Abstract
Objectives
Ascertain and quantify abnormality of the melanopsin-derived portion of the pupillary light reflex (PLR) in patients with Parkinson’s disease (PD) and parkinsonism features based on a statistical predictive modeling strategy for PLR classification.
Methods
Exploratory cohort analysis of pupillary kinetics in non-disease controls, PD subjects, and subjects with parkinsonism features using chromatic pupillometry. Receiver operating characteristic (ROC) curve interpretation of pupillary changes consistent with abnormality of intrinsically photosensitive retinal ganglion cells (ipRGCs) was employed using a thresholding algorithm to discriminate pupillary abnormality between study groups.
Results
Twenty-eight subjects were enrolled, including 17 PD subjects (age range 64–85, mean 70.65) and nine controls (age range 48–95, mean 63.89). Two subjects were described as demonstrating parkinsonism symptoms due to presumed Lewy body dementia and motor system atrophy (MSA) respectively. On aggregate analysis, PD subjects demonstrated abnormal but variable pupillary dynamics suggestive of ipRGC abnormality. Subjects with parkinsonism features did not demonstrate pupillary changes consistent with ipRGC abnormality. There was no relationship between levodopa equivalent dosage or PD severity and ipRGC abnormality. The pupillary test sensitivity in predicting PD was 0.75 and likelihood ratio was 1.2.
Conclusions
ipRGC deficit is demonstrated in PD subjects; however, the degree and constancy of abnormality appear variable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by muscle rigidity, tremor, and, in advanced cases, loss of physical movement [1]. Ocular complications are well described in PD and include reduced retinal dopamine [2], the primary neurotransmitter for retinal phototransduction. [3]. The linkage between retinal dysfunction and PD is an intriguing field of research that has important relevance not only in regard to PD-related co-morbidities but as an etiologic component of the disease process [4, 5]. Mounting evidence supports a link between the retina and nigrostriatal function. For example, bright light therapy for PD has been found to be beneficial in treatment of sleep abnormality through a putative mechanism involving suppression of retinal melatonin [4–7]. Moreover, bright blue treatment demonstrates not only positive effects on sleep and mood but also on PD-related motor function [8]. These findings suggest that functionality of the nigrostriatal system is influenced by a system emanating from the retina to the pineal gland, denoting a key role of circadian entrainment as a component of nigrostriatal operation [9]. The putative link between the retina and nigrostriatal function supports the notion that retinal dysfunction and concomitant circadian dysregulation are etiologic features in PD that have potential as both biomarkers and treatment targets of PD. The role of circadian dysfunction as an etiologic component of PD is reinforced in studies by Willis et al. that show PD-related symptomatology can be reversed experimentally by direct intravitreal delivery of l-dopa in amounts considered inadequate to result in systemic effects [9, 10]. These studies have implicated the retina not only as an associated neural system affected by PD but also as a component of the disease etiology itself [9, 11]. The importance of appropriate functionality of retinal supported circadian homeostasis is also denoted by exacerbation of PD symptoms in an animal model following enucleation [11].
Light-dark cycles maintained by the retina emanate from a subset of retinal ganglion cells known to be inherently photosensitive and colloquially termed intrinsically photosensitive retinal ganglion cells (ipRGCs) [12]. ipRGCs play a key role in the operation of the suprachiasmatic nucleus in regard to melatonin secretion and circadian homeostasis [13, 14]. The function of ipRGCs is based on the chromophore melanopsin, with peak sensitivity at 470 nm [15]. The response of ipRGC is energy dependent, with higher levels of irradiance leading to greater ipRGC activity [16]. The latter property of ipRGC is intriguing in regard to PD as studies have demonstrated the benefit of bright short-wavelength exposure in ameliorating some of the symptoms of PD-related complications [6]. ipRGCs also mediate other non-image-forming (NIF) visual responses such as the pupillary light reflex (PLR) via co-release of neurotransmitters glutamate and pituitary adenylate cyclase-activating peptide (PACAP) from their synaptic terminals.
Functionality of ipRGC can be reflected in pupillary dynamics [12, 17]. The hallmark finding of pRGC function is persistence of the pupillary response (PPR) denoted by prolonged pupillary constriction following extinguishing a light stimulus of suitable wavelength and intensity to activate the chromophore melanopsin within ipRGCs. A persistent potentiation of the pupil response following cessation of a suitable stimulus is the hallmark of healthy ipRGC function clinically [12]. The circadian dysregulation demonstrated in PD as well as the response to bright blue light in regard to PD therapy suggest a key link between functionality of ipRGC and PD [18, 19]. The purpose of this report is to assess the characteristics of the PPR in PD subjects and those with parkinsonism features versus a control cohort in regard to interpretation of PPR abnormality as a biomarker ipRGC dysfunction indicative of PD pathobiology.
Methods
This was an exploratory cohort study designed to examine differences in PPR in patients with PD, parkinsonism, and controls. Patients were enrolled by consecutive sampling. Inclusion criteria include prior diagnosis of PD with treatment of one or more dopaminergic agonists. Patients with parkinsonism features but without definitive PD diagnosis were also enrolled for exploratory analysis. Patients taking drugs known to impact circadian rhythm including lithium, benzodiazepines, steroidal and nonsteroidal anti-inflammatory drugs, and vitamin B12 were excluded from the study. Patients were screened prior to testing for the presence of ocular abnormalities including amblyopia, cataract reducing correctable vision to less than 20/25 in either eye, glaucoma, or any form of retinopathy such as that related to diabetes or age-related macular degeneration. We did not exclude pseudophakic subjects due to the fact retinal irradiance in phakic patients was normalized and corrected for media loss based on the method of van deKraats and van Norren [20] This correction factor obviates confounding of pupillary findings arising from deviation of inter-subject retinal illuminance. Overall, one PD subject and two control subjects were pseudophakic. The degree of PD severity was evaluated by the Hoehn and Yahr grading system [21].
Pupillometry
The right eye was dilated with 2.5% phenylephrine and 1% tropicamide. Dilation of the right eye was employed in an effort to maintain consistent retinal illumination within and between subjects during stimulation. Following eye dilation, the ipRGC-driven pupil response was measured via the consensual post-illumination pupil response (PIPR) in the left eye. Stimuli presented to the right eye consisted of long-wavelength (red) and short-wavelength (blue) narrowband 5-s pulses of light. This establishes the adequacy of the irradiance level used in this study to induce ipRGC action. Light stimuli were generated using a custom-built Maxwellian-view optical system consisting of narrow bandpass filters of 25-mm-diameter short wavelength; “blue” light, λmax = 470 nm; full width half maximum (FWHM) = 10 nm and long wavelength; “red” light, λmax = 640 FWHM = 10 nm, imaged to the plane of the pupil in conjunction with appropriate neutral density filters to alter power output to 8 or 30 μW (ILT 9000, International Light Technology). The stimulus pencil was constrained using an aperture of 10 mm in diameter.
The experimental setup included use of an infrared-sensitive XIMEA MQ013RG-E2 machine vision camera whose parameters were controlled by the bundled XIMEA CamTool software, which also enables initiation of photo and video capture which was attached to a Haag Streit 900 model slit lamp with a suitable C mount. Patients were placed securely in the biomicroscope to maintain alignment in Maxwellian view while the dilated right eye was stimulated. The stimuli for both long and short wavelengths were based on a spectral irradiance of 8 and 30 μW·cm−2·nm−1 resulting in irradiance stimuli of 11.421 and 11.995 log photons·cm−2·s−1 at 640 nm and 14.621 and 15.195 log photons·cm−2·s−1 at 470 nm determined at the corneal plane respectively. Given the older age of the participants, retinal irradiances were estimated based upon established corrections for age-related changes in the optical density of the media of the eye for stimuli greater than 3° in diameter [20]. The pupillary light reflex was determined by averaging three consensual pupil recordings of 40 s (5-s pre-stimulus, 5-s stimulus, and 30-s post-stimulus). Pupillary dynamics were assessed using the XIMEA CamTool software in conjunction with Adobe Premiere Pro v.2 video capture and Image J imaging software (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD, USA). Nerve fiber layer thinning has been associated with PD; therefore, assessment of retinal nerve fiber layer thickness and retinal morphology was assessed in the stimulated eye using an automated optical coherence tomography (OCT) instrument (Zeiss Cirrus Model 5000 OCT, Carl Zeiss AG, Oberkochen, Germany) [22].
Statistics
All data were normalized to the average pupillary diameter of the first 5 s of imaging and expressed as percentage baseline units. In order to ascertain the degree of data variability, both control and PD subject pupillary size/time curves were converted to interpretative AUC data for assessment of data scatter and trend. Analysis of the median pupillary area from time zero to time 40 s between high or low energy levels and short vs long-wavelength stimulation was conducted with a Mann-Whitney U test.
In order to illustrate the classification accuracy of the PLRs, we performed receiver operating characteristic (ROC) curve analysis. Given that the ROC-AUC provides information on the discriminability of PPR, a classification can be conducted based on the difference from control subjects. From the overall analysis of ROC-AUC in the control group, a threshold AUC statistic of 0.65 was deemed valid as a cut-off for identifying normal vs abnormal PPR in the established binary model. The relative low AUC findings with short-wavelength high-irradiance stimulation in the control cohort were consistent with normal PPR. Comparative AUC values (and confidence intervals) provided a p value (alpha = 0.05) that addressed the likelihood of generating an AUC statistic as great or greater than 0.65 assuming no difference in the AUC between control and PD data. To adjust for multiple comparisons, we employed the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli using a false discovery rate (Q value) of 6.5% (GraphPad v 9.0). A chi-square analysis was employed to demonstrate sensitivity, specificity, and likelihood ratio of the PPR in association with PD. Covariance between AUC and levodopa equivalent daily dosage and PD grade severity was determined using a Pearson product-moment correlation coefficient (r). The two subjects with parkinsonian features were assigned to the overall PD subject cohort for the purposes of data analysis as pupillary abnormalities have been previously described in patients with non-PD parkinsonian features [23].
Results
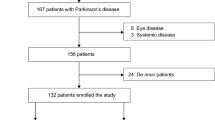
Overall, 17 PD subjects, 2 parkinsonian subjects, and 9 controls were enrolled. The two parkinsonian patients were described as demonstrating parkinsonism symptoms due to presumed Lewy body dementia and motor system atrophy (MSA) respectively. A description of patient demographics is found in Table 1. Difference in age between PD and control cohorts was not statistically significant (p = 0.0750).
Aggregate AUC analysis of pupillary tracings demonstrated between control and PD cohorts with high-irradiance short-wavelength stimulus demonstrated relative lack of PPR in the PD cohorts (mean AUC of PD cohort = 2684 vs 2436 for controls, p = 0.0144, Mann-Whitney U test, alpha = 0.05, Fig. 1). In contrast to controls, the PD cohort also demonstrated latency in pupillary recovery (Fig. 1). Mean pupillary area vs time data between short (470 nm) and long (640 nm) wavelengths at low irradiance in control and PD cohorts is shown in Fig. 2. Lack of PPR in the control and PD cohorts is consistent with the known high-irradiance photochemical requirement for melanopsin engagement (Fig. 2). Pupillometry data between short and long wavelengths at high irradiance in control and PD cohorts is seen in Fig. 3. High-irradiance short-wavelength stimulus produces a discernable PPR persistent to the 40-s time interval in the control cohort while the long-wavelength high-irradiance stimulus lacks PPR effect. These observations are consistent with the photochemical action spectrum of melanopsin and ipRGC activity. Lack of PPR in the PD cohort with short-wavelength high-irradiance stimuli is consistent with ipRGC abnormality (Fig. 3). A notable aberration in pupil latency at both short- and long-wavelength stimuli is demonstrated in the PD cohort (Figs. 2 and 3). The mean percent baseline pupillary area at the ending 40-s time interval for the high-irradiance short-wavelength stimulus is 66.45% in controls and 80.81% in PD subjects (p = 0.0012, Mann-Whitney, Fig. 3).
Pupillary tracing between control (left) and PD cohorts. Figures represent the aggregate of pupil area vs time tracings of the overall study cohort. Mean AUC of control and PD data = 2436 and 2684, respectively, p = 0.0144 (Mann-Whitney, alpha = 0.05). The overall flat tracings in the control cohort with relative low AUC following extinguishing the stimulus is the hallmark of appropriately functioning ipRGCs. Relative higher AUC data in the PD cohort is indicative of aberrant PPR and ipRGC abnormality
Mean pupillary area vs time data between short (470 nm) and long (640 nm) wavelengths at high (30 μW) irradiance in control and PD cohorts. Left: High-irradiance short-wavelength stimuli in the control cohort produces the expected discernable PPR persistent to the 40-s time interval while the long-wavelength high-irradiance stimulus lacks PPR effect. Right: Lack of PPR in the PD cohort with short-wavelength high-irradiance stimuli is consistent with ipRGC abnormality. Mean percent baseline pupillary area at the ending 40-s time interval for the high-irradiance short-wavelength stimulus = 66.45% in controls vs 80.81% for PD subjects (p = 0.0012, Mann-Whitney). A notable aberration in pupil latency at both short- and long-wavelength stimuli is demonstrated in the PD cohort
Tables 2 outlines the description of the study cohort in terms of levodopa equivalent dosage and PD severity grade. Tables 3, and 4 illustrate the predictive properties of ROC-AUC analysis to discriminate normal and abnormal PPR in PD and control cohorts respectively. Of the 19 subjects in the PD cohort, 8 were found to demonstrate abnormal PPR while one PD subject was deemed as demonstrating “borderline” PPR abnormality (Table 3). Interestingly, in the control cohort, 3 subjects were determined to demonstrate abnormal PRR (Table 4). Metrics describing PPR test sensitivity, specificity, and likelihood ratio in identifying PD are shown in Table 5. No relationship was found between levodopa equivalent daily dosage (LEDD) or PD severity grade and pupillary time AUC (r = 0.3551, p= 0.1358; r= 0.2410; p = 0.3184 respectively). Although there was no statistical difference in the mean retinal nerve fiber layer thickness between the PD and control cohorts, the PD cohort demonstrated a trend toward decreased retinal ganglion cell layer thickness (Table 1).
Discussion
Findings of the present study demonstrate variable abnormality in the PLR in a cohort of PD subjects typified by an aberrant PPR following short-wavelength, high-irradiance stimuli. Based on knowledge of the photochemical requirements of melanopsin activation [24], these findings are consistent with ipRGC abnormality as part of the melanopsin-derived contribution of the PLR. Our findings support that of prior studies which have also disclosed abnormal PPR in PD subjects [25]. In contrast however to prior studies examining ipRGC abnormality in PD patients, the present work included a cohort with a relatively higher Hoehn and Yahr grade indicating an overall higher level of disease severity [26]. Although prior studies have suggested that retinal nerve fiber layer thickness is reduced in relation to disease severity, our findings do not show a similar relationship [25]. Furthermore, our findings did not show a relationship between the PPR and PD severity or levodopa equivalent dose as was noted in prior studies [25]. Additionally, this study supports the notion that PPR abnormality found in PD is not due to morphologic abnormality of the retinal nerve fiber layer as denoted by the similarity in retinal nerve fiber layer thickness between disease and control groups reinforcing findings of prior studies [25]. Retinal nerve fiber compromise has been associated with abnormality in electroretinography findings in PD patients; however, the relationship between electroretinography and pupillary function in PD has not been established [27]. Observations in this study may be explained in several scenarios; disease pathobiological variability; systematic error in the clinical identification of a PD “gold standard”; and methodologic limitations of empirical ROC-AUC analysis for biomarker analysis.
Circadian neurohormonal secretion disrupted by ipRGC abnormality is now regarded as a rational component of neurodegenerative disease pathobiology [28]. Dysfunction of dopaminergic sub-populations in the retina as a result of PD has been shown to correlate with PD-related sleep, locomotor, and visual dysfunction [29]. Curiously, ipRGC are both pre- and postsynaptic to dopaminergic amacrine (DA) cells via reciprocal synapses; inhibitory DA signals are mediated through GABA co-released from DA along with excitatory dopamine signaling [30]. Dopamine has functions in the light-adaptation process by upregulating melanopsin transcription in ipRGCs and thus increasing the photoreceptor’s sensitivity [30]. DA releases dopamine extracellularly during daylight, with light adaptative effects typified by enhancing the activity of cone cells and increasing sensitivity to color and contrast while suppressing rod cell and retinal melatonin activity. Thus, due to diminished retinal dopamine, PD patients are considered to be in a persistent state of dark adaptation [31].
As noted, in this study, there was no correlation between LEDD and PPR abnormality. This finding may be interpreted that either ipRGC dopaminergic dependency acts in a stochastic binary rather than graded fashion or retinal dopamine levels among the PD cohort lacked sufficient variability for statistical significance. Binary expression of ipRGC pupil abnormality in PD may explain the lack of a relationship between LEDD and PD severity seen in this and other studies [25]. It is evident that in aggregate analysis, the identification of PPR abnormality is shown in the PD cohort. However, with the statistical approach in this study, its apparent significant heterogeneity in PPR abnormality exists not only in the PD cohort but also in the control group as well. The presence of abnormal PPR in the control group confounds the sensitivity and specificity of the PPR as a viable biomarker for PD. However, one cannot rule out the possibility that abnormality of PPR in the control cohort is a representative prodromal marker of an evolving neurodegenerative state. Furthermore, the variability in the depth of the PPR abnormality in PD patients is also notable suggesting either a graded degradation in ipRGC function in PD subjects.
The significance of the lack of correlation between PPR and PD disease grade may also lay in the dual nature by which ipRGC and conventional photoreceptors operate in relation to circadian entrainment. Photoreceptors, including ipRGCs as well as rods and cones, contribute to circadian oscillation in two distinct ways, as oscillator components or as mediators of luminous flux that do not involve rhythmicity, a phenomena termed masking [32]. In mammals, light is the most important zeitgeber synchronizing circadian rhythm. Luminous flux can therefore impact human activity in two ways, either by synchronizing an endogenous oscillator, modulated through ipRGCs, which in turn operationalize diurnal activity via a zeitgeber pacemaker, or through masking [33]. Conceptually, masking describes behavioral changes due to exogenous sources of illumination that are non-zeitgeber in origin. For example, exposure to illumination during periods of typical zeitgeber darkness will cause increased activity in a diurnal mammal (positive masking) while decreased illumination during periods of typically zeitgeber daylight results in decreased activity, or negative masking [33]. Positive masking can therefore be considered as a means to enhance activity by light in a diurnal species, while negative masking is used to describe suppression of activity by dark in a diurnal species and by light in a nocturnal species. Therefore, due to masking effects, PD patients with abnormal PPR may indeed display PPR aberration and consequent ipRGC efferent abnormality with resultant sleep and motor abnormality modified by effects of masking in a manner that may not be predictable or expected.
Addressing the lack of PPR abnormality in the two patients with parkinsonian features, previous studies have shown that patients with MSA indeed demonstrate abnormal PLR typified by deficits in constriction and dilation velocities [34]. However, these studies did not examine for PPR abnormality, only dynamics of pupillary constriction and velocity [34]. Although patients with MSA have been shown to have abnormality in measures of dynamic pupillometry, in the present study, neither patient with Lewy body dementia nor MSA demonstrated abnormal PPR typical of ipRGC deficit. Ideally, use of a PLR assessment to distinguish parkinsonian features of MSA or Lewy body dementia from PD would be of significant value. However, based on findings of the present study, it does not appear abnormality of PPR has sufficient sensitivity to be used clinically in this manner
Limitations of this study include a small sample size and the cross-sectional nature of the study design. Assessment of effect size denoted in this study suggests the study is underpowered to determine the true diagnostic accuracy of an abnormal PPR as a rational biomarker for PD. Furthermore, to estimate classification accuracy using standard ROC methods, a gold standard PD PPR status must be ascertained clearly without ambiguity, which is a dilemma as binary PD diagnosis disease classification may be unattainable or inaccurate. Nevertheless, use of ROC-AUC analysis as shown in this study provides a mechanism to both classify and illustrate PPR abnormality in both a qualitative as well as quantitative manner. Future studies that leverage analytical methodology that improve signal strength interpretation in estimating both the presence and extent of PLR abnormality in PD patients are required [34]. In summary, our PD cohort clearly demonstrates aberrant PPR albeit in a variable manner suggesting PD-related disruption of melanopsin-driven circadian entrainment with accompanying downstream pathobiological consequences occurs in either a graded fashion, or in a manner which eludes detection by current protocol of pupillary assessment.
Data availability
All data associated with this study are freely available online.
Code availability
GraphPad Prism version 9.0.0 GraphPad Software, San Diego, CA, USA, www.graphpad.com
References
Davie CA (2008) A review of Parkinson’s disease. Br Med Bull 86:109–127
Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2009) The retina in Parkinson’s disease. Brain 132:1128–1145
Harnois C, Di Paolo T (1990) Decreased dopamine in the retinas of patients with Parkinson’s disease. Investig Ophthalmol Vis Sci 31:2473–2475
Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA (1984) Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41:72–80. https://doi.org/10.1001/archpsyc.1984.01790120076010
Terman M, Terman JS (2005) Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr 10:647–663
Willis GL, Turner EJD (2007) Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int 24:521–537. https://doi.org/10.1080/07420520701420717
Endo T, Matsumura R, Tokuda IT, Yoshikawa T, Shigeyoshi Y, Node K, Sakoda S, Akashi M (2020) Bright light improves sleep in patients with Parkinson’s disease: possible role of circadian restoration. Sci Rep 10:7982. https://doi.org/10.1038/s41598-020-64645-6
Paus S, Schmitz-Hübsch T, Wüllner U, Vogel A, Klockgether T, Abele M (2007) Bright light therapy in Parkinson’s disease: a pilot study. Mov Disord 22:1495–1498. https://doi.org/10.1002/mds.21542
Willis GL (2008) Intraocular microinjections repair experimental Parkinson’s disease. Brain Res 1217:119–131. https://doi.org/10.1016/j.brainres.2008.03.083
Willis GL (2008) Parkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci 19:245–316
Willis GL, Kelly AMA, Kennedy GA (2008) Compromised circadian function in Parkinson’s disease: enucleation augments disease severity in the unilateral model. Behav Brain Res 193:37–47. https://doi.org/10.1016/j.bbr.2008.04.017
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. https://doi.org/10.1126/science.1067262
Skene DJ, Lockley SW, Arendt J (1999) Melatonin in circadian sleep disorders in the blind. NeuroSignals 8:90–95. https://doi.org/10.1159/000014575
Skene DJ, Arendt J (2007) Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med 8:651–655. https://doi.org/10.1016/j.sleep.2006.11.013
Rodgers J, Hughes S, Pothecary CA, Brown LA, Hickey DG, Peirson SN, Hankins MW (2018) Defining the impact of melanopsin missense polymorphisms using in vivo functional rescue. Hum Mol Genet 27:2589–2603. https://doi.org/10.1093/hmg/ddy150
Lucas RJ, Hattar S, Takao M et al (2003) Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299:245–247. https://doi.org/10.1126/science.1077293
Gamlin PDR, McDougal DH, Pokorny J et al (2007) Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res 47:946–954. https://doi.org/10.1016/j.visres.2006.12.015
Smilowska K, Van Wamelen DJ, Schoutens AMC et al (2019) Blue light therapy glasses in Parkinson’s disease: patients’ experience. Parkinson’s Dis 2019:1–4. https://doi.org/10.1155/2019/1906271
Willis GL, Moore C, Armstrong SM (2014) Parkinson’s disease, lights and melanocytes: looking beyond the retina. Sci Rep 4. https://doi.org/10.1038/srep03921
van de Kraats J, van Norren D (2007) Optical density of the aging human ocular media in the visible and the UV. J Opt Soc Am A Opt Image Sci Vis 24:1842. https://doi.org/10.1364/josaa.24.001842
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression, and mortality. Neurology 17:427–441
Mailankody P, Lenka A, Pal PK (2019) The role of optical coherence tomography in parkinsonism: a critical review. J Neurol Sci 403:67–74. https://doi.org/10.1016/j.jns.2019.06.009
Park KW, Choi N, Ryu H-S, Kim MS, Lee E-J, Chung SJ (2019) Pupillary dysfunction of multiple system atrophy: Dynamic pupillometric findings and clinical correlations. Parkinsonism Relat Disord 65:234–237
Adhikari P, Zele AJ, Feigl B (2015) The post-illumination pupil response (PIPR). Investig Ophthalmol Vis Sci 56:3838–3849. https://doi.org/10.1167/iovs.14-16233
Joyce DS, Feigl B, Kerr G, Roeder L, Zele AJ (2018) Melanopsin-mediated pupil function is impaired in Parkinson’s disease. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-26078-0
Sengupta P, Dutta K, Ghosh S, Mukherjee A, Pal S, Basu D (2018) Optical coherence tomography findings in patients of Parkinson’s disease: an Indian perspective. Ann Indian Acad Neurol 21:150–155
Unlu M, Duygu, Sevim G et al (2018) Correlations among multifocal electroretinography and optical coherence tomography findings in patients with Parkinson’s disease. Neurol Sci 39:533–541. https://doi.org/10.1007/s10072-018-3244-2
Lax P, Ortuño-Lizarán I, Maneu V, Vidal-Sanz M, Cuenca N (2019) Photosensitive melanopsin-containing retinal ganglion cells in health and disease: implications for circadian rhythms. Int J Mol Sci 20. https://doi.org/10.3390/ijms20133164
Korshunov KS, Blakemore LJ, Trombley PQ (2017) Dopamine: a modulator of circadian rhythms in the central nervous system. Front Cell Neurosci 11:1–17. https://doi.org/10.3389/fncel.2017.00091
Do MTH (2010) Intrinsically photosensitive retinal ganglion cells. Physiol Rev 90:1547–1581. https://doi.org/10.1152/physrev.00013.2010.Intrinsically
Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG (2019) Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol 39:31–59. https://doi.org/10.1007/s10571-018-0632-3
Panda S, Sato TK, Castrucci AM et al (2002) Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298:2213–2216. https://doi.org/10.1126/science.1076848
Mrosovsky N (1999) Masking: history, definitions, and measurement. Chronobiol Int 16:415–429
Park KW, Choi N, Ryu HS, Kim MS, Lee EJ, Chung SJ (2019) Pupillary dysfunction of multiple system atrophy: dynamic pupillometric findings and clinical correlations. Parkinsonism Relat Disord 65:234–237. https://doi.org/10.1016/j.parkreldis.2019.05.003
Tabashum T, Zaffer A, Yousefzai R, Colletta K, Jost MB, Park Y, Chawla J, Gaynes B, Albert MV, Xiao T (2021) Detection of Parkinson’s Disease Through Automated Pupil Tracking of the Post-illumination Pupillary Response. Front Med 8
Funding
Partial funding for this project was provided by the Richard Perritt Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by the Edward Hines Jr. Institutional Review Board and was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate and consent for publication
Consent for participating in this study as well as consent for publication was obtained from all study subjects prior to participation in any study-related protocol.
Conflict of interest
The authors declare no competing interests.
Informed consent
Freely provided informed consent documentation acknowledging subject understanding of the purpose, procedures and risks involved in this research study was obtained from all study subjects prior to study participation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaynes, B.I., Zaffer, A., Yousefzai, R. et al. Variable abnormality of the melanopsin-derived portion of the pupillary light reflex (PLR) in patients with Parkinson’s disease (PD) and parkinsonism features. Neurol Sci 43, 349–356 (2022). https://doi.org/10.1007/s10072-021-05245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05245-8