Abstract
Yeast extract addition to reconstituted apple juice had a positive impact on the development of the malolactic starter culture used to ensure malolactic fermentation in cider, using active but non-proliferating cells. In this work, the reuse of fermentation lees from cider is proposed as an alternative to the use of commercial yeast extract products. Malolactic enzymatic assays, both in whole cells and cell-free extracts, were carried out to determine the best time to harvest cells for use as an inoculum in cider. Cells harvested at the late exponential phase, the physiological stage of growth corresponding to the maximum values of specific malolactic activity, achieved a good rate of malic acid degradation in controlled cider fermentation. Under the laboratory conditions used, malic acid degradation rates in the fermentation media turned out to be near 2.0 and 2.5 times lower, compared with the rates obtained in whole-cell enzymatic assays, as useful data applicable to industrial cider production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To ensure successful achievement of malolactic fermentation (MLF) in cider and wine, inoculation with high numbers of non-proliferating cells of a suitable malolactic bacterial strain has turned out to be a useful manner to overcome difficulties, since cell growth is not necessary to accomplish MLF [8,15]. The use of concentrated apple juice and the improving sanitary conditions in industrial cellars have significantly reduced the presence of the desired microbiota naturally present [22]. Cider is a difficult medium for the growth of malolactic bacteria, so sluggish, stuck or failed MLFs are not infrequent events in cidermaking. Low pH, the presence of sulfur dioxide, ethanol and other inhibitors, nutrient deficiency and interactions between yeast and malolactic bacteria can be mentioned as negative factors affecting the completion of MLF [25]. Massive inoculation with the optimal number of selected cells requires a step in which the production of high quantities of biomass is necessary. The cultivation media should contain the essential nutrients to support the growth of these bacteria, yielding high cell densities in a short period of time, but at the same time it should facilitate an easy adaptation of cells to the hostile conditions to be found in the fermenting must. From an industrial practical approach, the culture medium should be easy to prepare and inexpensive.
The application of starter culture technologies to induce MLF in winemaking (not yet established for cider production) has shown failures, generally caused by a lack of adaptation of cultures. Since the loss of viability of cells is high when they are inoculated directly into wine, starter cultures generally need one or more reactivation steps and/or adaptations prior to use, although freeze-dried starter cultures for direct inoculation into wine have been proposed [19, 20]. The use of a high density of non-proliferating cells permitted deacidification of wine, using the same strains which usually failed to induce MLF when they were inoculated at low densities [15]. The same authors indicated that the minimal inoculation concentrations of non-proliferating cells to achieve optimal degradation of malic acid in wine were about 1×107 colony-forming units (CFU)/ml at pH 3.5 and 1×108 CFU/ml at pH 3.1.
Nutrients in yeast extract (YE) promoted the growth of Oenococcus oeni that conducted MLF [7, 23], but a considerable influence of the type of autolysate, bacterial strain and composition of the culture medium was observed. Much work has been done in synthetic media. Differences are found in the effectiveness of various commercial YE in promoting the growth of lactic acid cultures when added at different concentrations to a synthetic medium [5]. Commercial products are usually excellent but expensive, so industrial producers could find it useful to test other products which have a lower cost. The use of fermentation lees as a nitrogen and carbon supplement in order to improve the growth of starter cultures could be considered, in addition to being an industrial waste bioremediation procedure.
In the present work, O. oeni cells were grown under different nutrient conditions to test their efficiency as starter cultures for MLF in cider: (1) in reconstituted apple juice, (2) in the same apple juice supplemented with commercial YE and (3) in the juice supplemented with two types of cellular extracts obtained from the lees of fermentations (based on yeast cells and on yeast cells plus malolactic bacteria, respectively). To this end, a laboratory procedure to recover fermentation lees was undertaken. Malolactic enzymatic activity assays were performed to optimize the time to harvest bacterial cells for inoculation into cider, following a sequential model of inoculation of microorganisms. Finally, high numbers of cells obtained under these conditions were used as inocula to conduct MLF during cider production.
Materials and methods
Microorganisms
A commercial active/dried yeast strain of Saccharomyces cerevisiae subsp. bayanus (strain Pasteur Institute, Paris, 1969, “Champagne”; supplied by Novo Ferment, Switzerland) was used. The malolactic bacterium was previously isolated in the cellar of the Asturian cidermaking company Sidra Escanciador and was identified as an O. oeni strain [13].
Fermentation conditions
Concentrated (bright, enzymatically treated) apple juice (70.8 ° Brix) supplied by Covillasa (La Almunia Doña Godina, Zaragoza, Spain), was reconstituted with distilled water (1:6) to a specific gravity of approximately 1,060 g/l. Juice was sterilized with a tangential flow filtration device (Pellicon; Millipore, USA) using a polyvinylidene difluoride membrane (pore size 0.22 μm, Pellicon 2 Durapore membrane cassette filter; Millipore, USA), connected to a peristaltic pump.
Fermentations were carried out in pre-sterilized 1-l cylindrical flasks, filled to capacity, without agitation, in duplicate.
Active/dried yeast preparation was rehydrated in sterile apple juice and grown under aerobic conditions at 250 rpm on a shaker (New Brunswick Scientific, USA) at 28 °C for 18 h. To start the alcoholic fermentation, the apple must was inoculated with yeast at a final concentration of 106 CFU/ml. The temperature was maintained at 15 °C until completion of the alcoholic fermentation (when the specific gravity reached approximately 1,005 g/l).
To start the MLF, at the end of the alcoholic fermentation, the temperature was fixed at 22 °C and a high-number cell inoculum was added to the flasks, adjusted to 107 CFU/ml. For use as an inoculum, O. oeni cells were collected from each cultivation medium (see next section) at the end of the exponential phase, centrifuged (3,000 g), washed twice in saline solution and then resuspended in the fermentation media.
Preparation of lees cell extracts
Lees were collected after the alcoholic fermentation or, alternatively, after the completed cider fermentation and were centrifuged (3,000 g), and washed twice in saline solution. The method for cell lysis consisted of treating the cell pellets with 35% HCl at 100 °C for 8 h. After neutralization, the mixture was filtered through a cellulose filter paper (0.151 mm width, 73 g/m2) to separate debris and was lyophilized until used.
Culture conditions
Pure cultures of malolactic bacteria were grown in apple juice, prepared as previously described. Alternatively, apple juice was supplemented with commercial YE (0.5% w/v; Biokar, France) and with the lyophilized cell extracts obtained at concentrations ranging from 0.5% to 1% (w/v). Cultures were incubated at 30 °C without shaking, due to the microaerophilic nature of this bacterium.
Microbial counts
The growth of lactic acid bacteria in pure cultures was monitored by measuring the optical density at 660 nm (OD660). Calibration curves of OD660 units versus cell concentration (CFU/ml) and dry weight (g/l) were previously obtained. Lactic acid bacteria were followed throughout fermentations in each flask by counting viable cells. Serial dilutions were performed in saline solution, plating duplicated dilutions onto MRS medium (Biokar, France) supplemented with cycloheximide at 100 ppm to inhibit yeast growth. Plates were incubated at 30 °C for 5 days before enumeration.
Analytical methods
Total nitrogen content was determined by the method of Kjeldahl [1]. Protein and amino acid contents were determined by the Bradford [3] and Rosen [21] methods, respectively.
Samples collected periodically from each flask were filtered immediately through membrane (pore size 0.45 μm). Specific gravity was measured using a picnometer.
For chromatographic analysis, duplicate 2-ml samples were frozen (−20 °C).The organic acids in samples were determined by HPLC (Alliance model 2690; Waters, USA), equipped with a photodiode array detector (model 996; Waters), as previously described [2, 12]. Solvents and reagents were HPLC-grade. Analytical-grade organic acids (malic acid, lactic acids) were used as standards (Sigma–Aldrich). The sugar content in samples was determined by HPLC, as previously described [13].
Malolactic activity enzymatic assays
For whole-cell assays, cell suspensions were harvested at different times (middle, late exponential phases, stationary phase) by centrifugation (8,000 g, 15 min) and washed twice with tartrate-phosphate buffer, pH 3.5. The buffer was prepared as previously described [6], but using Na2HPO4 instead of K2HPO4. The pellet was resuspended in 5 ml of buffer. Aliquots of cell suspension (1 ml or 2 ml, giving 109 CFU/ml or 2×109 CFU/ml, respectively) were added to 25 ml of buffer (final volume) in 50-ml Erlenmeyer flasks. The head-space was flushed with N2 during the assay and suspensions were initially equilibrated at the reaction temperature for 10 min. In the reaction, linearity was constant through time, as tested with two different cell concentrations.
The reaction was started by adding 1 ml of a 600 mM l-malic acid stock solution (pH 3.5) to the flasks (24 mM, final concentration) at 30 °C (reaction temperature). Samples (1 ml) were withdrawn from the reaction mixture at 1 min after starting and then at every 15 min, over a period of 3 h. To stop the reaction, 100 μl of 1.2 N HClO4 were immediately added; and samples were then filtered through membranes (0.45 μm pore size; Whatman, UK). One blank without substrate and another without cells were prepared. The malic and lactic acids in samples were determined as described above. The results corresponded to the mean value of two determinations (coefficient of variation ≤6%).
Results were expressed as the specific activity (x μmol l-malic acid degraded per minute, per milligram dry weight).
For cell-free extracts, culture volumes containing at least 5×109 CFU/ml harvested at different physiological stages (middle, late exponential phases, stationary phase) were collected by centrifugation (8,000 g, 15 min) and washed twice with 0.1 M phosphate buffer, pH 6. The pellet was then resuspended in 1 ml of the same buffer and disrupted with an ultrasonic disintegrator. The cell-free extract was separated from bacterial debris by centifugation (10,000 g, 15 min, 4 °C). The protein in crude extracts were determined by the Bradford method [3] using Bradford reagent (Sigma) and cell extracts was used immediately for the determination of malolactic activity. The assay was performed at 30 °C, in 0.1 M sodium phosphate buffer, pH 6, containing 0.1 mM MnCl2, 0.5 mM NAD and 0.1 mg protein/ml. Assays were confirmed by adding 0.2 mg protein/ml to the reaction mixture. The headspace was flushed with N2 during the assay and suspensions were initially equilibrated at 30 °C for 10 min. The reaction was started by adding 20 mM l-malic acid (adjusted at pH 6). Samples were withdrawn at each 5 min for 1 h. One blank without substrate and another without cells were used. The reaction was stopped by HClO4 addition and samples were filtered prior to analysis in duplicate. The results corresponded to the mean value of two determinations (coefficients of variation ≤6%). Results were expressed as x μmol l-malic acid degraded per minute, per milligram protein.
Results and discussion
Effect of apple juice supplementation on bacterial growth
Figure 1a–d shows the growth of O. oeni and pH evolution (after inoculation with yeast and malolactic bacteria) in reconstituted apple juice (a) and in the same juice supplemented with either commercial YE (b) or cell extract obtained exclusively from yeast lees (c; i.e. from lees recovered after completion of the alcoholic fermentation without malolactic bacteria) or cell extract from lees recovered after completion of the alcoholic and MLF in apple juice (d).
Rate of growth (circles) and change in pH (crosses) in malolactic bacteria cultures using reconstituted apple juice and supplemented juices. a Reconstituted apple juice (RAJ), b RAJ supplemented with commercial yeast extract (0.5%), c RAJ supplemented with cell extract obtained from yeast lees recovered after completion of alcoholic fermentation, d RAJ supplemented with cell extract obtained from lees recovered after completion of alcoholic and malolactic fermentations. CFU Colony-forming units
In the conditions tested, cells reached maximum densities after approximately 96 h of incubation. In the supplemented media, biomass levels obtained in apple juice (a) were approximately 7-, 4- and 3-fold higher in b, c and d, respectively).
The highest growth-promoting effect observed was obtained after the addition of commercial YE (0.5%). Determination of total soluble nitrogen, protein and quantitative amino acid content in the media for the cultivation of the malolactic bacteria was performed (Table 1). Although higher contents of total nitrogen, protein and amino acids were added when the apple juice was supplemented with cell extract recovered from yeast plus bacterial lees, less growth was obtained compared with the commercial YE; and the growth obtained was very similar to that obtained when juice was supplemented with cell extract recovered from yeast lees only. Although the content of amino acids was quantitatively higher, it might not have contained sufficient amounts of the essential amino acids or vitamins required for growth [23], probably supplied by the commercial YE. It was reported that several commercial YE were tested and evaluated for their growth-promoting properties on lactic acid bacteria, fractionated by ultrafiltration [9]. In that work, results showed that the source of YE had a great effect on growth (higher with bakers’ YEs than with brewers’ YEs). Commercial YE is also rich in vitamins (especially the B group). The treatment applied to the fermentation lees in the present work, carried out at laboratory level, could be substituted by another method of lysis, more suitable to an industrial level and retaining the vitamin fraction in a more active form. In addition, further work is needed to identify the factors in YE promoting growth.
pH effect
pH has an important effect on the growth of lactic acid bacteria[4], since acidification of the growth medium produces a drop in internal and external pH which finally inhibits growth.
pH fluctuated in batch cultures (Fig. 1) without external control. In all cases, pH increased after 48 h. This phase corresponded to malic acid consumption (pKa1=3.40 for malic acid) and the expected lactic acid production (pKa=3.86 for lactic acid), as detected by chromatographic analysis (data not shown). Then, pH values decreased in the medium in relation to the extent of growth (Fig. 1). The rate of malate utilization by O. oeni was maximum at low pH (<4.5), meanwhile glucose and fructose catabolism were inhibited [11, 17]. The optimal pH for sugar catabolism and growth was observed at 5.5 [6]. Our results also confirmed that bacterial cultures growing at low pH began to degrade malic acid during the early stage of growth, without consumption of sugars during this phase, until depletion of this acid in the media (data not shown). At low pH, a preference for malate over glucose as an energy source was previously described [11].
Malolactic activity in free-cell extracts
The rate of malolactic activity depends on the bacterial cell density, the specific malolactic activity and the physiological stage of the cells [24]. Yeast autolysates cause only a small increase in the rate of MLF by lactic acid bacteria[24].
Assays were carried out using clarified crude extracts from cells grown in reconstituted apple juice only and in juice supplemented with commercial YE (0.5%), collected at different physiological stages.
In free-cell extracts (Fig. 2), maximum specific malolactic activity was reached at the late exponential phase, both in the absence and presence of commercial YE. The results obtained in this case revealed an increase in the specific malolactic activity from 2-fold to nearly 3-fold in the supplemented juice.
Malolactic activity in whole cells
Assays were also carried out using whole cells. In this way, the enzyme could be studied in a system which more closely resembled its state, in vivo, being influenced by the permeability of the membrane towards the substrate.
Specific malolactic activities in whole cells harvested at different physiological stages are shown in Fig. 3 (grown in apple juice or in apple juice supplemented with 0.5% commercial YE). A slight increase was found in the specific malolactic activity in whole cells grown in the presence of YE.
As shown in Figs. 2 and 3, both specific activities were maximum at the late exponential phase in apple juice. When YE was added to apple juice, the same pattern was obtained. Other studies revealed that maximum malolactic activity was reached in the beginning of the exponential phase [14], in the middle of the exponential phase [18] or during the stationary phase. In all cases, experiments were carried out in synthetic media.
MLF assays
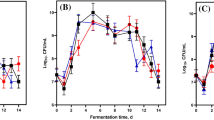
The composition of the culture medium influences the adaptation of the malolactic bacteria to the stress conditions in the fermenting must [10]. O. oeni cells grown in optimized media under laboratory-controlled conditions failed to induce MLF [11, 16]. Thus, cider fermentation assays were performed with controlled inoculation of the malolactic strain grown under the conditions tested, after culture centrifugation. On the basis of the results obtained in the enzyme assays, cells were harvested at the late exponential phase, in which maximum values for malolactic activity were reached. Starter cultures of the O. oeni strain, prepared as described, successfully conducted the malolactic conversion in cider in all cases (Fig. 4a–d). Degradation of malic acid accompanied by the expected increment in lactic acid concentration in the fermentation media could be detected in a short and similar period of time. A slightly higher malic consumption rate was achieved when cells were grown in apple juice supplemented with commercial YE (0.5%). In each case, counts of malolactic bacteria confirmed the maintenance of the inoculation level during the bioconversion phase. Cells corresponding to the exponential phase of growth and showing the highest specific malolactic activity failed to start MLF in wine [14]. It is noteworthy that, in our case (regarding cider production and under the fermentation conditions applied), the cells harvested at the late exponential phase (where the specific malolactic activity was highest) were able to conduct the malolactic bioconversion, showing a good malic acid degradation rate in the assays. When the rates of malic acid degradation in the fermentation media using cells grown in apple juice only and in juice supplemented with commercial YE (Fig. 4a, b) were expressed as x μmoles l-malic acid per minute·per milligram dry weight, they were 2.6-fold and 2.1-fold lower, respectively, than those obtained in the enzymatic assays using whole cells corresponding to the late exponential phase of growth, perhaps due to the presence of inhibitory compounds in the fermentation media. Keeping the same culture conditions, the geometric shape of the fermenter (cylindrical) and the auto-induced agitation used in this work, a good scale-up to pilot-plant and/or industrial fermenters must be expected.
Malolactic fermentations (FML) in cider, using cells grown under the conditions tested. a RAJ only, b RAJ supplemented with commercial yeast extract, c RAJ supplemented with cell extract obtained from yeast lees recovered after completion of an alcohol fermentation, d RAJ supplemented with cell extract obtained from yeast and bacterial lees recovered after completion of alcoholic and malolactic fermentations. Black squares Malic acid consumption, white squares lactic acid production
Since this work was performed at the laboratory level, an alternative method should be optimized in order to recover fermentation lees suitable to improve starter-culture development for industrial fermentations.
References
AOAC (2002) Official methods of analysis, 17th edn. AOAC, Maryland
Blanco D, Morán MJ, Gutiérrez MD, Mangas JJ (1988) Application of HPLC to characterization and control of individual acids in apple extracts and ciders. Chromatographia 25:1054–1058
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Champagne CP, Gardner NJ (2002) Effect of process parameters on the production and drying of Leuconostoc mesenteroides cultures. J Ind Microbiol Biotechnol 28:291–296
Champagne CP, Gaudreau H, Conway J, Chartier N, Fonchy E (1999) Evaluation of yeast extract as growth media supplements for lactococci and lactobacilli by using automated spectrophotometry. J Gen Appl Microbiol 45:17–21
Cox DJ, Henick-Kling T (1989) The malolactic fermentation: a chemiosmotic energy-yielding (ATP) decarboxylation reaction. J Bacteriol 171:5750–5752
Edwards CG, Beelman RB (1989) Inducing malolactic fermentation in wines. Biotechnol Adv 7:333–360
Gao C, Fleet GH (1994) The degradation of malic acid by high density cell suspensions of Leuconostoc oenos. J Appl Bacteriol 76:632–637
Gaudreau H, Champagne CP, Conway J, Degré R (1999) Effect of ultrafiltration of yeast extracts on their ability to promote lactic acid bacteria growth. Can J Microbiol 45:891–897
Hayman DC, Monk P (1982) Starter culture preparation for the induction of malolactic fermentation in wine. Food Technol Aust 34:16–18
Henick-Kling T (1995) Control of malolactic fermentation in wine: energetics, flavour modification and methods of starter culture preparation. J Appl Bacteriol Symp [Suppl] 79:29S–37S
Herrero M, Cuesta I, García LA, Díaz M (1999a) Changes in organic acids during malolactic fermentation at different temperatures in yeast-fermented apple juice. J Inst Brew 105:191–195
Herrero M, Roza C de la, García LA, Díaz M (1999b) Simultaneous and sequential fermentations with yeast and lactic acid bacteria in apple juice. J Ind Microbiol Biotechnol 22:48–51
Krieger SA, Hammes WP, Henick-Kling T (1992) Effect on medium composition on growth rate, growth yield, and malolactic activity of Leuconostoc oenos LoZH1-t7-1. Food Microbiol 9:1–11
Maicas S, Natividad A, Ferrer S, Pardo I (2000a) Malolactic fermentation in wine with high densities of non-proliferating Oenococcus oeni. World J Microbiol Biotechnol 16:805–810
Maicas S, Pardo I, Ferrer S (2000b) The effects of freezing and freeze-drying of Oenococcus oeni upon induction of malolactic fermentation in red wine. Int J Food Sci Technol 35:75–79
Miranda M, Ramos A, Veiga-da-Cuhna M, Loureiro-Dias MC, Santos H (1997) Biochemical basis for glucose-induced inhibition of malolactic fermentation in Leuconostoc oenos. J Bacteriol 179:5347–5354
Naouri P, Chagnaud P, Arnaud A, Galzy P, Mathieu J (1989) Optimization of the conditions for preparing bacterial cultures for malolactic bioconversion. J Biotechnol 10:135–150
Nault I, Gerbaux V, Larpent JP, Vayssier Y (1995) Influence of pre-culture conditions on the ability of Leuconostoc oenos to conduct malolactic fermentation in wine. Am J Enol Vitic 46:357–362
Nielsen JC, Prahl C, Lonvaud-Funel A (1996) Malolactic fermentation in wine by direct inoculation with freeze-dried Leuconostoc oenos cultures. Am J Enol Vitic 47:42–48
Rosen H (1957) A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys 67:10–15
Scott JA, Swaffield CH (1998) Observations on the influence of temperature, dissolved oxygen and juice source on stored alcoholic cider flavour development. Food Biotechnol 12:13–26
Tracey RP, Britz TJ (1989) The effect of amino acids on malolactic fermentation by Leuconostoc oenos. J Appl Bacteriol 67:589–595
Versari A, Parpinello GP, Cattaneo M (1999) Leuconostoc oenos and malolactic fermentation in wine: a review. J Ind Microbiol Biotechnol 23:447–455
Wibowo D, Fleet GH, Lee TH, Eschenbruch RE (1988) Factors affecting the induction of malolactic fermentation in wine with Leuconostoc oenos. J Appl Bacteriol 64:421–428
Acknowledgements
This work was financed by CICYT (project MCT-00-AGL-0597) of the Science and Technology Ministry, Spain. The authors acknowledge the assistance of Mónica Alvarez, Alejandro Alvarez, Covadonga Quirós, Samuel Marty and Estefanía Noriega and the support of the Asturian cidermaking company Sidra Escanciador, S.A. (Villaviciosa, Principado de Asturias, Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrero, M., García, L.A. & Díaz, M. Malolactic bioconversion using a Oenococcus oeni strain for cider production: effect of yeast extract supplementation. J IND MICROBIOL BIOTECHNOL 30, 699–704 (2003). https://doi.org/10.1007/s10295-003-0102-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-003-0102-9