Abstract
Purpose
Cashew Apple Juice (CAJ), rich in reducing sugars, is used to make a Geographical Indication alcoholic beverage named “Feni,” with a unique aroma. However, the alcohol yield from the reducing sugar (YP/S) of CAJ has been assessed to be as low as 0.24 g/g against the theoretical yield of 0.51 g/g. This poor yield of alcohol is mainly attributed to the inadequate monitoring and control of the fermentation process.
Methods
With the aim to improve ethanol production from CAJ, the current work involved identifying the potent strains, evaluating their alcohol production potential through sugar and ethanol tolerance studies, and studying the effect of C/N ratio on ethanol production by supplementing readily available urea as the nitrogen source.
Results
The isolates Saccharomyces cerevisiae and Pichia kudriavzevii showed reducing sugar tolerance up to 25% w/v but varied in their ethanol production capabilities. Furthermore, the kinetic models describing the ethanol inhibition on the growth rate and ethanol production revealed that the maximum concentration of ethanol beyond which the cell growth was completely inhibited (Pm) was 80 g/L, and the maximum ethanol production above which cells do not produce ethanol (P’m) was 96 g/L. P. kudriavzevii showed a 41.6% increase in the ethanol yield with a YP/S value of 0.34 g/g, ethanol concentration of 51 g/L and the productivity was 0.71 g/L/h.
Conclusion
The current study suggests that high sugar and ethanol-tolerant P. kudriavzevii could be used as a potent starter culture for producing alcohol and cashew Feni from cashew apple juice.
Statement of Novelty
Feni, a widely acclaimed alcoholic beverage, is produced by the distillation process of fermented cashew fruit juice. The enduring tradition holds a profound presence within the societal structure of the region and possesses significant cultural significance. Various individuals contribute at different stages of the Feni-making process, including family members, neighbours, and employees. In the current era characterised by modernity, it is imperative to have a solid understanding of the scientific principles that form the foundation of manufacturing processes. Therefore, researchers have drawn ideas from yeast-based ethanol production, implementing slight modifications to the beverage manufacturing process while preserving its core conventional techniques. This study seeks to assess the performance of two yeast strain isolates, namely Saccharomyces cerevisiae and Pichia kudriavzevii, in enhancing Feni production. These isolates were derived from fermented cashew apple juice. Tolerance testing were carried out to evaluate the suitability of sugar and ethanol for the purpose of ethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cashew nuts contribute to more than 80% of the total income of cashew farmers in India; however, net profit has declined in recent years due to rapid changes in the worldwide weather pattern. Studies reveal that most cashew farmers face challenges in disposing of significant quantities of waste generated during cashew processing, including cashew apple pulp, shell nut oil, and cashew shells. As a result, cashew producers have been motivated to explore the production of value-added products utilizing all cashew processing industry residues. The agro-industrial wastes from cashew industries hold the potential to serve as a substrate for the microbial synthesis of products such as mannitol, lactic acid, dextransucrase, and biosurfactants [1,2,3,4,5] and bio-ethanol [6, 7].
Conventionally many fruits like bananas, papaya, mango, pineapple, cashew apple, and grapes have been extensively utilized for alcohol production [8,9,10]. Their distinctive aroma get infused into the fermented liquor or distillate, creating unique fruit-based alcoholic beverages. The fibrous cashew apple produces highly nutrient-rich juice and contains a staggering amount of fermentable sugars, minerals, and vitamins [11]. In fact, Goa is the only state in India where the cashew apple is effectively utilized for “Feni” production through spontaneous fermentation using native yeast species found in the fruit. In addition to its primary use in producing this GI-tagged alcoholic beverage, a considerable portion of the distilled alcohol was also redirected towards hand-sanitizer applications, driven by the increased demand for sanitization products during the COVID-19 pandemic. Concurrently, there has been a substantial surge in the demand for alcohol as a sustainable fuel source. However, in recent years, the rising labor costs and marginal turnover from the Feni business have compelled some farmers to abandon their Feni-making operations and concentrate solely on processing cashew nuts. Also, a vast number of cashew farms having only rudimentary fermentation and distillation facilities for alcohol production from cashew apple juice could not compete with large-scale distillers, which maintain improved Feni-production facilities. According to a report, only a small proportion of the fruits are used for Feni production, with approximately 70% of the whole fruits being discarded as residual pulp, which is currently disposed of in nearby areas without any additional processing or treatment [12].
Among the various strains employed for ethanol production, Saccharomyces cerevisiae stands out as the most widely utilized. Before considering any yeast strain for industrial applications, it is crucial to conduct an evaluation of specific physiological characteristics, including sugar tolerance, ethanol tolerance, and invertase activity [13, 14]. Pichia-genus yeasts are renowned for their versatility in utilizing both pentose and hexose sugars as substrates for bioethanol synthesis [15]. In contrast, the industrial yeast Saccharomyces cerevisiae, commonly used in industry, lacks the capability to metabolize pentose sugars. This pivotal distinction positions Pichia as the superior choice for second-generation bioethanol production [16]. The selection of the fermentation microorganism plays a crucial role in achieving optimal bioethanol yield. As highlighted by Ansanay-Galeote et al. (2001), yeasts confront depletion during fermentation due to exposure to various environmental stressors, including high ethanol concentrations, increased temperatures, and osmotic pressure [17]. Also, the use of appropriate mathematical models, especially kinetic models, is essential for evaluating the performance of bioethanol in fermentation. Kinetic modeling follows an iterative process that increases in complexity and accuracy as assumptions and parameters are incorporated into the model.
The present study aims to evaluate the ethanol production capabilities of two yeast strains, Saccharomyces cerevisiae and Pichia kudriavzeviiisolated from fermented CAJ. This assessment involves comprehensive investigation into their sugar tolerance and ethanol tolerance. Additionally, the study explores the use of urea as a cost-effective nitrogen source to augment ethanol production. Furthermore, the study investigates the kinetics of ethanol production under anaerobic batch conditions, focusing specifically on the isolate Pichia kudriavzevii. The proposed model aims to elucidate the kinetic pattern of ethanol inhibition on the specific growth and ethanol fermentation rates.
Materials and Methods
Raw Material
Fresh cashew apple fruits (Red colour variety-Vengurla grade), specifically cultivated for yielding superior nut qualities, were obtained from the local farm Navika c°ashew nursery (15.75° N, 73.86° E), Mopa, North Goa. The nuts were detached, and the fruits (peduncle) were carefully placed in plastic boxes and transported to the laboratory. Upon arrival at the lab, the fruits were rinsed thoroughly several times in tap water to remove the dirt and other impurities. The fruits were then cut into small pieces and the juice was extracted by using juice blender (Bajaj, 500 W). The fluorescent dye (Kadamba Green 264) used to visualize the yeast strains was kindly provided by Fluoresight Bioprobes Pvt Ltd, Goa.
Isolation of Yeast Strains
To isolate the yeast strain from the fermented CAJ, a 1 mL sample of the juice from a test tube was carefully transferred into 9 mL of normal saline solution (0.85% w/v). Dilutions up to 10-6 (CFU/mL) were obtained by sequentially adding 1 mL from the previous dilutions to the next. Subsequently, 0.1 mL of sample obtained from 10-6 (CFU/mL) dilution was uniformly spread onto Potato Dextrose Agar (PDA; Hi-media) by spread-plating technique. The plates were kept in Scigenics, Model: Orbitek Incubator (Chennai, India) at 28 °C for 2 days. The colonies that appeared on agar plates were sub-cultured for further analysis and characterization.
Morphological Characterization by Confocal Microscope Image
Isolated colonies of yeasts were assessed for their colony characteristics, mainly shape, color, margin, texture [18], and microscopic characteristics (yeast cell shape, pseudohyphae, and presence/absence of budding) [19] by post-staining method. Briefly, the yeast cultures were evaluated for log phase growth. Serial dilutions were done until the appropriate cell concentration (10-6) was reached. To obtain the cell pellet, the grown culture was centrifuged at 7000 rpm for five minutes and the supernatant was discarded. The obtained pellet was dissolved in 50-100µl of deionized water. A thin smear was prepared and gradually warmed over a flame and cooled to room temperature on a clean glass slide. A 100 µM working solution of Kadamba Green (KG) 264 was used to stain the cell smear for 30 min at room temperature and the surplus staining solution was discarded. The stained slide was air dried and mounted with glycerol using the cover slip. A 405nm violet excitation laser was used for confocal microscopy (Model: Olympus Corporation FV3000) under 100x magnification with zoom factor. The emission filter was set to 500-550nm.
Identification by DNA Sequencing
The selected yeast isolates DNA was extracted, and the gene fragment was subjected to amplification by using PCR. In 25µL of the PCR reaction was contained 12.5 µL of PCR master mix (Sigma), 50ng of DNA template, and 50 pmol of each primer ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATG-3’) were used [20]. Amplified PCR products were purified and sequenced commercially (Bioresource Biotech Pvt Ltd, Pune). The obtained sequences were BLAST searched in the NCBI database. The isolates that showed more than ≥ 0.98 identity values were confirmed as yeast species [21].
Estimation of Reducing Sugars by DNS Method
The reducing sugar (glucose) concentration was estimated by using DNS assay [22]. Briefly, 1.5 mL of the sample was added to 1.5 mL of DNS reagent. The solution was mixed thoroughly and boiled for 5 min at 100 ̊ C, and subsequently added 0.5 mL of Rochelle salt and kept on ice for 5 min. The total amount of reducing sugars was estimated using a UV spectrophotometer at 540 nm.
Estimation of Ammonia, Nitrate and Nitrite Content
The content of ammonium, nitrate, and nitrite was assessed using ammonium and nitrate strips (Quantofix test strip) and reflectometry. To establish an exact concentration measurement, each strip was immersed in the supernatant that had been adequately diluted, bringing it within the mid-range [23].
Effect of Optimized Carbon and Nitrogen Ratio on Microbial Growth and Ethanol Production of potent ethanol tolerant yeast isolates
The fresh CAJ used in the current study showed a maximum NH4 concentration of 1250 mg/L for a maximum reducing sugar concentration of about 150 g/L, which results in the initial C/N ratio of about 60:1. Thus a protocol was employed to investigate the effects of varying C/N molar ratios (60:1, 50:1, 40:1, 20:1, and 10:1) on biomass concentration and ethanol production for the potent ethanol tolerant of isolates 1 and 2 grown in fermented cashew juice. The nitrogen content in the juice was varied by supplementing appropriate quantities of urea, since it is a widely available and inexpensive as compared to other organic nitrogen source such as peptone, tryptone, and yeast extract [24]. The kinetics of biomass growth and the DNS analysis were carried out at the optimal C/N ratio, resulting in maximum ethanol production. The alcohol content was measured using Electronic Ebulliometer, BulTech 2000 (Bulgaria).
Evaluating Ethanol Tolerance of Yeast Strains
The medium used had the following composition (for 1 L medium): 10 g glucose, 1.5 g yeast extract, 2.5 g NH4Cl, 5.5 g Na2HP04, 3 g KH2P04, 0.25 g MgSO4, 0.01 g CaCl2, 5 g citric acid, and 2.5 g sodium citrate [25]. The medium was sterilized at 121 °C for 15 min in an autoclave and cooled. Absolute ethanol was added, constituting varying percentages (2.5%, 5%, 7.5%, 10%, 12.5% & 15%,v/v). A flask without ethanol served as a control. The flasks were inoculated with 100 µL of 24 h actively growing cell suspension of respective cultures and incubated in Scigenics, Model: Orbitek (Chennai, India) at 30 °C for 96 h in an orbital shaker incubator set at 150 rpm. The optical density (OD) at A600nm of each flask was measured using a UV–Vis spectrophotometer (Model: UV-2600 Make: Shimadzu, Japan) using sterile medium as the blank.
An increase in the optical density with the increasing incubation time of the culture was considered as the evidence of growth. The concentration at which the growth of the yeast was just inhibited was considered the highest concentration of ethanol that the strain could tolerate [11, 13].
Sugar Tolerance of Yeast Strains
The isolated yeast strains were screened for sugar tolerance in the medium used had the following composition (for 1 L medium): 1.5 g yeast extract, 2.5 g NH4Cl, 5.5 g Na2HP04, 3 g KH2P04, 0.25 g MgSO4, 0.01 g CaCl2, 5 g citric acid, and 2.5 g sodium citrate [25] and glucose in varying concentrations of 100 g, 150 g, 200 g, and 250 g. The medium was sterilized at 121 °C for 15 min in an autoclave and cooled.
To a sterile medium, the cell concentration of 1 × 106 cfu/ml suspension (12 h old culture) was added to an individual flask containing different glucose concentrations. The flasks were incubated in an orbital shaker incubator at 150 rpm, 30 °C for 96 h. The absorbance at A600 nm of each flask were measured using a UV–Vis spectrophotometer (Model: UV-2600 Make: Shimadzu, Japan) for every 12 h up to a maximum observation period of 96 h.
Kinetic Models for Ethanol Production (Synthetic Medium)
The modified Monod model was used to evaluate the effect of product inhibition on growth and fermentation rates described by Luong 1985 [25]. The following model equations are as follows:
For P ≠ 0, the Eqs. (1) and (2) can be rearranged as follows:
Where,
P – ethanol concentration (g/L).
Pm - ethanol concentration above which cells do not grow (g/L).
\(\text{P}{\prime }\)m - ethanol concentration above which cells do not produce ethanol (g/L).
µi – specific growth rate of microorganisms in the presence of ethanol (l/h).
µo – maximum specific growth rate of microorganisms at zero ethanol concentration (l/h).
υi – specific rate of ethanol production in the presence of ethanol (g /g-1.h).
υo – maximum specific rate of ethanol production at zero ethanol concentration (g /g-1.h).
α and β are the dimensionless constant defined in equations.
Fermentation Efficiency
Microorganisms convert fermentable sugars into ethanol and carbon dioxide during alcohol fermentation. Equation 5 demonstrates the general chemical formula for alcoholic fermentation using glucose as the primary carbon substrate.
For complete conversion, one mole of glucose leads to two moles of ethanol and two moles of carbon dioxide, according to Eq. (5). The yield of ethanol to consumed sugar (g/g) was defined as:
The ethanol productivity (g/.L.h) was calculated as the ratio of ethanol concentration (g/L) at the fermentation time (t, h) as given in Eq. (7)
Results and Discussion
Isolation, Identification, and Characterization of Potent Yeast Strain
In recent years, genetic breeding initiatives have significantly improved the reducing sugar concentrations in a number of fruits; however, during the fermentation process the yeast cells should be able to tolerate such a high concentration of reducing sugars for optimal ethanol production [26]. The sugar and ethanol tolerance levels vary from genus to genus of yeasts and hence there is a strong need to isolate a potent ethanol tolerant yeast strains for enhancing the ethanol yield through fermentation process. Thus, with an objective to improve the ethanol yield in the final fermented CAJ. Based on the morphological characteristics depicted in Fig. 1, it appears that the two isolated strains are yeast species and tested for physiological properties such as ethanol and sugar tolerance in this study. The chosen yeast exhibited smooth, white colonies with glossy surfaces when grown on Potato Dextrose Agar (PDA). The microscopic features consisted of oval cells exhibiting budding. The phylogenetic tree analysis revealed that the isolates 1 and 2 under this study were identified as Pichia kudriavzevii and Saccharomyces cerevisiae (Figs. 1 and 2) with a similarity percentage of identification over 99%. This finding aligns with the existing literature, as the yeasts isolated from oranges in the greater Mekong subregion had same morphological traits [27]. Undoubtedly, yeast is widely distributed and has the ability to thrive on various substrates [14]. To confirm these results, a polymerase chain reaction (PCR) was carried out and the amplified PCR products were run in the agarose gel. Figure 2 illustrates the amplicon photograph of both the isolates with respect to the existing genetic database that shows similar bands of 517 bp and 797 bp for P. kudriavzevii and S. cerevisiae respectively with the reference ladder of 100 bp in the 1% agarose gel. While Saccharomyces cerevisiae has been reported to be the potent ethanol producer by a number of researches [13, 14, 26]. The P. kudriavzevii isolate has become a promising substitute for the commonly employed S. cerevisiae in the production of second-generation bioethanol, attracting substantial attention. Significantly, it has exceptional inherent resilience to stress caused by lignocellulosic inhibitors, osmotic stress, and high temperatures. Furthermore, this specific yeast strain exhibits the ability to attain increased ethanol production while effectively overcoming the negative effects caused by high ethanol concentrations. In recent research, a strain of the thermotolerant yeast P. kudriavzevii was tested for its capacity to ferment steam-exploded wheat straw hydrolysate into high-gravity bioethanol. In lignocellulosic hydrolysates, the isolated strain enhanced ethanol production by ≥ 24% [27]. In order to boost Feni production, we have also employed the strain P. kudriavzevii in this investigation, which was isolated from fermented cashew apple juice.
Ethanol Tolerance Studies
In this study, the mineral medium was used to evaluate the ethanol tolerance of yeast strains isolated from fermented cashew apple juice. From an economic standpoint, the ability to withstand various environmental stresses, such as osmolarity and ethanol, is paramount when selecting yeast strains for effective ethanol fermentation. Since the plasma membrane of unicellular organisms plays a vital role in their interactions with the environment, its properties are likely to significantly influence the cells’ tolerance to the changes that occur during fermentation. Hence, the ethanol tolerance capacity of a yeast strain is inherently linked to its specific type [11, 13, 14].
In the present study, the maximum percentage of ethanol (v/v) which inhibited the growth of Pichia kudriavzevii and Saccharomyces cerevisiae were evaluated to be 10% and 5%, respectively. A 10% ethanol concentration completely inhibited the growth of the Pichia kudriavzevii isolates. This is because ethanol inhibits yeast growth, cell division, and reduces cell volume and specific growth rate. Furthermore, high concentrations of ethanol diminish cell vitality and increase cell mortality. When selecting yeast strains for ethanol fermentation, it is crucial to emphasise their ability to withstand various environmental challenges, such as osmolarity and ethanol. The plasma membrane of unicellular organisms plays a vital role in their interactions with the environment, as it is expected to significantly affect the cells’ resistance to the changes that take place during fermentation. Hence, the ethanol tolerance capability of a yeast strain is inherently linked to its specific type [14]. Figures 3 and 4 illustrate the biomass growth of the isolates P. kudriavzevii and S. cerevisiae in increasing ethanol concentration. As a general trend, increase in ethanol concentration decreased the growth of the biomass and increased the lag phase in both the strains.
The ethanol tolerance by cells is correlated to their ability to withstand osmotic pressure. Osho (2005) demonstrated this aspect of strain tolerance using four strains isolated from fermenting cashew apple juice, which showed significant growth in a medium containing 9% (v/v) ethanol. By employing such efficient yeast isolates with high ethanol tolerance to enhance ethanol yields during fermentation, it is possible to reduce distillation costs and consequently increase the overall profitability of the process [28]. In another investigation, Techaprin et al. (2017) found that most of the yeast isolates were able to withstand ethanol concentrations of up to 10% (v/v). The ethanol tolerance of the P. kudriavzevii strain identified in this current study aligns with the previously published findings [29] and hence should be suitable for producing alcoholic beverages like double-distilled Feni. P. kudriavzevii showcases notable resilience in the face of diverse stressors, including osmotic stress [30], thermal stress [31], and challenges posed by lignocellulosic inhibitors [32]. Furthermore, this yeast boasts impressive ethanol yields and exceptional resistance to end-product inhibition induced by high ethanol concentrations.
Ethanol tolerance in yeast isolates has been reported in the range of 15–20% (v/v). In recent study, wild yeast strains from Ethiopian sugar factories were isolated for bioethanol production using sugarcane molasses as the substrate. Seven strains, including Meyerozyma caribbica (MJTm3, MJTPm4, SHJF), S. cerevisiae TA2, and Wickerhamomyces anomalus (MJTPm2, 4m10, HCJ2F), showed ethanol tolerance up to 18%. Strain MJTm3 of M. caribbica exhibited an even higher tolerance of 20% [33]. On the other hand, seven yeast strains were isolated from locally fermented foods in Indonesia. Among them, Strain F08b displayed exceptional resilience to high temperatures and ethanol, withstanding up to 17% ethanol concentration. Another isolate, F01, thrived in high ethanol environments, reaching an impressive 18% ethanol tolerance level [34]. In another investigation, the experiments were conducted on studying thermotolerant yeast strains to improve the production of ethanol at high temperatures. A total of 222 yeast isolates were isolated from soil samples collected in sugarcane fields in Thailand. Two yeast strains, specifically P. kudriavzevii NUPHS33 and NUPHS34, shown exceptional resistance to ethanol, tolerating concentrations as high as 15% (v/v). Additionally, these strains indicated the capacity to thrive at temperatures reaching 45 °C. Remarkably, these strains demonstrated markedly enhanced ethanol production capacities in comparison to the industrial strain S. cerevisiae TISTR5606 [30]. These findings highlight the potential of these yeast strains for robust bioethanol production in industrial applications.
In our study, since P. kudriavzevii showed higher ethanol tolerance than S. cerevisiae, it was chosen for further model-based prediction of important kinetic parameters towards improved fermentation process. The influence of alcohol concentration on specific growth rate of P. kudriavzevii is presented in Table 1.
Sugar Tolerance Studies
Sugar utilization during fermentation is an essential physiological property of yeast strains used for industrial ethanol production [11]. Also, depending upon the type of cultivar, the reducing sugar concentration in the juice of freshly harvested cashew apples has been found to be in the range of 100 to 150 g/L, and this demands the study on sugar tolerance of the yeast cells for a successful fermentation. In the current study, increasing the sugar concentration, decreased the growth rate of the biomass but increased the final ethanol concentration (Fig. 5a and b). Although two isolates could withstand the highest sugar concentration (25%, w/v), the growth observed in the 25% sugar-containing medium was much less than in the medium containing 15% sugar concentrations.
At high sugar concentrations, increase in osmotic pressure of fermenting medium inhibits growth of yeasts. During fermentation, this abrupt shift in physiological conditions places yeast cells under water and ethanol stress. The highest growth was observed at 10% (w/v) sugar concentration within the first 12 h of incubation for both isolates. The tolerance of isolates was reduced with increasing sugar concentrations—the present investigation also agrees with other reports [11,12,13]. Yeasts utilize sugars as a significant source of carbohydrates to produce alcohol and acid during the incubation period. In this study, the inhibitory effect of sugar was observed on both isolates, irrespective of the fact that sugar is the chief carbon source required for yeast growth. As a result, increased sugar concentrations showed a reduction in the optical density of the culture. Although, the 25% sugar concentration produced a high alcohol content (8.51% v/v) with the isolate Pichia kudriavzevii however from the (Fig. 6), it is noted that the YP/S value of 0.027 g/g at 25% (w/v) sugar concentration was significantly less as compared to YP/S of 0.037 g/g at 15 % (w/v). The influence of varying glucose concentration on specific growth rate of P. kudriavzevii is presented in Table 2.
In a recent study, researchers examined the sugar tolerance of osmotolerant yeasts and yeast-like moulds sourced from apple orchards and apple juice processing facilities in China. They identified a diverse pool of 66 osmotolerant isolates, representing 23 distinct species. Among these, Kluyveromyces marxianus, Hanseniaspora uvarum, Saccharomyces cerevisiae, Zygosaccharomyces rouxii, Candida tropicalis, and Pichia kudriavzevii were particularly prominent. These strains demonstrated a remarkable capacity to thrive in varying concentrations of glucose, similar to the sugar concentration of 50% (w/v) typically found in apple juice products. Notably, Zygosaccharomyces rouxii stood out as the most robust, displaying an impressive tolerance to a high glucose concentration of 70% (w/v). This study highlights the remarkable adaptability of these strains to challenging, sugar-rich environments [35].
One crucial reason for screening yeasts for ethanol and sugar tolerance is that not all yeast isolates withstand higher levels of ethanol and could produce ethanol at the required concentrations. The isolate P. kudriavzevii showed tolerance to moderate to higher concentrations of sugar and ethanol when compared to the isolate S. cerevisiae. Hence the isolate P. kudriavzevii can be considered a suitable organism for further evaluating the suitability for producing Feni (an alcoholic beverage from fermented cashew apple juice) with higher alcohol content.
Ethanol Inhibition Kinetics of Pichia Kudriavzevii
The experimental data for ethanol and sugar tolerance studies are fitted in the model equations for biomass growth and ethanol production rate mentioned under Sect. 2.9 of this paper and the obtained kinetics parameters are estimated and compared with the reported literature data as shown in Table 3.
Ghose & Tyagi established the kinetic relationships of growth, substrate utilization, and product formation during ethanol fermentation of cellulose hydrolysate and predicted the maximum ethanol concentration above which cells inhibit the growth (Pm) and alcohol production rate (P’m). The proposed model predicted that the maximum ethanol concentration above which cells do not grow was 80 g/L. This Pm value was compared with the literature data and it is 87 g/L [36]. The suggested model also predicted that 96 g/L would be the maximum ethanol production level over which cells would cease to produce ethanol. The value of P’m that Ghose and Tyagi anticipated was 114 g/L [36]. The fact that the best-fit value for P was almost 1, showing a straightforward linear relationship between vi/vo and P, with an average R2 value of 0.972, is noteworthy (Fig. 7a).
Bazua and Wilke’s data reported that the estimated P and P’m values were 107.8 and 114 g/L and obtained best-fit values of 93.84 and 93.1 g/L, respectively. The estimated values of the empirical constants were 1.65 and 1.81, respectively. With an R2 value of 0.988, the best-fit P value in the current research was very close to 1, demonstrating a linear connection between µi/µo and P (Fig. 8a). It was determined that the obtained empirical constants α and β were found to be 0.99 and 1.05, respectively. Straight-line results were also observed when ln (1-vi/vo) or ln (1- µi/µo) vs. ln P with R2 values of 0.76 and 0. 982, respectively (Figs. 7b and 8b). The predicted Pm and P’m values of this study obtained from the modified Monod equation are presented in Table 4.
In a recent study, Ghods (2020) performed the kinetics of bioethanol production from xylose using Pichia stipitis with the development of a MATLAB mathematical model (based on the combined Andrews and Levenspiel’s model with oxygen limitation and inhibition terms included), considering the effects of substrate, oxygen, biomass, and product concentrations on sugar utilization, growth rate, and ethanol formation. Different aeration levels (0.0, 0.1, and 0.2 vvm) and different initial biomass concentrations (0.3, 1.5, and 3 g L-1) were tested, in which the combination of aeration at 0.1 vvm and a biomass concentration of 3 g/L resulted in the highest ethanol yield and productivity of 0.45 g/g and 0.75 g/L/h. respectively [39]. The flocculant yeast S. cerevisiae CCA008, cultured on cashew apple juice, was used to produce ethanol in another investigation, and the effects of stirring speed, substrate, starting cell concentrations, and temperature were investigated. Results have demonstrated that the model herein proposed was capable of accurately describing the ethanol production by S. cerevisiae flocculant yeast taking into account the influence of operational conditions like substrate concentration of 70–170 g/L temperature from 26 to 42 °C, especially the effect of the stirring speed from 80 to 800 rpm with an apparent mean error (9.77%) for the fermentation rate [40].
Costa et al. (2012) conducted a study to assess the efficiency of batch and fed-batch fermentation using the strain S. cerevisiae (F13A) in an aerated stirred tank reactor (STR) for producing ethanol from concentrated carob pulp extracts. The study also examined the kinetics of sugar consumption and the impact of ethanol inhibition. In the fed-batch fermentation experiments, the highest concentration of ethanol reached was 130 g/L. The theoretical yield was between 0.47 and 0.50 g/g, and each batch run resulted in an ethanol concentration of 100-110 g/L. The conversion efficiency was 90–95% [38].
The proposed model suggested that the ethanol concentration at which cells do not produce ethanol was slightly higher than that above which cells do not grow in the medium. P and P’m values were estimated to be 80 and 96 g/L, respectively. In contrast, study reported that the value of P’m was predicted to be 240 g/L. However, the value of P was not obtained [37]. Such information will be valuable in determining whether the cells can produce ethanol even after they stop growing. Therefore, the physiological circumstances of the microorganism and the medium in which they can grow and produce ethanol are the causes of the variations in the given experimental results. The suggested kinetic model seems to be beneficial for anticipating the rates of microbial growth and ethanol production.
Effect of Optimized C/N Ratio on the Microbial Growth and Ethanol Production from CAJ using the Potent Ethanol Tolerant Strain P. Kudriavzevii
The use of affordable carbon sources is currently viewed as a practical approach. As a result, the raw materials needed for industrial ethanol production must satisfy several criteria, including affordability, low contaminant levels, quick fermentation rates, high yields, and constant availability [2]. This study investigated the effect of various C/N ratios on the biomass concentration and the ethanol production of the isolate P. kudriavzevii from fermented cashew apple juice (CAJ). The organic nitrogen source urea was supplemented with CAJ for ethanol production since the juice has a low protein content. However, they are rich in reducing sugars, minerals and vitamins [24].
On an average, microbes consisted of about 50% carbon and 6–13% nitrogen, with carbon serving as the primary substrate for cell development. Meanwhile, nitrogen sources played a crucial role in the production of enzymes, proteins, and nucleic acids, all of which are essential for the formation of new cells [41]. Urea, Yeast extract, tryptone, and peptone are examples of organic nitrogen sources, whereas ammonium sulfate, ammonium nitrate, and ammonium chloride are examples of inorganic nitrogen sources. Since the total nitrogen content in the cashew apple juice was much less 600 mg/L obtained in the present study, the addition of nitrogen supplements in the juice could enhance the production of ethanol. The adequate nitrogen levels in the medium are crucial as they provide the necessary components for yeasts to synthesise proteins, carry out enzymatic reactions, and maintain overall cellular health. These factors collectively enhance the efficiency of ethanol production during fermentation. Nevertheless, it is crucial to uphold an appropriate balance, since both excessive and inadequate nitrogen levels can have an adverse impact on fermentation and the production of ethanol [41].
In a study, the author reported that Carbon-to-nitrogen ratios (C/N) ranging from 15 to 70 on a mass basis have been employed for various aerobic and anaerobic microorganisms. However, deviating from this ideal range can lead to complications. A high C/N ratio results in a nitrogen deficiency, thereby impeding the growth rate due to an inadequate number of cells to sustain an active microbial biomass. Conversely, a low C/N ratio with high nitrogen content can lead to the evolution of toxic ammonia, effectively halting the fermentation process. This emphasizes the crucial role of both macro and micronutrients in enabling proper microbial metabolism, necessitating their presence in precise ratios and concentrations within the substrate. Additionally, it underscores the significance of the initial C/N ratio as a pivotal factor influencing growth and production rates [41].
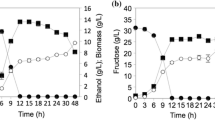
The present work investigated the effect of various C/N ratios, such as 60:1, 50:1, 40:1, 20:1, and 10:1, on the biomass concentration and ethanol production by P. kudriavzevii. Results revealed that the ethanol concentration of 6.78% (v/v) was obtained when the juice was supplemented with the urea at the optimal C/N molar ratios of 40:1. Figure 9(a &b) shows the effect of C/N ratio on the biomass and reducing sugar concentration and it is found that 40:1 (C/N) ratio gives better production. The C/N ratio significantly influences ethanol production in microbial fermentation. It plays a vital role in promoting the efficient growth and reproduction of yeast by aiding the conversion of the carbon source into ethanol. Optimizing the C/N ratio not only reduces inhibitory effects but also improves the nutrient utilization, resulting in increased ethanol production per substrate. The ideal ratio mentioned above enhances the yeast’s ability to withstand stressors that arise during fermentation, hence ensuring a steady and continuous increases in ethanol production. On the other hand, an extremely low C/N ratio can result in increased cell mass production but a simultaneous decrease in ethanol concentration. As a result, maintaining an optimal C/N ratio is crucial for reducing nitrogen usage, maximizing ethanol yield, and promoting cost-effectiveness in the fermentation process [40, 41, 44].
Effect of C/N ratio with P. kudriavzevii isolate (a) Growth curve of optimized 40:1 (C: N) ratio and the respective reducing sugar concentration. The points in the graph are the average of the two experimental values with standard deviation of ± 5% limit (b) Ebulliometer analysis for the effect of different C/N ratios on Ethanol production
In recent work, the researcher reported that the addition of an inorganic nitrogen source in the form of ammonium sulfate to fresh longan juice (FLJ) could bring the C/N molar ratio down to 39.07 ± 0.48 or decrease by 30.12 ± 5.77 times with non-supplemented FLJ. Culturing Candida tropicalis TISTR 5306 in this medium could produce 12.9 ± 0.5 g/L dried biomass and 22.3 ± 1.1 g/L ethanol [42]. In 2019, the same research group experimented with cultivating the isolate C. tropicalis TISTR 5306 with FLJ supplemented with ammonium sulfate as the nitrogen source for ethanol production. Results revealed that 24.0 ± 1.1 g/L of ethanol concentration was obtained from the optimal C/N molar ratio of 21.88 ± 0.20 [43].
Manikandan and Viruthagiri (2010) evaluated the coculture fermentation of Aspergillus niger and S. cerevisiae in batch cultivation using 6% w/v of tapioca flour as the carbon source and peptone and yeast extract as the nitrogen sources. Investigating the effects of C/N ratios ranging from 3.5 to 35.2 on ethanol and biomass concentration levels, the ideal C/N ratio of 35.2 was found. The maximum ethanol concentration was 8.85 g/L, while the maximum biomass concentration was 15.3 g/L at this C/N ratio [44]. In the current study, the isolate P. kudriavzevii produces the most ethanol concentration at a maximum rate of 51 g/L with a YP/S value of 0.34 g. g− 1, the productivity of 0.71 g/L/h with a theoretical conversion efficiency of 0.51, and an optimal C/N ratio of 40:1. These findings demonstrated the distinction between the facultatively fermentative nature of Candida yeasts and the Crabtree-positive S. cerevisiae in the partitioning of the C—metabolism between cell growth and fermentative products like ethanol [42]. The various parameters of ethanol production by P. kudriavzevii using CAJ as a carbon source supplemented with urea are presented in Table 5.
* The efficiency (η) was calculated based on the theoretical yield value of 0.51 g/g and increase in the ethanol yield obtained in this study with a YP/S value of 0.34 g/g
Conclusion
The vast potential lies within cashew apples and the residual pulp waste generated after juice extraction. These resources can be harnessed effectively through microbial fermentation, yielding a range of valuable products such as bioethanol, hydrolytic enzymes, lactic acid, biosurfactants, wine, and Feni. Extensive literature research and on-site visits to cashew farming sites make it evident that achieving a transformative improvement in the industry hinges on adopting holistic cultivation practices and efficient waste management, especially concerning cashew apples. Furthermore, there is an urgent need for a substantial upgrade in processing techniques to enhance the quality, marketability, and international export potential of Feni production, which serves as the mainstay of India’s cashew processing sector.
In this research, potent ethanol tolerant yeast strains were isolated from fermented cashew apple juice (CAJ) and characterized using conventional morphological methods. The homology and phylogenetic analysis revealed that the identified isolates belonged to P. kudriavzevii and S. cerevisiae. Notably, these strains demonstrated a remarkable tolerance to stressful conditions, including ethanol and sugar tolerance tests. The isolate P. kudriavzevii exhibited significantly higher ethanol and sugar tolerances, viz., 10% (v/v) and 25% (w/v), respectively. As a result, P. kudriavzevii presents itself as a promising candidate for ethanol production under high osmotic pressure conditions. Moreover, optimizing the cultivation of P. kudriavzevii in cashew apple juice with urea supplement using a C/N molar ratio of 40:1 resulted in significant increase in ethanol titres. This configuration produced a maximum ethanol concentration of 51 g/L, with a YP/S value of 0.34 g/g, a productivity rate of 0.71 g/L/h, and a theoretical conversion efficiency of 0.51 g/g. Furthermore, the study explored the kinetics of ethanol inhibition during alcohol fermentation. The findings revealed that the maximum ethanol concentration, beyond which cell growth was inhibited, was 80 g/L. Additionally, the threshold for maximum ethanol production, beyond which cells did not produce more ethanol, was found to be 96 g/L. In conclusion, this study suggests that employing mixed cultures, such as Saccharomyces cerevisiae and non-Saccharomyces yeasts supplemented with organic nitrogen sources, may offer a feasible approach to enhance ethanol yield derived from fermented cashew apple juice.
Data Availability
Not Applicable.
References
Fontes, C.P.M.L., Honorato, T.L., Rabelo, M.C., Rodrigues, S.: Kinetic study of mannitol production using cashew apple juice as substrate. Bioprocess. Biosyst Eng. 32(4), 493–499 (2009). https://doi.org/10.1007/s00449-008-0269-6
Silveira, M.S., Fontes, C.P.M.L., Guilherme, A.A., Fernandes, F.A.N., Rodrigues, S.: Cashew Apple Juice as substrate for lactic acid production. Food Bioprocess. Technol. 5(3), 947–953 (2012). https://doi.org/10.1007/s11947-010-0382-9
Honorato, T.L., Rodrigues, S.: Dextransucrase stability in cashew apple juice. Food Bioprocess. Technol. 3(1), 105–110 (2010). https://doi.org/10.1007/s11947-008-0053-2
Rocha, M.V.P., Oliveira, A.H.S., Souza, M.C.M., Gonçalves, L.R.B.: Natural cashew apple juice as fermentation medium for biosurfactant production by Acinetobacter calcoaceticus. World J. Microbiol. Biotechnol. 22(12), 1295–1299 (2006). https://doi.org/10.1007/s11274-006-9175-8
Rocha, M.V.P., et al.: Production of biosurfactant by Pseudomonas aeruginosa grown on cashew apple juice. Appl. Biochem. Biotechnol. 137–140 (2007). https://doi.org/10.1007/s12010-007-9050-6
Rocha, M.V.P., Rodrigues, T.H.S., de Albuquerque, T.L., Gonçalves, L.R.B., de Macedo, G.R.: Evaluation of dilute acid pretreatment on cashew apple bagasse for ethanol and xylitol production. Chem. Eng. J. 243, 234–243 (2014). https://doi.org/10.1016/j.cej.2013.12.099
Pinheiro, Á.D.T., et al.: Optimization and scale-up of ethanol production by a flocculent yeast using cashew apple juice as feedstock. Brazilian J. Chem. Eng. 37(4), 629–641 (2020). https://doi.org/10.1007/s43153-020-00068-0
Velásquez-Arredondo, H.I., Ruiz-Colorado, A.A., De Oliveira, S.: Ethanol production process from banana fruit and its lignocellulosic residues: Energy analysis. Energy. 35(7), 3081–3087 (2010). https://doi.org/10.1016/j.energy.2010.03.052
Parameswari, K., Hemalatha, M., Priyanka, K., Kishori, B.: Isolation of yeast and ethanol production from papaya (Carica papaya) and grape (Vitis vinifera) fruits, Int. J. Sci. Eng. Res, no. February, 2015, [Online]. Available: http://www.ijser.org/researchpaper%5CIsolation-of-yeast-and-ethanol-production-from-papaya.pdf
Degner, S.F., et al.: Golden wine produced from mixed juices of passion fruit Int. J. Food Sci. Technol, 49(December), 764–774 (2010)
Ranade, I.: Identification and screening of the Yeast Flora from Indian Cashew Apple for Sugar and ethanol tolerance. Int. J. Biotechnol. Wellness Ind. 259–265 (2012). https://doi.org/10.6000/1927-3037.2012.01.04.6
S R, V.K., Vermani, A., Rangarajan, V., Manjare, S.D.: Production of Feni Beverage from Cashew Apple and Assessment of Bioethanol Production Potential from the residual pulp, pp. 1–28, (2023). https://doi.org/10.1007/978-3-031-04195-2_185-1
Osho, A.: Ethanol and sugar tolerance of wine yeasts isolated from fermenting cashew apple juice. Afr. J. Biotechnol. 4(7), 660–662 (2005). https://doi.org/10.5897/AJB2005.000-3119
Kechkar, M., et al.: Isolation and identification of yeast strains from sugarcane molasses, dates and figs for ethanol production under conditions simulating algal hydrolysate. Brazilian J. Chem. Eng. 36(1), 157–169 (2019). https://doi.org/10.1590/0104-6632.20190361s20180114
Mussatto, S.I., Machado, E.M.S., Carneiro, L.M., Teixeira, J.A.: Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy. 92, 763–768 (2012). https://doi.org/10.1016/j.apenergy.2011.08.020
Radecka, D., Mukherjee, V., Mateo, R.Q., Stojiljkovic, M., Foulquié-Moreno, M.R., Thevelein, J.M.: Looking beyond Saccharomyces: The potential of non-conventional yeast species for desirable traits in bioethanol fermentation. FEMS Yeast Res. 15(6), 1–13 (2015). https://doi.org/10.1093/femsyr/fov053
Ansanay-Galeote, V., Blondin, B., Dequin, S., Sablayrolles, J.M.: Stress effect of ethanol on fermentation kinetics by stationary-phase cells of Saccharomyces cerevisiae. Biotechnol. Lett. 23(9), 677–681 (2001). https://doi.org/10.1023/A:1010396232420
Spencer, J., et al.: Yeast identification: Reassessment of assimilation tests as sole universal identifiers. Lett. Appl. Microbiol. 53(5), 503–508 (2011). https://doi.org/10.1111/j.1472-765X.2011.03130.x
Haw, B.P., Asma, I., Eugene, O., Sasidharan, S.: Phenotyping identification of candida albicans for the production of in house helicase for nucleic acid-based detections for fast diagnosis. Res. J. Pharm. Biol. Chem. Sci. 4(2), 576–583 (2013)
Taverna, C.G., et al.: Comparative analyses of classical phenotypic method and ribosomal RNA gene sequencing for identification of medically relevant Candida species. Mem. Inst. Oswaldo Cruz. 108(2), 178–185 (2013). https://doi.org/10.1590/0074-0276108022013009
Barghouthi, S.A.: A Universal Method for the identification of Bacteria based on General PCR primers. Indian J. Microbiol. 51(4), 430–444 (2011). https://doi.org/10.1007/s12088-011-0122-5
Miller, G.L.: Use of Dinitrosalicylic Acid Reagent for determination of reducing Sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
Ganesan N.G., Rangarajan, V.: A kinetics study on surfactin production from Bacillus subtilis MTCC 2415 for application in green cosmetics. Biocatal. Agric. Biotechnol. 33, 102001 (2021). https://doi.org/10.1016/j.bcab.2021.102001
Honorato, T.L., Rabelo, M.C., Gonçalves, L.R.B., Pinto, G.A.S., Rodrigues, S.: Fermentation of cashew apple juice to produce high added value products. World J. Microbiol. Biotechnol. 23(10), 1409–1415 (2007). https://doi.org/10.1007/s11274-007-9381-z
Luong, J.H.: Ethanol inhibition in Alcohol Ferment. Biotechnol. Bioeng. XXVII, 280–285 (1985)
Kannan, V., Rangarajan, V., Manjare, S.D., Pathak, P.V.: Microbial production of value-added products from cashew apples - an economical boost to cashew farmers. J. Pure Appl. Microbiol. 15(4), 1816–1832 (2021). https://doi.org/10.22207/JPAM.15.4.71
Hoppert, L., Kölling, R., Einfalt, D.: Investigation of stress tolerance of Pichia kudriavzevii for high gravity bioethanol production from steam–exploded wheat straw hydrolysate. Bioresour Technol. 364 (October, 2022). https://doi.org/10.1016/j.biortech.2022.128079
Chandrasena, G., Keerthipala, A.P., Walker, G.M.: Isolation and characterisation of Sri Lankan yeast germplasm and its evaluation for alcohol production. J. Inst. Brew. 112(4), 302–307 (2006). https://doi.org/10.1002/j.2050-0416.2006.tb00735.x
Techaparin, A., Thanonkeo, P., Klanrit, P.: High-temperature ethanol production using thermotolerant yeast newly isolated from Greater Mekong Subregion. Brazilian J. Microbiol. 48(3), 461–475 (2017). https://doi.org/10.1016/j.bjm.2017.01.006
Pongcharoen, P., Tawong, W., Kucharoenpsaibul, S.: Enhanced high temperature ethanol production using newly isolated thermotolerant yeast Pichia kudriavzevii NUPHS from Thailand. ScienceAsia. 47(1), 47–56 (2021). https://doi.org/10.2306/scienceasia1513-1874.2021.009
Li, C., Li, L., Yang, X., Wu, Y., Zhao, Y., Wang, Y.: Effect of inorganic salt stress on the thermotolerance and ethanol production at high temperature of Pichia kudriavzevii. Ann. Microbiol. 68(5), 305–312 (2018). https://doi.org/10.1007/s13213-018-1339-x
Sunkar, B., Bhukya, B.: Bi-phasic hydrolysis of corncobs for the extraction of total sugars and ethanol production using inhibitor resistant and thermotolerant yeast, Pichia kudriavzevii. Biomass Bioenerg. 153, 106230 (2021). https://doi.org/10.1016/j.biombioe.2021.106230
Hawaz, E., et al.: Isolation and characterization of bioethanol producing wild yeasts from bio-wastes and co-products of sugar factories. Ann. Microbiol. 72(1) (2022). https://doi.org/10.1186/s13213-022-01695-3
Nurcholis, M., Setiawan, A., Kusnadi, J., Maligan, J.M.: Isolation of thermo-tolerant and ethanol-tolerant yeast from local fermented foods and their potential as bioethanol producers. IOP Conf. Ser. Earth Environ. Sci. 924(1) (2021). https://doi.org/10.1088/1755-1315/924/1/012077
Wang, H., Hu, Z., Long, F., Niu, C., Yuan, Y., Yue, T.: Characterization of osmotolerant yeasts and yeast-like molds from Apple Orchards and Apple Juice Processing Plants in China and Investigation of their spoilage potential. J. Food Sci. 80(8), M1850–M1860 (2015). https://doi.org/10.1111/1750-3841.12946
Ghose, T.K., Tyagi, R.D.: Rapid ethanol fermentation of cellulose hydrolysate. II. Product and substrate inhibition and optimization of fermentor design. Biotechnol. Bioeng. 21(8), 1401–1420 (1979). https://doi.org/10.1002/bit.260210808
Helene, G., Héléne, B., Galzy, P.: Inhibition of alcoholic fermentation. Biotechnol. Genet. Eng. Rev. 2(1), 365–382 (1984). https://doi.org/10.1080/02648725.1984.10647805
Lima-Costa, M.E., Tavares, C., Raposo, S., Rodrigues, B., Peinado, J.M.: Kinetics of sugars consumption and ethanol inhibition in carob pulp fermentation by Saccharomyces cerevisiae in batch and fedbatch cultures. J. Ind. Microbiol. Biotechnol. 39(5), 789–797 (2012). https://doi.org/10.1007/s10295-011-1079-4
Nosrati-Ghods, N., Harrison, S.T.L., Isafiade, A.J., Tai, S.L., February: 107754, https://doi.org/10.1016/j.bej.2020.107754. (2020)
Pereira, A.S., Pinheiro, Á.D.T., Rocha, M.V.P., Gonçalves, L.R.B., Cartaxo, S.J.M.: A new approach to model the influence of stirring intensity on ethanol production by a flocculant yeast grown on cashew apple juice. Can. J. Chem. Eng. 97(S1), 1253–1262 (2019). https://doi.org/10.1002/cjce.23419
Rughoonundun, H., Mohee, R., Holtzapple, M.T.: Influence of carbon-to-nitrogen ratio on the mixed-acid fermentation of wastewater sludge and pretreated bagasse. Bioresour Technol. 112, 91–97 (2012). https://doi.org/10.1016/j.biortech.2012.02.081
Nunta, R., et al.: Ethanol and phenylacetylcarbinol production processes of Candida tropicalis TISTR 5306 and Saccharomyces cerevisiae TISTR 5606 in fresh juices from longan fruit of various sizes. J. Food Process. Preserv. 42(11), 1–11 (2018). https://doi.org/10.1111/jfpp.13815
Nunta, R., et al.: Batch and continuous cultivation processes of Candida tropicalis TISTR 5306 for ethanol and pyruvate decarboxylase production in fresh longan juice with optimal carbon to nitrogen molar ratio. J. Food Process. Eng. 42(6), 1–17 (2019). https://doi.org/10.1111/jfpe.13227
Manikandan, K., Viruthagiri, T.: Optimization of C/N ratio of the medium and fermentation conditions of ethanol production from tapioca starch using co - culture of aspergillus Niger and Sachormyces Cerevisiae. Int. J. ChemTech Res., 2, 2, (2010)
Acknowledgements
The authors are thankful to Department of Science and Technology (DST) Goa for the research funding and the management of BITS Pilani K. K. Birla Goa campus for their support during this project. Special thanks are extended to all the Feni manufacturers who have generously provided the cashew apple pulp for the current research work. We thank Fluoresight Bioprobes Pvt Ltd. for providing the Kadamba Green (KG) 264 fluorescent dye utilized in our research work.
Funding
Funding was provided by Department of Science and Technology (DST) Goa (Grant no. 6-198-2016).
Author information
Authors and Affiliations
Contributions
Vinoth Kannan S R: Conceptualization, Investigation and Data collection, Writing - original draft, review & editing. Vivek Rangarajan: Validation, Supervision, Writing - review & editing. Sampatrao Dagu Manjare: Conceptualization, Supervision, Funding acquisition, Resources, Writing-review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
S R, V., Rangarajan, V. & Manjare, S.D. Investigating the Potential of Ethanol and Sugar Tolerant Yeast Strain Isolates from Fermented Cashew Apple Juice for Improved Feni Production. Waste Biomass Valor 15, 4885–4898 (2024). https://doi.org/10.1007/s12649-024-02475-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-024-02475-7