanner J (2013) Application of fluorescence in situ hybridization for the study and characterization of nitrifying bacteria in nitrifying/denitrifying wastewater treatment plants. Environ Technol 34(16):2415–2422
Daims H, Nielsen JL, Nielsen PH, Schleifer K-H, Wagner M (2001) In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol 67(11):5273–5284
Dionisi HM, Layton AC, Harms G, Gregory IR, Robinson KG, Syler GS (2002) Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl Environ Microbiol 68(1):245–253
Dytczak MA, Londry KL, Oleszkiewicz JA (2008) Activated sludge operational regime has significant impact on the type of nitrifying community and its nitrification rates. Water Res 42:2320–2328
Eyice Ö, Ince O, Ince BK (2015) Monitoring the abundance and the activity of ammonia-oxidizing bacteria in a full-scale nitrifying activated sludge reactor. Environ Sci Pollut Res 22:2328–2334
Ficara E, Musumeci A, Rozzi A (2000) Comparison and combination of titrimetric and respirometric techniques to estimate nitrification kinetics parameters. Water SA 26(2):217–224
Fiocchi N, Ficara E, Bonelli S, Canziani R, Ciappelloni F, Mariani S, Pirani M, Ratini P, Mazouni D, Harmand J (2008) Automatic set-point titration for monitoring nitrification in SBRs. Water Sci Technol 58(2):331–336
Gerardi MH (2002) Nitrification and denitrification in the activated sludge process. Environ Protection Magazine Series. Wiley, New York
Hallin S, Lydmark P, Kokalj S, Hermansson M, Sorensson F, Jarvis A, Lindgren P-E (2005) Community survey of ammonia-oxidizing bacteria in full-scale activated sludge processes with different solids retention time. J Appl Microbiol 99:629–640
Harms G, Layton AC, Dionisi HM, Gregory IR, Garret VM, Hawkins SA, Robinson KG, Sayler GS (2003) Real time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ Sci Technol 37:343–351
ISO 8192. (2007) Water quality—test for inhibition of oxygen consumption by activated sludge for carbonaceous and ammonium oxidation
Loy A, Maixner F, Wagner M, Horn M (2007) ProbeBase an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res 35:800–804
Mamais D, Andreadakis A, Noutsopoulos C, Kalergis C (1996) Causes of, and control strategies for, Microthrix parvicella bulking and foaming in nutrient removal activated sludge systems. Water Sci Technol 37:4–5
Massone AG, Rozzi A (1997) Nitrification and toxicity on line control in an industrial wastewater treatment plant. In: Proc Instrumentation Control and Automation IWAT Brighton, 6–9 June
Miana P, Grando L, Caravello G, Fabris M (2002) Microthrix parvicella foaming at the Fusina WWTP. Water Sci Technol 42:499–502
Ramadori R, Rozzi A, Tandoi V (1980) An automated system for monitoring the kinetics of biological oxidation of ammonia. Tech Note Water Res 14:1555–1557
Siripong S, Rittmann BE (2007) Diversity study of nitrifying bacteria in full-scale municipal wastewater treatment plants. Water Res 41:1110–1120
Wagner M, Rath G, Amann R, Koops HP, Schleifer KH (1995) In situ identification of ammonia-oxidizing bacteria system. Appl Microbiol 18:251–264
Wang J, Li Q, Qi R, Tandoi V, Yang M (2014) Sludge bulking impact on relevant bacterial populations in full-scale municipal wastewater treatment plant. Process Biochem. doi:10.1016/j.procbio.2014.08.005
Wanner J (1994) Activated sludge, bulking and foaming control. Technomic Publishing Co. Lancaster, Basle
Zeng W, Bai X, Zhang L, Wang A, Peng Y (2014) Population dynamics of nitrifying bacteria for nitritation achieved in Johannesburg (JHB) process treating municipal wastewater. Bioresour Technol 162:30–37
Zou S, Yao S, Ni J (2014) High-efficient nitrogen removal by coupling enriched autotrophic-nitrification and aerobic-denitrification consortiums at cold temperature. Bioresour Technol 161:288–296
Acknowledgments
The authors would like to thank Dr. Elena Romano, Centre of Advanced Microscopy (CAM) “P. Albertano”, Department of Biology, University of Rome ‘Tor Vergata’, for her skillful assistance in the use of the facility and Prof. David Jenkins, University of California at Berkeley, USA, for his critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(A). Annual trends SVI and temperature during an 11-year period. (B) Annual trends of SVI and MLVSS/MLSS% during an 11-year period. (PDF 335 kb)
Supplementary Fig. 2
Organic (A) and nitrogen (B) loadings applied to the plant during the period between Jan-11 and Oct-13. (PDF 13 kb)
ESM 3
(PDF 28 kb)
ESM 4
(PDF 29 kb)
Rights and permissions
About this article
Cite this article
Badoer, S., Miana, P., Della Sala, S. et al. Nitrifying bacterial biomass and nitrification activity evaluated by FISH and an automatic on-line instrument at full-scale Fusina (Venice, Italy) WWTP. Environ Sci Pollut Res 22, 19786–19792 (2015). https://doi.org/10.1007/s11356-015-5140-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5140-0