Abstract
Pseudomonas fluorescens NCIM 2100 was studied for its potential to produce extracellular biosurfactant in the nutrient broth medium supplemented with diesel (2%) as an additional carbon source. Biosurfactant production was checked based on oil spread and drop collapse test. Emulsification index test was conducted to determine the emulsifying ability of the crude biosurfactant produced. The emulsification index, minimum surface tension and production of crude bioemulsifier were found to be 90.38% with diesel, 34.40 ± 0.03 mN/m and 2.80 ± 0.45 g/L respectively. Maximum product accumulation occurred during stationary phase of the bacterial growth curve. Crude biosurfactant produced was stable, withstanding a wide temperature (37–80 °C) and pH range (7–10.5), with an E-24 Index value greater than 50%. Produced biosurfactant has an efficient emulsifying ability. Most of the emulsions formed with tested aliphatic hydrocarbons and ester-based vegetable oils were stable. Emulsions formed with different hydrophobic substrates were oil-in-water (o/w) in nature.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Biosurfactants are surface active compounds of microbial origin (Panjiar et al. 2015). Biodegradable nature, low toxicity, chemical and functional diversity makes these compounds a suitable substitute for chemical surfactants (Panjiar et al. 2013). Presence of both hydrophilic and hydrophobic moieties causes its accumulation at the air–water or oil–water interface, thereby reducing surface and interfacial tension respectively (Vijayakumar and Saravanan 2015). Bioemulsifiers are group of biosurfactants having efficient emulsification properties, generally comprising of high molecular weight biosurfactants (Lovaglio et al. 2015). They form microemulsions where hydrophobic substrates can disperse in aqueous phase, forming oil-in-water (o/w) emulsions, or water can disperse in oil as continuous phase resulting into water-in-oil emulsions (w/o). Oil-in-water emulsions having characteristic aqueous continuous phase have found applications in different industries like cosmetics, food, pharmaceutical, agriculture and petroleum (Randhawa and Rahman 2014). Industrial process like microbial enhanced oil recovery (MEOR) requires the use of thermostable biosurfactants (Pacwa-Płociniczak et al. 2011). The genus Pseudomonas has been extensively studied for its biosurfactant producing potential (Aparna et al. 2012; Rebello et al. 2013). Although the research related to large-scale biosurfactant production has considerably increased during recent years, target biotechnological production of these compounds at industrial scale is yet to achieve, which is the requirement of the present time (Rebello et al. 2013). The purpose of the present work is to determine the biosurfactant production capability of Pseudomonas fluorescens NCIM 2100 and its application in formation of stable oil-in-water emulsions.
Materials and Methods
Maintenance of the Microorganism and Preparation of Seed Culture
The bacterial culture, P. fluorescens NCIM 2100 was obtained from the culture collection of Department of Bio-Engineering, Birla Institute of Mesra, Ranchi, Jharkhand, India, and was regularly subcultured after an interval of one month and maintained on nutrient agar slants at 4 °C for future use. Preparation of seed culture was performed by transferring a loopfull of microorganism from the stored slant into 6 mL of sterilized nutrient broth followed by incubation at 37 °C and 120 rpm in a shaker incubator (New Brunswick Scientific, USA) for 12–15 h.
Detection of Biosurfactant in the Culture Broth
Detection of biosurfactant in the culture broth of P. fluorescens NCIM 2100 was determined by inoculating 1% (v/v) of homogenous seed culture (OD600 ~ 1) in nutrient broth medium supplemented with diesel (2%) followed by incubation at 37 °C and 120 rpm. Hydrophobic substrate, diesel, was used to induce the production of biosurfactant. Culture supernatant was obtained after centrifugation at 5000 rpm for 20 min at 25 °C. Biosurfactant production in the culture supernatant was checked using multiple screening tests like oil spread (Morikawa et al. 2000), drop collapse (Jain et al. 1991) and emulsification index (E-24 index) test (Cooper and Goldenberg 1987), as described earlier by Panjiar et al. (2015). The percentage of emulsification index is calculated by using Eq. (1). Sodium dodecyl sulphate (SDS) and phosphate buffer saline (PBS) were used as positive and negative controls respectively.
Surface tension measurement of the culture supernatant and whole culture broth was performed by the dynamic contact angle tensiometer DCAT21 (Dataphysics, Germany) using the Wilhelmy plate method. Sterile nutrient broth was used as control (Nitschke et al. 2004).
Bacterial Growth and Biosurfactant Production Study
The growth pattern of P. fluorescens NCIM 2100 was determined both in the presence and absence of diesel as described earlier by Panjiar et al. (2015). Microbial growth (OD at 600 nm) and biosurfactant production, measured in terms of E-24 Index, was determined simultaneously by withdrawing samples from the culture broth.
Biosurfactant Extraction from the Culture Broth
Biosurfactant produced by P. fluorescens NCIM 2100 into the broth culture supernatant was acid hydrolyzed with concentrated hydrochloric acid to precipitate it, followed by overnight incubation at 4 °C. During acidification, biosurfactant present in protonated form become less soluble in aqueous solution. The precipitated bioemulsifier was collected by centrifugation at 5000 rpm for 20 min at 4 °C. Collected bioemulsifier was washed twice with distilled water, dried at 65 °C and weighed to calculate its production (Nitschke et al. 2004).
Stability Study of Extracellular Crude Biosurfactant
Effect of pH and Temperature
Stability of the extracellular crude biosurfactant was determined by measuring the E-24 Index of the culture supernatant after subjecting it to different pH and temperature ranges. pH values of 2–10 was adjusted by hydrochloric acid (1N)/sodium hydroxide (1N). Temperatures considered for study were 4, 10, 20, 40, 60 and 80 °C (Khopade et al. 2012).
Emulsifying and Emulsion Stabilizing Capacity of the Extracellular Biosurfactant
Emulsifying ability of the produced biosurfactant was examined by studying E-24 Index with different hydrophobic substrates (hexane, heptane, octane, hexadecane, dodecane, benzene, toluene, xylene, diesel, kerosene and oils of soybean, mustard, groundnut, olive and coconut). The emulsion formed by vortexing hydrophobic substrates and culture supernatant was left to stand for 1 h, and was considered as starting time, 0 h. Emulsification Index (E-24 Index, %), relative emulsion volume (EV, %), emulsion stability (ES, %) and emulsified organic phase (EOP, %) were calculated at 24 h intervals up to 72 h, 1 month and then after 3 months’ intervals from Eqs. (1) to (4) respectively (Batista et al. 2006; Portilla-Rivera et al. 2010).
where TOP is total volume of organic phase and NEOP is non-emulsified organic phase. The emulsions stabilized by culture supernatant were also compared with those formed by 1% (w/v) solution of the chemical surfactant SDS in deionized water.
Nature and Droplet Size Distribution Study of the Emulsions Formed
Nature of the emulsion, whether it was o/w or w/o, was determined by the addition of small amount of powder water soluble dye (methylene blue) on the surface of the tested emulsion kept on the slide. If the external continuous phase was aqueous (emulsion of o/w type), the dropped dye got diffused immediately throughout the aqueous phase of the emulsion. On contrary, if the emulsion was of w/o type, the dropped dye remained as clump on the surface of the tested emulsion (http://www.surfatech.com/pdfs/emulsions.pdf).
Optical microscope was used to measure the size of the emulsion’s droplets (Portilla-Rivera et al. 2010). A drop of emulsion was placed in a glass slide and was observed through a 10× objective lens of the microscope. Radii of the observed droplets were measured through standard micrometric procedure. Images of several regions from each slide were taken to capture the representative structure of emulsion droplets.
Results and Discussion
P. fluorescens NCIM 2100 was investigated to produce surface active compounds called biosurfactants.
Detection of Biosurfactant in the Culture Broth
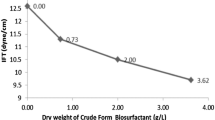
Sensitive detection methods like the drop collapse and oil spread tests are included together for primary screening of biosurfactant production (Satpute et al. 2008), whereas E-24 index test was conducted to determine the emulsifying ability of the produced biosurfactant (Dhail and Jasuja 2012). The drop containing culture supernatant collapsed within 10 s, thereby indicating the presence of biosurfactant. Diameter of clear zone was observed to be greater than 5 cm, further confirming the surfactant nature of the culture supernatant. Maximum E-24 Index observed was 90.38% with diesel, which is in conformity with Willumsen and Karlson (1997), stating a promising bioemulsifier should have an E-24 Index greater than 50%. Biosurfactant produced by P. fluorescens NCIM 2100 could reduce the surface tension value of the culture broth to 34.40 ± 0.03 mN/m, which was around the threshold value of 40 mN/m or lower (Olivera et al. 2003). Significant reduction of surface tension was not observed may be due to production of polymeric biosurfactant having reasonable emulsification abilities as compared to surface activity (Willumsen and Karlson 1997; Plaza et al. 2006). Extracellular nature of the produced biosurfactant was confirmed by the similar surface tension values of culture broth and culture supernatant.
Bacterial Growth and Biosurfactant Production Study
Simultaneous study was conducted for measuring bacterial growth (A600) and biosurfactant production (E-24) at different time intervals (Fig. 1). Biosurfactant was not detected in the nutrient broth without diesel, which is control sample. Considerable bioemulsifier production started after 32 h of incubation period at the exponential phase of bacterial growth curve, when grown in production medium. Highest production (E-24 Index of 90.38%) was attained after 72 h of incubation, at its stationary phase of nutrient limiting condition. Singh and Tripathi (2013) have described maximum production of rhamnolipid biosurfactant during stationary phase of the studied bacterial strain Pseudomonas stutzeri. Different authors have also reported biosurfactant production during their stationary phase (Batista et al. 2006; Sriram et al. 2011). 2.80 ± 0.45 g/L of biosurfactant was produced, as determined by acid precipitation method. The precipitate was again dissolved in distilled water and pH was set to 7 by adding sodium hydroxide (1N) and it was checked for the presence of bioemulsifier by emulsification index test. The remaining culture supernatant was also checked and found to give negative result for emulsification index test.
Stability Study of Extracellular Crude Biosurfactant
Effect of pH and Temperature
Crude bioemulsifier produced by P. fluorescens NCIM 2100 is stable at pH range of 7–10.5 (Fig. 2a) and temperature range of 37–80 °C (Fig. 2b) with E-24 Index value greater than 50%. Thermal and pH stability of the crude biosurfactant render its applicability in process like MEOR (Singh and Tripathi 2013).
Emulsifying and Emulsion Stabilizing Capacity of the Extracellular Biosurfactant
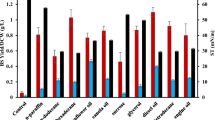
It was evaluated by determining E-24 Index (%), EV (%), ES (%) and EOP (%) with different hydrophobic substrates namely hexane, heptane, octane, hexadecane, dodecane, benzene, toluene, xylene, diesel, kerosene, soybean, mustard, groundnut, olive and coconut oil at 0, 24 h, 1 month and 3 months’ intervals. Biosurfactant produced by P. fluorescens NCIM 2100 could efficiently emulsify all the tested aliphatic and ester-based oils but was less capable of emulsifying aromatic hydrocarbons. From Fig. 3a–d, it was apparent that emulsions formed with diesel and other aliphatic hydrocarbons had a greater E-24 index (90.38%), EV values (75.98%) after olive oil (98.07% E-24 Index and 97.97% EV). All the emulsions formed were 100% stable up to 24 h, with the entire organic layer converted into emulsion. However, less emulsion stability was detected in case of mustard and coconut oil.
Emulsifying ability and stability results of biosurfactant by studied bacteria were found to be comparable to the chemical surfactant used (SDS) with 75% of E-24 Index, EV and 100% of ES and EOP. PBS as negative control was found to have 0% of E-24 Index, EV, ES and EOP.
Study by Aparna et al. (2012) has reported different E-24 Index values with sunflower oil, crude oil, gasoline, n-hexadecane, kerosene, hexane and benzene by the isolate Pseudomonas sp. 2B, when grown in modified proteose peptone glucose ammonium salt medium. Maximum E-24 Index value of 84% was found with sunflower oil, while the produced biosurfactant was not able to emulsify benzene. The results were found to be similar as in the present study.
Nature and Droplet Size Distribution Study of the Emulsions Formed
Emulsions formed with different hydrophobic substrates by the produced biosurfactant, was found to be o/w in nature. Optical images of emulsion apparently showed the oil droplets dispersed in continuous aqueous phase (Fig. 4b). Figure 4c showed the compact o/w emulsion of olive oil in water.
Standard micrometric procedure was used to measure the droplet size of the emulsions. Figure 4a represents the distribution of droplets diameter for different emulsions. It was observed that compact and stable emulsions comprised of more than 75% of droplets of diameter between 0 and 24.9 µm. Whereas loose and unstable emulsions contain 5.5–36 and 7.5–21% of droplets of diameter 50–99.9 and 100–150 µm respectively. Moreover, it was also observed that compact emulsions were polydispersed in nature, characterized by droplets of different diameter whereas loose emulsions are more homogeneous with larger droplet size, which is in accordance with the findings of Portilla-Rivera et al. (2010).
Conclusion
P. fluorescens NCIM 2100 was examined for its biosurfactant-producing property in the nutrient-rich medium containing diesel as an additional carbon source. Stationary phase of the bacterial growth was observed to be the most optimal for maximum biosurfactant accumulation into the medium. Stability of the crude biosurfactant at broad pH and temperature range and its ability to efficiently emulsify aliphatic hydrocarbons and ester-based vegetable oils, forming stable oil-in-water emulsions, suggests its potential use in petroleum, food, cosmetic industry and as cleaning agents. It may have environmental applications for mineralization of recalcitrant hydrocarbons in contaminated areas. Moreover, the use of obtained biosurfactant in its crude form conform the requirement of cost reduction.
References
Aparna A, Srinikethana G, Smitha H (2012) Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf B 95:23–29
Batista SB, Mounteer AH, Amorim FR, Tótola MR (2006) Isolation and characterization of biosurfactant/bioemulsifier-producing bacteria from petroleum contaminated sites. Bioresour Technol 97:868–875
Cooper DG, Goldenberg B (1987) Surface active agents from two Bacillus species. Appl Environ Microbiol 53:224–229
Dhail S, Jasuja ND (2012) Isolation of biosurfactant-producing marine bacteria. Afr J Environ Sci Technol 6:263–266
Jain DK, Thompson DKC, Lee H, Trevors JT (1991) A drop collapsing test for screening surfactant producing microorganisms. J Microbiol Methods 13:271–279
Khopade A, Biao R, Liu X, Mahadik K, Zhang L, Kokare C (2012) Production and stability studies of the biosurfactant isolated from marine Nocardiopsis sp. B4. Desalination 285:198–204
Lovaglio RB, Silva VL, Ferreira H, Hausmann R, Contiero J (2015) Rhamnolipids know-how: looking for strategies for its industrial dissemination. Biotechnol Adv. doi:10.1016/j.biotechadv.2015.09.002
Morikawa M, Hirata Y, Imanaka T (2000) A study on the structure function relationship of the lipopeptide biosurfactants. Biochem Biophy Acta 1488:211–218
Nitschke M, Ferraz C, Pastore GM (2004) Selection of microorganisms for biosurfactant production using agroindustrial wastes. Braz J Microbiol 35:81–85
Olivera NL, Commendatore MG, Delgado O, Esteves JL (2003) Microbial characterization and hydrocarbon biodegradation potential of natural bilge waste microflora. J Ind Microbiol Biotechnol 30:542–548
Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget ZZ, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12:633–654
Panjiar N, Gabrani R, Sarethy IP (2013) Diversity of biosurfactant producing Streptomyces isolates from hydrocarbon-contaminated soil. Int J Pharm Bio Sci 4:524–535
Panjiar N, Ghosh Sachan S, Sachan A (2015) Screening of bioemulsifier-producing micro-organisms isolated from oil-contaminated sites. Ann Microbiol 65:753–764
Plaza GA, Zjawiony I, Banat IM (2006) Use of different methods for detection of thermophilic biosurfactant producing bacteria from hydrocarbon-contaminated and bioremediated soils. J Petrol Sci Eng 50:71–77
Portilla-Rivera OM, Torrado AM, Domínguez JM, Moldes AB (2010) Stabilization of kerosene/water emulsions using bioemulsifiers obtained by fermentation of hemicellulosic sugars with Lactobacillus pentosus. J Agric Food Chem 58:10162–10168
Randhawa KKS, Rahman PKSM (2014) Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front Microbiol 5:454–460
Rebello S, Asok AK, Joseph SV, Joseph BV, Jose L, Mundayoor S, Jisha MS (2013) Bioconversion of sodium dodecyl sulphate to rhamnolipid by Pseudomonas aeruginosa: a novel and cost-effective production strategy. Appl Biochem Biotechnol 169:418–430
Satpute SK, Bhawsar BD, Dhakephalkar PK, Chopade BA (2008) Assessment of different screeningmethods for selecting biosurfactant producing marine bacteria. Indian J Mar Sci 37:243–250
Singh DN, Tripathi AK (2013) Coal induced production of a rhamnolipid biosurfactant by Pseudomonas stutzeri, isolated from the formation water of Jharia coalbed. Bioresour Technol 128:215–221
Sriram MI, Kalishwaralal K, Deepak V, Gracerosepat R, Srisakthi K, Gurunathan S (2011) Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids Surf B 85:174–181
Vijayakumar S, Saravanan V (2015) Biosurfactants-types, sources and applications. Res J Microbiol 10:181–192
Willumsen PA, Karlson U (1997) Screening of bacteria isolated from PAH-contaminated soils for production of biosurfactants and bioemulsifiers. Biodegradation 7:415–423
Acknowledgements
Neha Panjiar acknowledges Council of Scientific and Industrial Research (CSIR), New Delhi for the research fellowship provided [09/554(0023)/2010-EMR-I] and corresponding authors acknowledge the University Grant Commission (UGC), Government of India for the financial support [F. No. 40-160/2011(SR)]. The authors are grateful to Central Instrumentation Facility (CIF) and Department of Bio-Engineering at Birla Institute of Technology, Mesra, Ranchi for providing culture and instrumentation facilities necessary to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Panjiar, N., Sachan, S.G., Sachan, A. (2017). Biosurfactant Production by Pseudomonas fluorescens NCIM 2100 Forming Stable Oil-in-Water Emulsions. In: Mukhopadhyay, K., Sachan, A., Kumar, M. (eds) Applications of Biotechnology for Sustainable Development. Springer, Singapore. https://doi.org/10.1007/978-981-10-5538-6_13
Download citation
DOI: https://doi.org/10.1007/978-981-10-5538-6_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5537-9
Online ISBN: 978-981-10-5538-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)