Abstract
Endoscopic thoracic sympathectomy is routinely used to treat severe hyperhidrosis. It is usually performed at the T2–T3 level of the nerve, but may produce less severe compensatory hidrosis if performed at a lower level.
This study evaluates the outcome of 1,274 patients who underwent endoscopic thoracic sympathectomy for plamar, plantar, axillary or facial hyperhidrosis/blushing. Half of the patients were clamped at the T2–T3 level and half were clamped at the T3–T4 level. Postsurgical symptoms and side effects were assessed by interview.
All of patients with palmar hyperhidrosis were cured or improved. Patients with plantar and axillary hyperhidrosis were more likely to be improved at T3–T4 level clamping. Patients with facial hyperhidrosis were more likely to be cured at T2–T3 level, but did show improvement at the T3–T4 level. Overall satisfaction was higher in the T3–T4 group. Some degree of mild compensatory sweating occurred in all patients. However, severe compensatory sweating was more common in the T2–T3 group. Around 2% of patients requested a reversal of their surgery.
Endoscopic thoracic sympathectomy is a safe and effective treatment for hyperhidrosis. Clamping at the T3–T4 level has a more successful outcome. In particular, it appears to reduce the incidence of severe compensatory hidrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential or primary hyperhidrosis affects between 0.5 and 1% of the population [25]. It is characterized by profuse sweating on the palmar surface of the hands, armpits, groin and feet, which can have psychological consequences. It is a problem of sympathetic dysregulation [4, 6, 8, 12, 14, 22, 24, 27, 32] that may have an underlying genetic component [14].
To control their sweat production, patients initially try non-surgical therapies, such as anticholinergic drugs, topical astringents and/or absorbing powders, biofeedback, iontophoresis or botulinum toxin injections [26, 29–31]. However, unless their symptoms are mild, these approaches are rarely successful and the problem persists.
After unsuccessfully attempting at least one non-surgical therapy [22, 23], many patients then seek a surgical approach to manage their hyperhidrosis. Surgical sympathectomies have been carried out for more than 100 years [10], and have been available for the treatment of hyperhidrosis in the last decades [1, 5, 11, 16]. In the 1990s, advancement in endoscopic techniques revolutionized sympathetic surgery, allowing the surgeon to view the sympathetic ganglia through a small incision.
Instead of permanently transecting or cauterizing the sympathetic trunk, some surgeons now apply clamps [17]. These clamps generate pressure on the sympathetic ganglia, which blocks the transmission of sympathetic impulses. The advantage of the clamps is that they can be removed [18], or repositioned [3], if the postsurgical side effects are intolerable. The clamping method (the term “clamping” is used as “clipping” could be conceived as cutting) may be more successful than permanent sympathetic cauterization [18] and has no greater incidence of adverse effects [23].
Endoscopic thoracic sympathectomy is now the standard procedure chosen by physicians for the treatment of severe hyperhidrosis [9, 11, 13, 15, 21]. It is safe and successful in almost 98% of cases, only 1–2% of patients experience recurrence of their hyperhidrosis [16, 23, 28]. All patients develop some degree of compensatory sweating after surgery. In most this is mild and tolerable; however, in 3–5% of patients it is severe and intolerable [7]. Endoscopic thoracic sympathectomy is minimally invasive, reducing postoperative pain, and can be performed on an outpatient basis [23, 28].
Although the surgical techniques have been refined, there is still debate as to exactly what level the sympathectomy should be performed. In retrospective analysis of a large cohort of patients undergoing endoscopic thoracic sympathectomy for hyperhidrosis, the results of clamping at either the T2–T3 level or T3–T4 level are compared.
Materials and methods
Subjects
A total of 1,274 patients (29.3 ± 9.5 years, range 10.4–61.7) with essential hyperhidrosis underwent endoscopic thoracic sympathectomy. 618 patients (48.5%) were clamped at the T2–T3 level (1.5% of these were clamped only at the T2 level). The remaining 656 patients (51.5%) were clamped at the T3–T4 level (five cases also included additional T2 clamping for facial symptoms). The level of clamping was based on the chronological order of the cases. From June 1999 to December 2001 patients received T2–T3 clamping, and from October 2001 to March 2004 T3–T4 clamping, with some overlap during the first few months of introducing the T3–T4 technique.
Prior to surgery in an attempt to control their hyperhidrosis, 83.4% had tried topical medications (e.g., Drysol), 31.2% had tried the Drionic electrical iontophoresis device, 11.9% had tried anticholinergic agents, 11.3% had tried anti-anxiety medications, 5.2% had tried beta-blockers, and 5.2% had tried Botox. Table 1 summarizes patient characteristics.
The majority of patients (91.7%) had palmar sweating, 88.8% had plantar sweating, 19.3% had axillary sweating, 12.6% had facial sweating and 8.7% had facial blushing. 7.9% (n = 100) had facial symptoms alone; 94% of these patients were clamped at the T2–T3 level.
All patients gave written surgical consent. Patients received only the standard clinical procedure. After surgery, patients were followed up by a standard practice telephone interview. All data were stored on an electronic database and analyzed with patient identification removed.
Surgical procedures
All surgical procedures were performed at an outpatient surgical center. Briefly, an 8 to 10 mm incision was made in the fourth intercostal space of the axillary fold (beneath the pectoralis muscle), to initiate insufflation with a surgical CO2 insufflator. Adult patients were insufflated with 0.6 l CO2, while in pediatric patients, 0.4–0.5 l CO2 was used. Through the incision, a 10/11 mm trocar (TroGARD Finesse Dilating Trocar System, CONMED Corp., Utica, NY) and a 10 mm endoscope with a working channel (Olympus 10 mm operating scope A5240, Olympus America Inc., Melville, NY) were inserted. A custom designed 5 mm diameter metal nerve hook was introduced and used to elevate the nerve. The hook was specifically designed to allow precise positioning of the jaws of the clamp applicator on the nerve, while at the same time limit the damage to the surrounding tissues. Typically, two clamps (5 mm autosuture endoscopic clips) were applied at the upper level (either T2 or T3) and two clamps below the lower rib (either T3 or T4), so that the whole segment, between the two ribs, was eliminated. After the pleural space was exsufflated, the lung was inflated back to the chest wall and skin sites were closed. The procedure was then repeated on the other side.
The entire procedure was complete in less than 30–45 minutes. After 60–90 minutes of recovery, chest X-rays were taken to rule out a pneumothorax or hemothorax and check the positioning of the clamps. Following this, patients were discharged. As the procedure inflicted limited tissue damage, postoperative pain was typically mild to moderate and treated with oral pain medications (e.g., ibuprofen or codeine-containing drugs).
Postoperative outcome scores
Data was collected by review of medical charts, telephone interviews, and e-mail or mail correspondence. During an interview, patients were asked to state whether they considered their symptoms to be ‘cured’, ‘improved’, or ‘unchanged’. Based on a series of questions, patient satisfaction was rated on a 5-point scale ranging from ‘very unsatisfied’ to ‘very satisfied’. Any occurrence of gustatory sweating, compensatory sweating, and recurrence was noted. Based on the patient’s comments, ‘severe’ compensatory hidrosis was indicated when the patient mentioned that sweating interfered with their normal activity, for example, when clothing had to be changed two or three times per day. ‘Moderate’ compensatory hidrosis was noted when the patient indicated that their sweating was not bothersome. ‘Mild’ compensatory hidrosis was considered when the patient did not mention dampness or made only a brief reference to their sweating. The effect of compensatory hidrosis on their quality of life was rated as ‘mild’, ‘moderate’ or ‘severe’. Finally, the patients were asked whether they would recommend the procedure to a friend. In those few cases where a second, or “redo” operation was performed, the final outcome was reported.
Data analysis
Unless otherwise stated, all data are expressed as mean ± SD or as percentages. Descriptive and inferential statistical analyses were performed, using both parametric and nonparametric procedures, as appropriate. Comparisons of categorical/ordinal variables were performed using Chi-Square. Satisfaction ratings were grouped simply as ‘satisfied’ or ‘unsatisfied’ for statistical comparison. Continuous variables were compared using an independent group t-test. Criterion for statistical significance was set at P ≤ 0.05, two-tailed.
Results
Not all patients could be reached for follow-up. In total, 95% of patients with palmar symptoms and 100% of patients with only facial symptoms were followed up. Treatment efficacy was assessed in 1,199 of the 1,274 patients (94%). Mean time to follow up was 17.9 ± 9.3 months for the T2–T3 group and 8.2 ± 4.6 months for the T3–T4 group.
Postsurgical outcomes
Symptoms
A total of 99.5% of patients considered their palmar hyperhidrosis to be cured. The remaining 0.5% considered themselves “improved” but not completely cured.
The majority of patients with plantar sweating, at both clamping levels, stated that they were ‘improved’ but not ‘cured’. Patients clamped at the T3–T4 level reported more improvement in plantar sweating compared to those clamped at the T2–T3 level (P < 0.01).
Of the 239 patients with axillary sweating, those clamped at the T3–T4 level had a higher rate of improvement (P < 0.01).
Most of the 218 patients with facial sweating or blushing, reported being improved or cured. However, those clamped at the T2–T3 level were more likely to report being cured than those clamped at the T3–T4 level (57.1% vs. 3.5%; P < 0.001)
When the 100 patients with only facial symptoms are examined separately, 50% (three out of six) of the T3–T4 clamped patients reported that they were “unchanged”, compared to only 3.2% (3 out of 94) of the T2–T3 clamped patients. Table 2 shows sympathectomy level and symptoms outcomes for all patients.
Patient satisfaction
Most patients were satisfied with the outcome of their sympathectomy (Table 3). When both groups were combined, the overall satisfaction rate was 96.2%. The unsatisfied rate was highest in patients who underwent surgery for facial symptoms alone. When these facial symptom patients were excluded, the satisfaction rate rose to 97.6%. Those clamped at the T3–T4 level showed a slightly higher rate of satisfaction compared to those clamped at the T2–T3 level (98.6% vs. 93.8%, P < 0 .001). However, patients clamped at the T3–T4 level had a higher rate (75.9%) of being ‘very satisfied’ compared to the T2–T3 group (53.5%, P < 0.001). 84.6% of patients said they would recommend the procedure to a friend.
Complications
Surgical complications were rare. There was no incidence of hemothorax and only four cases (0.3%) of pneumothorax (three in the T2–T3 group). There were no cases of gustatory sweating in the T3–T4 group, however, the prevalence was 2.1% in the T2–T3 group (P < 0.001). Symptoms reoccurred in a small number of patients (1.4%). Eleven patients (0.9%) eventually had their operation redone. Recurrence and redo rates did not differ between groups.
Compensatory hidrosis
As expected, there was some degree of compensatory hidrosis in all patients. Severe compensatory hidrosis was uncommon and occurred in 5.5% of all patients. However, only 1.4% of patients indicated that their compensatory hidrosis was severe enough to interfere with everyday life.
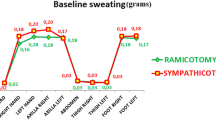
As shown in Figure 1, the rate of mild compensatory sweating was highest in the T3–T4 group while moderate and severe compensatory sweating were more common in the T2–T3 group (P < 0.001) (severe = 8.2% and 2.7% for T2–T3 and T3–T4, respectively).
As compensatory hidrosis may develop over time and the follow up time in the T2–T3 group was longer, secondary analysis using only those patients who were followed up after 12 months (396 patients in the T2–T3 group and 109 patients in the T3–T4 group) was performed. Whilst the number of patients who reported severe compensatory hidrosis increased, the rate remained higher in the T2–T3 group (9.9% vs. 5.5%, T2–T3 vs. T3–T4; P < 0.001).
Around 2% (n = 25) of patients (3.6% of the T2–T3 patients and 0.5% of the T3–T4 patients) were unsatisfied with their surgery, most due to compensatory hidrosis, and underwent removal of the clamps. Despite being happy with their postsurgical sweating, two males in the T2–T3 group had the clamps removed as they could no longer achieve their desired heart rates during exercise.
A later study of 31 patients who underwent reversal of clamps at our institution found that 80.6% reported that they were improved after follow-up, although no objective assessment of how their sweating resumed was obtained. More recent experience by the author suggests that the actual improvement rate after clamp removal may be lower (65–70%).
Discussion
Implications
Endoscopic thoracic sympathectomy is a safe treatment for hyperhidrosis. These results indicate that T3–T4 level clamping has the highest rate of postsurgical success and the lowest rate of moderate to severe compensatory hidrosis.
Palmar hyperhidrosis was nearly always cured. An equal percentage of patients were cured when clamped at the T2–T3 level or the T3–T4 level. Plantar or axillary hyperhidrosis was more likely to be improved, rather than cured, and the rate of improvement was higher at the T3–T4 level. Facial symptoms were more likely to be cured with T2–T3 clamping, but they were improved at the T3–T4 level. Overall postoperative satisfaction was higher in the T3–T4 group, and this was probably due to the lower rate of moderate or severe compensatory hidrosis. Recurrence rates were lowest in the T3–T4 group. These results suggest that T3–T4 clamping is preferable to T2–T3 for palmar, plantar and axillary hyperhidrosis.
Until recently, patients with palmar hyperhidrosis or facial hyperhidrosis/blushing were clamped at the same level. However, surgical indications have changed [22, 23]. In theory, as most of the sympathetic innervation to the head area originates from the T2 ganglia, it is thought that the T2 should be sympathectomized to treat craniofacial hyperhidrosis and/or facial blushing. Patients with facial symptoms clamped at the T2–T3 level are more likely to be cured, but more likely to suffer from more severe compensatory hidrosis. Whereas, patients with facial symptoms clamped at T3–T4 are improved (but not cured), but do have a lower risk of severe compensatory hidrosis. The overall outcome favors T3–T4 clamping. In patients with facial symptoms alone, sympathectomy is rarely successful and symptoms can worsen over time. As in our clinical practice, perhaps we should all avoid sympathectomy in patients with only facial symptoms.
Postsurgical side effects
After sympathectomy, we assume that all patients experience some degree of compensatory sweating, due to the disruption of sympathetic activity. The rate of severe compensatory hidrosis is lower using the clamping method [2, 23]. The white rami comunicantes and its axons from cells in the intermedolateral column can be damaged when permanent sympathecomy techniques, such as cauterization, are used [2]. As a consequence, cell bodies in the spinal cord may die or reorganize, and the resultant neuronal remodeling may lead to enhance sympathetic tone in other areas. With the clamping technique used in this study, the dissection was minimal and lateral coagulation is only used when the clamps are activated, which reduces the risk of damage to the delicate surrounding areas. Moreover, the specific design of the custom nerve hook, used in these surgeries, further reduces surrounding tissue damage. This may also explain why the rate of severe compensatory hidrosis is lower with the clamping method [2, 23].
In theory, after sympathectomy, there is decreased sympathetic activity from the lower ganglia to the head, which may then cause increased sweating in other body areas to maintain adequate temperature regulation. When the sympathectomy is carried out below T2, there may be more intact sympathetic supply to the head and less anhydrosis in the upper body, and as a result less compensatory hidrosis. This theory is supported by these results, as severe compensatory hidrosis was lower in the T3–T4 group compared to the T2–T3 group.
Gustatory sweating is also a relatively common side effect after interrupting sympathetic activity [23]. However, it is normally mild and tolerable. The pathophysiology of gustatory sweating is not completely known, but may be due to collateral sprouting at the stellate ganglion or parasympathetic sprouting of the 9th and 10th nerves [19]. Clamping has reduced the rate of gustatory sweating, and clamping at the T3–T4 level further reduces its incidence. In this study, none of the patients in the T3–T4 group reported this side effect. Minimal tissue damage and intact T2 nerves may explain the absence of postsurgical gustatory sweating.
Disrupted sympathetic activity after sympathectomy can lead to Horner’s syndrome [1]. Lower level clamping at T3–T4 probably explains the absence of Horner’s syndrome in our patients, as sympathetic innervation to the eye remained intact.
One complication that did occur in two of these patients was the inability to reach a desired maximal heart rate during exercise. This has previously been reported [20].
Limitations
The outcome of the patients was subjective, and not measured objectively by quantitively assessing postsurgical sweating. However, interviews are routinely used and data was entered prospectively into a database. This hopefully reduces the risk of bias.
The direct comparison of two different surgeries (i.e., sympathectomy levels) is often difficult when different surgeons are involved. However, in this study, the surgeon, the specific techniques and instrumentation were the same, thus it was just the location of the clamps that differed and not the “surgical skill”.
The lower rate of compensatory hidrosis in the T3–T4 group should be interpreted with caution as they were followed up sooner than the T2–T3 group.
Conclusions
Endoscopic thoracic sympathectomy using the clamping method is a safe and effective treatment for hyperhidrosis. Recurrence rates are low and patient satisfaction is high. All patients experience some degree of mild compensatory hidrosis; however, rates of severe compensatory hidrosis are low using the clamping method. Clamping at the T3–T4 level has a higher rate of success and a lower risk of severe compensatory hidrosis.
References
Adar R, Kurchin A, Zweig A, et al. (1977) Palmar hyperhidrosis and its surgical treatment: a report of 100 cases. Ann Surg 186:34–41
Atkinson JLD, Fealey RD (2003) Sympathotomy instead of sympathectomy for palmar hyperhidrosis: minimizing postoperative compensatory hyperhidrosis. Mayo Clin Proc 78:167–172
Chou SH, Kao EL, Lin CC, Huang MF (2006) The outcome of ganglion clipping in hyperhidrosis, blushing. Clin Auton Res 16(3):240–242. Epub 2006 May 12
De Marinis M (2005) Oculosympathetic hyperactivity in idiopathic hyperhidrosis. Clin Auton Res 15(6):417–418
Drott C (1994) The history of cervicothoracic sympathectomy. Eur J Surg 572:5–7
Drott C, Class G, Olsson-Rex L, et al. (1998) Successful treatment of facial blushing by endoscopic transthorascopic sympathectomy. Br J Dermatol 138:639–643
Fredman B, Zohar E, Shachor D, et al. (2000) Video assisted transthoracic sympathectomy in the treatment of primary hyperhydrosis. Friend or foe? Surg Laparosc Endosc Percutan Tech 10:226–229
Goetz RH (1948) Physiology of the sympathetic nervous system with special reference to cardiovascular disorders. Int Abst Surg 87:417–439
Gossot D, Toledo L, Fritsch S, Celerier M (1997) Thoracoscopic sympathectomy for upper limb hyperhidrosis: looking for the right operation. Ann Thorac Surg 64:975–978
Hashmonai M, Kopelman D (2003) History of sympathetic surgery. Clin Auton Res 1:I6–9
Hashmonai M, Kopelman D, Schein M (1994) Thoracoscopic versus open supraclavicular upper dorsal sympathectomy; a prospective randomized trial. Eur J Surg 572(suppl):13–16
Kao MC, Chen YL, Lin JY, et al. (1996) Endoscopic sympathectomy treatment for craniofacial hyperhidrosis. Arch Surg 131:1091–1094
Kao MC, Lin JY, Chen YL, et al. (1996) Minimally invasive surgery: video endoscopic thoracic sympathectomy for palmar hyperhidrosis. Ann Acad Med Singapore 25:673–678
Kaufman H, Saadia D, Polin C, Hague S, Singelton A, Singelton A (2003) Primary hyperhidrosis: evidence of autosomal dominant inheritance. Clin Auton Res 13:96–98
Kopelman D, Hashmonai M, Ehrenreich M, et al. (1996) Upper dorsal thoracoscopic sympathectomy for palmar hyperhidrosis: improved intermediate term results. J Vasc Surg 24:194–199
Kux M (1978) Thoracic endoscopic sympathectomy in palmar and axillary hyperhidrosis. Arch Surg 113:264–266
Lin CC, Mo LR, Lee LS, et al. (1998) Thoracoscopic T2 block by clipping: a better and reversible operation for the treatment of hyperhidrosis palmaris: experience with 326 patients. Eur J Surg 164(Suppl580):13–16
Lin T-S, Huang L-C, Wang N-P, et al. (2001) Video-assisted thoracoscopic T2 sympathetic block by clipping for palmar hyperhidrosis: analysis of 52 cases. J Laparoendosc Adv Surg Tech 11:59–62
Nesathurai S, Harvey DT, Schatz SW (1995) Gustatory facial sweating subsequent to upper thoracic sympathectomy. Arch Phys Med Rehabil 76:104–107
Noppen M, Dendale P, Hagers Y, et al. (1996) Changes in cardiocirculatory anatomic function after thoracoscopic upper dorsal sympaticolysis for essential hyperhidrosis. J Auton Nerve Syst 60:115–120
Noppen M, Herregodts P, D‘haens J, et al. (1996) A simplified T2–T3 thoracoscopic sympaticolysis technique for the treatment of essential hyperhidrosis: short-term results in 100 patients. J Laparo-endosc Surg 6:151–159
Reisfeld R, Nguyen R, Pnini A (2000) Endoscopic thoracic sympathectomy for treatment of essential hyperhidrosis syndrome: experience with 650 patients. Surg Laparosc Endosc Percutan Tech 10:5–10
Reisfeld R, Nguyen R, Pnini A (2002) Endoscopic thoracic sympathectomy for hyperhidrosis. experience with both cauterization and clamping methods. Surg Laparosc Endosc Percutan Tech 12:255–267
Rex LO, Drott C, Claes G, et al. (1998) The Boras experience of endoscopic thoracic sympathectomy for palmar, axillary, facial hyperhidrosis and blushing. Eur J Surg 164(suppl 580):23–26
Ro KM, Cantor RM, Lange KL, Ahn SS (2002) Palmer hyperhidrosis: evidence of genetic origin. J Vasc Surg 35:382–386
Shelly WB, Talanin NY, Shelly ED (1998) Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol 38:227–229
Shih CJ, Wu JJ, Lin MT (1983) Autonomic dysfunction in palmar hyperhidrosis. J Anatom Nerv Syst 8:33–42
Singh B, Huffejee AA, Moodley J, et al. (1996) Endoscopic thoracic sympathectomy: the Durban experience. S Afr J Surg 34:11–16
Solomon BA, Hayman B (2000) Botulinum toxin type A therapy for palmar and digital hyperhidrosis. J Am Acad Dermatol 42:1026–1029
Stolman LP (1987) Treatment of excess sweating of the palms by iontophoresis. Arch Dermatol 123:893–896
Stolman LP (1998) Treatment of hyperhidrosis. Dermatol Clin 16:863–867
Telaranta T (1998) Treatment of social phobia by endoscopic thoracic sympathectomy. Eur J Surg 164(Suppl 580):27–32
Acknowledgments
Karen I. Berliner, Ph.D. for data analysis and help with manuscript editing. RR performed all surgeries.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reisfeld, R. Sympathectomy for hyperhidrosis: should we place the clamps at T2–T3 or T3–T4?. Clin Auton Res 16, 384–389 (2006). https://doi.org/10.1007/s10286-006-0374-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-006-0374-z