Abstract
Purpose
To compare pupillary autonomic dysfunction in multiple system atrophy (MSA) and Parkinson’s disease (PD).
Methods
We administered eye-drop tests to 40 MSA patients, 40 PD patients with similar disease duration, and 20 age-matched healthy controls. Pupillary supersensitivity to a parasympathomimetic agent (0.05% pilocarpine hydrochloride) and to a sympathomimetic agent (0.02% dipivefrine hydrochloride) was examined by assessing changes in pupil diameter.
Results
Pupillary supersensitivity to a parasympathomimetic agent (0.05% pilocarpine hydrochloride) and to a sympathomimetic agent (0.02% dipivefrine hydrochloride) was examined by assessing changes in pupil diameter. Pupillary supersensitivity to 0.05% pilocarpine was greatest among the PD patients (PD −23.1 ± 14.4%, MSA −12.4 ± 11.5%, control −9.5 ± 8.2%, p < 0.05) but was not correlated with disease duration. Pupillary sensitivity to 0.02% dipivefrine was significantly greater in the PD and MSA patients versus controls (PD 10.5 ± 12.0%, MSA 11.8 ± 11.0%, control 3.1 ± 5.8%, p < 0.05). MSA patients had pupillary sympathetic dysfunction from an early stage, whereas in PD patients it tended to gradually accelerate as the disease advanced. In MSA patients, pupillary sympathetic sensitivity to 0.02% dipivefrine was correlated with the severity of orthostatic hypotension during a head-up tilt test and with the elevation of systolic blood pressure during a noradrenaline infusion test. In PD patients, pupillary sympathetic sensitivity to 0.02% dipivefrine was correlated with a reduction of the heart-to-mediastinum (H/M) ratio using delayed-phase iodine-123 meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy.
Conclusion
These data indicate that eye-drop tests can reveal differences in the progression of pupillary autonomic dysfunction in patients with MSA and PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple system atrophy (MSA) is a sporadic neurodegenerative disorder characterized by a combination of Parkinsonism, autonomic failure, cerebellar dysfunction, and pyramidal tract involvement [1]. Autonomic insufficiency results in orthostatic hypotension, inadequate heart rate response to standing, male erectile dysfunction, constipation, and decreased sweating [2]. Neuronal degeneration is found in the putamen, substantia nigra, locus ceruleus, dorsal vagal nucleus, pontine nuclei, cerebellar cortex, inferior olive, Onuf’s nucleus, sacral autonomic nucleus, and intermediolateral column of the spinal cord [3–5]. Degeneration of sympathetic preganglionic neurons in the intermediolateral column of the spinal cord is thought to contribute to orthostatic hypotension in MSA [6]. Autonomic dysfunction in patients with MSA seems to be caused mainly by central autonomic failure [1].
In patients with Parkinson’s disease (PD), postganglionic sympathetic dysfunction is thought to mostly account for autonomic failure [1]. However, cardiovascular autonomic function tests cannot differentiate autonomic failure associated with PD from MSA [7]. Preganglionic sympathetic fibers originating from the thoracolumbar outflow innervate the pupil after synapsing in the superior cervical ganglion and the heart through the middle cervical ganglion. Therefore, pupillary autonomic dysfunction may exist in MSA patients.
We previously reported that the severity of pupillary postganglionic autonomic involvement was related to visual symptoms in PD patients [8]. Pupillary autonomic symptoms, including alternating Horner’s syndrome and iris atrophy, are present in MSA, and the biochemical abnormalities of pupils have been used to assess autonomic function in MSA patients [9]. However, there are no cross-sectional studies assessing pupillary autonomic dysfunction using eye-drop tests in patients with MSA and PD. In this study, we evaluated pupillary autonomic dysfunction in MSA patients using eye-drop tests in comparison to PD patients. We also evaluated the evolution of pupillary autonomic dysfunction in terms of disease progression between MSA and PD.
Subjects and methods
Subjects
We recruited 40 patients with probable MSA based on Gilman’s diagnostic criteria, 40 disease duration-matched patients with PD, and 20 healthy volunteers as controls (Table 1). Clinical diagnoses of MSA and PD were established by the consensus diagnostic criteria of Gilman et al. [2, 10] and the criteria of the United Kingdom Brain Bank, respectively. The disease severity of PD was assessed according to the Hoehn and Yahr (H–Y) stage. In order to match the disease durations of MSA patients, we recruited only PD patients whose disease duration was under 7 years. All patients underwent general physical and neurological examinations, laboratory tests, and brain MRIs to exclude other causes of Parkinsonism and cerebellar ataxia. Patients with ocular disease (e.g., cataract surgery, glaucoma), diabetes mellitus, or peripheral neuropathy were excluded. Patients’ anti-Parkinsonian therapies were continued if necessary. This study was approved by the Ethics Committee of Nagoya University Graduate School of Medicine, and informed consent was established before participation in the study.
Measurement of pupillary diameter
A pupillary diameter was measured with an infrared video pupillometer (Binocular Iriscorder C-7364, Hamamatsu Photonics Co. Ltd, Shizuoka, Japan) [11]. We measured the initial pupil diameter in each eye after 5 min of dark adaptation in all three groups of subjects. We measured one eye then the other, spend at least 2 min using iris scope.
Eye-drop tests
After the pupillary dynamics measurement, we administered two drops of 0.05% dilute pilocarpine (PL) to one eye and two drops of 0.02% dilute dipivephrine (DPE) to the other eye. PL is a miotic parasympathomimetic agent, and DPE is a sympathomimetic agent that causes mydriasis [12]. The optimal concentrations of PL and DPE, as well as the assessment times, were chosen as described previously [8]. Pupil diameter was recorded at 60 min after 0.05% PL administration and 120 min after 0.02% DPE administration. The pupil response was quantified with the ratio (D1 − D2)/D1 × 100. D1 and D2 represent the baseline pupil diameter and the drug-induced pupil diameter, respectively. We defined a change in pupillary diameter exceeding the mean plus two standard deviations (SD) in the control group as indicating supersensitivity.
Analyses of pupillary dysfunction in early and late stages
To investigate the progress of pupillary dysfunction in early- and late-stage PD and MSA, patients were put into two subgroups based on disease duration: less than 2 years (early stage) or 2–7 years (late stage) from onset. To determine whether the pupil assay using PL and DPE eye drops could be useful for the early diagnosis of MSA, the sensitivity and specificity of the diagnostic value to discriminate between MSA and PD at an early stage were assessed using receiver-operating characteristic (ROC) analysis.
Cardiovascular autonomic function tests
We administered a head-up tilt table test (HUT) to all patients. Patients were tilted up to 60° in a stepwise manner (20°, 40°, and 60° for 5 min each) after resting for at least 5 min in supine position [13]. Thirty-nine patients with MSA and 38 patients with PD were examined to assess sensitivity to a noradrenaline (NA) infusion test. Systolic blood pressure (SBP) was measured after a dilute NA solution was administered intravenously at a rate of 3 μg/min for 3 min [14, 15].
Iodine-123 meta-iodobenzylguanidine myocardial scintigraphy was also used in 38 patients with PD and 20 patients with MSA. Myocardial 123I-MIBG uptake was measured using the heart-to-mediastinal uptake ratio (H/M) according to methods described previously [16]. We investigated correlations between pupillary mydriatic change to 0.02% DPE and a decrease of SBP during HUT, elevation of SBP during the NA infusion test, and reduction of delayed-phase H/M ratio in PD and MSA patients.
Statistics
Statistical analysis was performed using the PRISM software package (Version 5.0; GraphPad Software, La Jolla, CA, USA). Values are presented as the means ± standard deviation (SD). A one-way ANOVA with the Kruskal–Wallis test and Dunn’s multiple comparison test were used to compare baseline pupil diameter and differences in pupillary change due to 0.05% PL and 0.02% DPE administration in the three groups of subjects. Relationships between pupillary change due to 0.02% DPE and the decrease of SBP during HUT, the elevation of SBP during the NA infusion test, and the durations of MSA and PD were analyzed using Pearson’s correlation coefficient. Statistical significance was set at a p value below 0.05.
Results
Clinical manifestations
Disease severity in patients with MSA was relatively early stage. No ocular clinical manifestations (Horner’s syndrome, alternating Horner’s syndrome, iris atrophy, corneal hypaesthesia, or diminished lacrimation) were found.
Measurement of pupillary diameter
There was no significant difference in the baseline pupil diameter between the MSA and PD groups (MSA 4.7 ± 0.8 mm, PD 5.0 ± 0.8 mm, control 5.1 ± 0.8 mm, p = 0.21).
Cardiovascular autonomic function tests
The head-up tilt table test showed that systolic BP decreased at the 60° angle compared with baseline: systolic BP was 24.4 ± 21.8 mmHg in the MSA group and 14.7 ± 18.4 mmHg in the PD group, a significant difference (p = 0.035). 23 patients with MSA and 15 patients with PD showed a 20 mmHg decrease, indicating the presence of orthostatic hypotension. There was also an elevation of systolic BP during the NA infusion test: systolic BP was 25.7 ± 16.7 mmHg in the MSA group and 24.8 ± 15.9 mmHg in the PD group, not a significant difference (p = 0.975). 16 patients with MSA and 18 patients with PD showed a 25 mmHg elevation, indicating the presence of supersensitivity. The delayed-phase H/M ratio was 1.76 ± 0.64 in the PD group (n = 38) and 2.70 ± 0.75 in the MSA group (n = 20).
Pupillary response to PL and DPE
Pupillary miotic response to 0.05% PL was highest in the PD subjects (PD −23.1 ± 14.4%, MSA −12.4 ± 11.5%, control −9.5 ± 8.2%, p < 0.05). Pupillary mydriatic response to 0.02% DPE in patients with PD and MSA was significantly greater than that of controls (PD 10.5 ± 12.0%, MSA 11.8 ± 11.0%, control 3.1 ± 5.8%, p < 0.05). We defined supersensitivity as a change in pupillary diameter exceeding the mean value plus 2 standard deviations (SD) in the control group; i.e., −26% pupillary miotic change at 60 min after 0.05% PL administration, or 14% pupillary mydriatic change at 120 min after 0.02% DPE administration. Of the 40 PD patients, 13 (32.5%) showed supersensitivity to PL, 11 (27.5%) showed supersensitivity to DPE, 6 (15.0%) patients showed supersensitivity to both drugs, and 22 (55.0%) patients showed supersensitivity to neither of the two drugs. Of the 40 MSA patients, 4 (10%) showed supersensitivity to PL, 16 (40%) showed supersensitivity to DPE, 3 (7.5%) showed supersensitivity to both drugs, and 23 (57.5%) showed supersensitivity to neither of the two drugs.
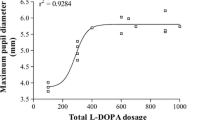
Pupillary miotic response to PL did not correlate with disease duration in PD patients. Pupillary mydriatic response to DPE was significantly correlated with disease duration in PD patients (r = 0.334, p < 0.05; Fig. 1) but not in MSA patients.
Analyses of pupillary response in early- and late-stage MSA and PD
Pupillary miotic responses to 0.05% PL were more marked in PD patients than in MSA patients at an early stage (PD −22.2 ± 14.1%, MSA −10.9 ± 11.4%, p < 0.05). In both PD and MSA patients, pupillary mydriatic responses to 0.02% DPE eye drops tended to be greater at a late stage versus those at an early stage. Pupillary mydriatic responses to 0.02% DPE were significantly marked in early stage MSA patients, but not so in early stage PD patients (MSA 9.5 ± 7.8%, control 3.1 ± 5.8%, p < 0.05; Table 2).
A ROC sensitivity curve was plotted against 1-specificity using pupillary miotic response to PL to discriminate PD from MSA, and by using pupillary mydriatic response to DPE to discriminate MSA from PD in early stage (Fig. 2). ROC analyses demonstrated that the area under the curve was 0.737 (p = 0.0045) for the PL eye-drop test and 0.633 (p = 0.65) for the DPE test.
Receiver-operating characteristic curve to discriminate PD from MSA. A ROC sensitivity curve was plotted against 1-specificity using pupillary miotic response to 0.05% PL eye-drop to discriminate PD from MSA in a subgroup of patients with disease duration of 2 years or less. The most discriminative cut-off point was set nearest to the upper left corner of the graph. ROC receiver operating characteristic, AUC area under the curve, PL pilocarpine
Correlation between pupillary mydriatic response to DPE and cardiovascular autonomic function tests
Pupillary mydriatic response to 0.02% DPE was significantly correlated with both a decrease of systolic BP during HUT (r = −0.48, p < 0.05) and an elevation of systolic BP during the NA infusion test (r = 0.4, p < 0.05) in patients with MSA, but it did not correlate in patients with PD (Fig. 3a, b).
Relationship between pupillary mydriatic response to dipivefrine and cardiovascular autonomic tests in MSA. a Correlation between pupillary mydriatic response to 0.02% DPE eye drops and decrease of systolic blood pressure (SBP) during a head-up tilt test. Pupillary mydriatic response to 0.02% DPE was positively correlated with a decrease of SBP during the head-up tilt test in MSA patients. b Correlation between pupillary mydriatic response to 0.02% DPE and elevation of systolic blood pressure during a noradrenaline infusion test in MSA patients. Pupillary mydriatic response to 0.02% DPE was significantly correlated with elevation of SBP during the NA infusion test in MSA patients. DPE dipivefrine
The delayed-phase H/M ratio accumulation was reduced in patients with PD, and was weakly and inversely correlated to pupillary mydriatic response to 0.02% DPE (r = −0.335, p < 0.05, Fig. 4), but there was no such correlation in patients with MSA (p = 0.11).
Discussion
Pharmacologic pupillary function tests are useful for evaluating denervation supersensitivity in patients with autonomic dysfunction, especially of the postganglionic variety [12]. Denervation supersensitivity seen in eye-drop tests is not only due to lesions in the postganglionic nerve, but to a lesser degree, by lesions more proximal in the nerve pathway [12]. A supersensitivity response to diluted PL eye-drop thus indicates dysfunction anywhere from the midbrain to the nerve endings in the iris sphincter [17]. DPE is an α-adrenoceptor agonist used to test for supersensitivity caused by a lesion anywhere in the peripheral sympathetic pathway, including pre- or post-ganglionic lesions.
We reported previously that eye-drop testing using PL and DPE in PD patients revealed a denervation supersensitivity resulting from dysfunction in the peripheral sympathetic and parasympathetic nervous system [8]. In the present study, we found that pupillary parasympathetic denervation supersensitivity can be detected in PD patients from an early stage of illness, but rarely in MSA patients. Sugiyama et al. [18] have also reported that pupillary supersensitivity to cholinergic agonists is prominent in early stages of PD.
In our previous study, we demonstrated that pupillary sympathetic denervation supersensitivity occurred mostly in PD patients at a relatively advanced stage (Hoehn–Yahr III) [8]. In another study, sympathetic supersensitivity was reported to be rare in early PD stages (H–Y I and II), usually becoming detectable only in stage III patients by eye-drop testing using DPE [18]. Sawada et al. demonstrated the presence of pupillary peripheral sympathetic dysfunction in PD patients in eye-drop tests using phenylephrine and cocaine [19]. In the present study, we found that sympathetic denervation supersensitivity becomes severe with advancing disease duration in PD patients. Furthermore, we found that sympathetic denervation supersensitivities were detected in early stage MSA patients.
As indicated by these previous reports, the parasympathetic denervation supersensitivity in PD may be localized to the Edinger–Westphal (E–W) nucleus and/or the ciliary ganglion [8, 18]. On the other hand, cholinergic deficits have been shown to be less severe in MSA than in PD [20]. In MSA, there have been occasional reports of neuronal loss in the E–W nucleus, while in PD, Lewy bodies are commonly found in the E–W nucleus [21]. Our results support the notion that pupillary parasympathetic dysfunction corresponds to pathologic involvement of the E–W nucleus. Other data suggest that cholinergic dysfunction in MSA can be severe and widespread [22]. Nonetheless, neither the pathologic involvement of the ciliary ganglion in PD and MSA patients, nor the pathologic site of the pupillary parasympathetic involvement in PD, has been described. Therefore, further pathological studies are necessary.
It has been reported that autonomic dysfunction in MSA patients primarily affects preganglionic sympathetic neurons and the hypothalamus [6, 23]. Previous neuropathological studies indicate that there is a loss of preganglionic fibers in MSA but that the postganglionic nerve fibers are sufficiently well preserved [21]. However, some studies suggest the presence of postganglionic autonomic dysfunction in MSA. As for the cardiac sympathetic nerve, Orimo et al. [3, 24] reported that a mild degeneration of the cardiac sympathetic nerve can occur in patients with MSA, and it was presumed that loss of excitatory preganglionic inputs from the intermediolateral cell column could reduce firing rates of sympathetic ganglionic cells. In the present study, a denervation supersensitivity detected in MSA patients following DPE eye drops also might reflect a functional disorder of postganglionic neurons resulting from a transsynaptic degeneration secondary to the degenerations of preganglionic neurons of the intermediolateral column.
The pupillary mydriatic response to 0.02% DPE correlated with the severity of cardiovascular autonomic dysfunction observed in the cardiovascular function tests, which mainly reflect preganglionic autonomic impairments of the sympathetic nervous system in MSA patients [6]. Pupillary mydriatic response to 0.02% DPE was weakly correlated with a reduction of 123I-MIBG cardiac accumulation, demonstrating postganglionic sympathetic dysfunction in the hearts of PD patients. Pupillary hypersensitivity due to the DPE eye-drop test presumably reflects sympathetic impairment, which is preganglionic in MSA and postganglionic in PD.
The clinical diagnostic differentiation between PD and MSA is often difficult in their early stages [1, 25]. A retrospective assessment of the consensus criteria concluded that sensitivity for probable MSA was poor and detecting early cases of MSA was problematic [26]. The ROC analysis in this study revealed that pupillary supersensitivity in the PL eye-drop test might serve as a useful aid for differentiating PD and MSA at an early stage. Although it is difficult to draw conclusions about a longitudinal process of disease evolution from analyses at single time points, pupil analysis using the combined PL and DPE eye-drop test may be a marker for distinguishing between MSA and PD at an early stage.
A limitation of this study is that we did not assess the effect of corneal permeability, which influences the pharmacologic reactivity of the pupil. There are large interindividual variations in the ability of pilocarpine or dipivefrine to penetrate a normal cornea. Horner syndrome can increase central corneal thickness. Corneal hypaesthesia, a documented finding in MSA patients, may affect drug absorption. The extent of exposure to light may influence pupillary cholinergic sensitivity. These and other potentially confounding variables should be controlled in subsequent studies. Another limitation is that the severity of PD is estimated by H–Y stage; however, in MSA patients, especially in MSA-C patients, it is impossible to estimate disease severity by using the H–Y scale. Thus, it is difficult to compare pupillary autonomic dysfunction in relation to disease severity in these two diseases.
In conclusion, there is a difference in progression of pupillary peripheral autonomic dysfunction between MSA and PD patients, and a dipivefrine eye-drop test is as useful as cardiovascular autonomic function tests to detect systemic autonomic dysfunction in MSA.
References
Wenning GK, Colosimo C, Geser F, Poewe W (2004) Multiple system atrophy. Lancet Neurol 3:93–103
Gilman S, Low PA, Quinn N et al (1999) Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 163:94–98
Daniel SE (1999) The neuropathology and neurochemistry of multiple system atrophy. In: Mathias CJ, Bannister SR (eds) Autonomic failure. Oxford University Press, Oxford, pp 321–328
Wenning GK, Tison F, Ben Shomo Y et al (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12:133–147
Gray F, Vincent D, Hauw JJ (1988) Quantitative study of lateral horn cells in 15 cases of multiple system atrophy. Acta Neuropathol 75:513–518
Oppenheimer DR (1980) Lateral horn cells in progressive autonomic failure. J Neurol Sci 46:393–404
Riley DE, Chelimsky TC (2003) Autonomic nervous system testing may not distinguish multiple system atrophy from Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:56–60
Hori N, Takamori M, Fumitada Y, Hirayama M et al (2008) Pupillary supersensitivity and visual disturbance in Parkinson’s disease. Clin Auton Res 18:20–27
Nirankari VS et al (1982) Ocular manifestations of Shy-Drager syndrome. Ann Ophthalmol 14:635–638
Daniel SE, Lees AJ (1993) The clinical features of Parkinson’s disease in 100 histologically proven cases. Adv Neurol 60:595–599
Ishikawa S (2001) Normal data on pupillary dynamics. Neuro-ophthalmol Jpn 18:154–156
Shirley A, Smith SA, Smith SE (1999) Pupil function: tests and disorders. In: Bannister R, Mathias CJ (eds) Autonomic failure. A textbook of clinical disorders of the autonomic nervous system, 4th edn. Oxford University Press, Oxford, pp 245–253
Mathias CJ, Bannister R (1999) Investigation of autonomic disorders. Autonomic failure. In: Mathias CJ, Bannister R (eds) Autonomic failure. A textbook of clinical disorders of the autonomic nervous system, 4th edn. Oxford University Press, Oxford, pp 171–175
Polinsky R, Kopin I, Ebert M, Weise V (1981) Pharmacologic distinction of different orthostatic hypotension syndromes. Neurology 31:1–7
Niimi Y, Ieda T, Hirayama M, Koike Y, Sobue G, Hasegawa Y, Takahashi A (1999) Clinical and physiologic characteristics of autonomic failure with Parkinson’s disease. Clin Auton Res 9:139–144
Hamada K, Hirayama M, Watanabe H, Kobayashi R, Ito H, Ieda T, Koike Y, Sobue G (2003) Onset age and severity of motor impairment are associated with reduction of myocardial 123I-MIBG uptake in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:423–426
Jacobson DM (1990) Pupillary responses to dilute pilocarpine in preganglionic 3rd nerve disorders. Neurology 40:804–808
Sugiyama T, Utsumi T (1990) Pupillary dynamics in Parkinson’s disease. Neuro-ophthalmology 10:1–7
Sawada H, Yamakawa K, Yamakado H, Hosokawa R, Ohba M, Miyamoto K, Kawamura T, Shimohama S (2005) Cocaine and phenylephrine eye drop test for Parkinson disease. JAMA 293:932–934
Spokes EGS, Bannister R, Oppenheimer DR (1979) Multiple system atrophy with autonomic failure. Clinical, histological and neurochemical observations in four cases. J Neurol Sci 43:59–82
Matthews MR (1999) Autonomic ganglia and preganglionic neurons in autonomic failure. In: Bannister R, Mathias CJ (eds) Autonomic failure. A textbook of clinical disorders of the autonomic nervous system, 4th edn. Oxford University Press, Oxford, pp 329–339
Khurana RK (1994) Cholinergic dysfunction in Shy-Drager syndrome: effect of the parasympathomimetic agent, bethanechol. Clin Auton Res 4:5–13
Shy GM, Drager GA (1960) A neurological syndrome associated with orthostatic hypotension: a clinicopathological study. Arch Neural 2:511–527
Orimo S et al (2007) Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol 113:81–86
Wenning GK, Ben Shomo Y, Hughes A, Daniel SE, Lees A, Quinn NP (2000) What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease. J Neurol Neurosurg Psychiatry 68:434–440
Osaki Y, Wenning GK, Daniel SE et al (2002) Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology 59:1486–1491
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, F., Hirayama, M., Nakamura, T. et al. Pupillary autonomic dysfunction in multiple system atrophy and Parkinson’s disease: an assessment by eye-drop tests. Clin Auton Res 20, 191–197 (2010). https://doi.org/10.1007/s10286-009-0051-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-009-0051-0