Abstract
The aim of the present study was to compare the effect of chitosan nanoparticle, QMix, and 17% EDTA on the penetrability of a calcium silicate-based sealer into dentinal tubules using a confocal laser scanning microscope (CLSM). Sixty mandibular premolar teeth were selected and randomly divided into three groups (n = 20) before root canal preparation according to the solution used in the final rinse protocol: chitosan, QMix, and EDTA groups. Twenty teeth of each group were filled with a TotalFill BC sealers’ single gutta-percha cone and with 0.1% rhodamine B. The specimens were horizontally sectioned at 3 and 5 mm from the apex, and the slices were analyzed in CLSM (4×). Total percentage and maximum depth of sealer penetration were measured using confocal laser scanning microscopy with using Image J analysis software. Dentinal tubule’s penetration depth, percentage, and area were measured using imaging software. Kruskal–Wallis test was used for statistical analysis. The level of significance was set at 5%. Results of Kruskal–Wallis analysis showed that there was a significant difference in the percentage and depth of sealer penetration among all groups at 3 and 5 mm level sections (P < 0.05). Within the groups, the minimum sealer penetration depth was recorded for chitosan nanoparticle group. Greater depth of sealer penetration was recorded at 5 mm as compared to 3 mm in all the groups. Within the limitation of the present study, it can be concluded that QMix and EDTA promoted sealer penetration superior to that achieved by chitosan nanoparticle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies showed that removal of the smear layer improved the success and quality of root canal treatment by increasing the penetration of root canal sealers into dentinal tubules during obturation [1, 2]. Traditionally, sodium hypochlorite and ethylene diamine tetra acetic acid (EDTA) solution have been used to remove the smear layer [3]. QMix (Dentsply Sirona, Baillagues, Switzerland) is a mixture of EDTA, chlorhexidine, and detergent. According to previous research, when QMix was used as the final irrigation solution, it removed the smear layer and provided a residual antimicrobial effect [4].
Chitosan is a cationic biopolymer obtained from deacetylation of chitin in alkaline media, with biocompatible, biodegradable, and environmentally friendly properties [5]. Due to its unique physicochemical and biological properties, chitosan has many uses, such as pharmaceutical, biotechnological, cosmetic, agricultural, biomedical, food processing, and textile applications [6]. In addition, chitosan has pendant-free functional –OH and –NH2 groups on a polymer chain that enable various modifications, which lead to improved mechanical strength and chemical stability [7]. One important modification using sodium tripolyphosphate has led to the production of chitosan nanoparticles. These nanoparticles are widely used in biomedical applications and drug delivery systems because of their distinctive physicochemical properties, such as sensitivity, specificity, and bioavailability [8]. Chitosan nanoparticles have also been used in endodontics due to their antibacterial and smear removal properties [9].
Bioceramic-based root canal sealers have advantageous physical and biological properties and are therefore commonly used. Previous research reported that bioceramic-based sealers composed of calcium phosphate and calcium silicate showed strong hydrophilic properties [10]. Another study demonstrated that bioceramic-based sealers had high tubular penetration capability, therefore making them suitable for use in obturation procedures [11]. TotalFill BC (FKG Dentaire, La-Chaux-de-Fonds, Switzerland) is a bioceramic-based sealer with high radiopacity. Based on an extensive literature search, there appear to be no studies of the effect of chitosan nanoparticle solution on dentinal tubule penetration of bioceramic-based root canal sealers. The aim of this study was to evaluate the effect of EDTA, QMix, and chitosan nanoparticle irrigation solutions on dentinal tubule penetration by TotalFill BC root canal sealer using confocal laser microscopy (CLSM). The null hypothesis of the study was that the different final irrigation solutions would have no effect on dentinal tubule penetration by TotalFill BC root canal sealer.
Materials and methods
Sample selection
After obtaining the approval of the ethics committee (No: 2017-82), 60 freshly extracted mandibular premolar teeth due to orthodontic reasons with mature apices were included in the study. Soft and hard tissues, and debris around the teeth were mechanically removed with the aid of a periodontal curette (Hu-Friedy Mfg. Co. Inc., Leimen, Germany). Radiographs of mesio-distal and bucco-lingual aspects were obtained. Teeth with canal calcification, endodontic treatment, internal or external resorption, fracture, and immature roots were replaced with new ones. Root had less than 5 ° curvature and teeth with similar length (19 ± 1 mm) were also selected for standardization. The crowns of the teeth were removed under water cooling using diamond burs (Diatech, Charleston, SC, USA) in the direction of the cemento-enamel junction, and samples with a root length of 12 ± 1 mm were obtained. The selected teeth were stored in 0.1% thymol solution at 5 °C until used in further studies.
Root canal preparation
A size #10K file (Dentsply Sirona, Baillagues, Switzerland) was inserted into the root canal until it was visible in the apical foramen, and the working length of each root canal was determined to be 1 mm shorter than this measurement. The root canals were prepared using a ProTaper Next NiTi file system (Dentsply Sirona) until the apical diameter 40/0.06 (size 40, 0.06 taper) was obtained. The system was operated at 300 rpm and 400 g cm−1 torque. A total of 20 ml of 6% sodium hypochlorite (CanalPro; Coltene-Whaledent, Allstetten, Switzerland) was used throughout the root canal preparation. A double-port 31-gauge needle (NaviTip Sideport; Ultradent Products Inc., South Jordan, UT, USA) was used for all irrigations. After rinsing all the canals using 2-ml distilled water each, the teeth were then randomly divided into three groups (n = 20), and the following final irrigation procedures were performed:
Group 1: EDTA
In this group, each tooth was irrigated for 60 s with a solution of 5 ml of 17% EDTA (CanalPro; Coltene-Whaledent) in total. To remove the residual irrigation solution after the final irrigation, all teeth were irrigated with 2 ml of distilled water, and the root canal was dried with paper points (DiaDent Group International Inc., Cheongju, Korea).
Group 2: QMix
In this group, each tooth was irrigated for 60 s with 5 ml of QMix solution (Dentsply Sirona) in total. To remove residual irrigation solution after the final irrigation, all teeth were irrigated with 2 ml of distilled water, and the root canal was dried with paper points (DiaDent Group International Inc.).
Group 3: Chitosan nanoparticles
Chitosan nanoparticles were prepared according to the literature [12]. Briefly, 1 g of chitosan was dissolved in 50 ml of acetic acid solution at room temperature (2%, w:w) and stirred overnight to obtain a clear solution. Freshly prepared sodium tripolyphosphate (3 ml, 2% TPP, v/v) was then added to the reaction media and stirred for 30 min. At the end of the reaction, chitosan particles were separated using centrifugation (10,000 rpm, 10 min) and examined under a scanning electron microscope (JSM-7001F; JEOL, Tokyo, Japan) to verify that they had a homogenous structure. SEM images indicated that the average dimensions of the chitosan nanocomposite ranged between 71 and 78 nm (Fig. 1).
Root canal obturation
Total Fill BC Sealer (FKG Dentaire) was mixed with 0.1% rhodamine B (Sigma-Aldrich, St. Louis, MO, USA) and placed in the canal 1 mm shorter than the working length using a 400-rpm lentulo spiral (Dentsply Sirona) for 5 s [13]. The canals were then obturated with BC Points (FKG Dentaire) using the single cone technique. The samples were temporarily restored with filling material (Coltosol; Coltene-Whaledent) and incubated at 37 °C in 100% humidity for 14 days to allow the root canal sealer to set.
Evaluation of dentinal tubule penetration
The specimens were cut using a 0.4-mm-thick diamond disk at low-speed under water cooling (Isomet 1000, Buehler, IL, USA). Two sections 1 ± 0.1-mm-thick were obtained a distance of 3 and 5 mm from the root apex. The sections were then polished with silicon carbide abrasive paper to produce a smooth surface and eliminate dentinal debris generated during the cutting procedures.
All the specimens were mounted onto glass slides and examined using a confocal laser microscope (Carl Zeiss LSM 510; Carl Zeiss Microscopy, Jena, Germany) at a wavelength of 575 nm and 4× magnification, with a zoom oil lens. In cases where the entire canal could not be examined in one image, further partial images were taken and then assembled as a single image using Pages software (Apple Inc., Cupertino, CA, USA). Digital images were imported into Image J analysis software (v. 1.44p; National Institutes of Health, Bethesda, MD, USA) to measure the total dentinal tubule penetration area. The dentinal tubule penetration area was measured in square millimeters (mm2). Each section was evaluated using a Zeiss LSM Image Browser, version 4.2.0.121 (Carl Zeiss Micro Imaging GmbH 1997–2006) and calibrated measuring tool. The maximum depth and total percentage of sealer penetration were measured as previously described [14].
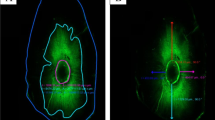
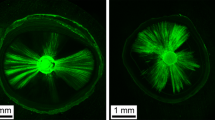
Figure 2 shows CLSM images of tooth sections at 3 and 5 mm levels. The images obtained from confocal microscopy were analyzed using Image J analysis software (National Institutes of Health). The circumference of the root canal wall was outlined and measured using the measuring tool available in the software. Areas along the canal walls that showed sealer penetration into dentinal tubules were then measured. The measured distances were divided by the total canal circumference to calculate the percentage of the area of canal wall covered by sealer. To measure the depth of penetration, the point of deepest penetration was measured from the canal wall to the point of maximum sealer penetration.
CLSM images representative of tag formation at the sealer–dentine interface in apical and coronal sections after different irrigation solutions. Longer tags with greater density can be observed in the specimens irrigated with QMix and EDTA solutions. A smaller number of tags can also be observed in the apical sections than in the coronal sections
Statistical analysis
The data were first analyzed using the Shapiro–Wilk test to verify the assumption of normality. The Kruskal–Wallis test was then performed for statistically analyzing the data using SPSS 21.0 (IBM-SPSS Inc., Chicago, IL, USA) software. The statistical significance level was set at 5%.
Results
The means and standard deviations of dentinal tubule penetration depths, areas, and percentages at 3 and 5 mm in the different groups are shown in Table 1.
Evaluation of sealer penetration depth
The Kruskal–Wallis analysis showed that the sealer penetration depth at 3 and 5 mm sections in all the groups was significantly different (P < 0.05). The penetration depth in all the groups was significantly higher in 5 mm sections than 3 mm sections (P < 0.05). In the between-group comparison, the sealer penetration depth was significantly lower in both the 3 and 5 mm sections of chitosan group as compared with the 3 and 5 mm sections of EDTA and QMix groups (P < 0.05).
Evaluation of sealer penetration area
When the intragroup penetration areas were compared, in all the groups, the area of sealer penetration was significantly higher in the 5 mm section than 3 mm section (P < 0.05). In the between-group comparison, the sealer penetration area was significantly lower in both the 5 and 3 mm sections in the chitosan group as compared with that in the EDTA and QMix groups.
Evaluation of sealer penetration percentage
When the percentage of intracanal penetration was compared, the percentage of sealer penetration was significantly higher in the 5 mm sections than 3 mm sections in both the EDTA and QMix groups (P < 0.05). There was no significant difference in the sealer penetration percentage values in the 5 mm and 3 mm sections in the chitosan group (P > 0.05). When the percentage of penetration between groups was compared, there was no significant difference in the 5 and 3 mm sections in the EDTA and QMix group. However, the percentage of penetration was lower in both 3 and 5 mm sections in the chitosan group as compared with that of the other two groups (P ˂ 0.05).
Discussion
Sealer penetration into dentinal tubules is considered a positive outcome to prevent bacterial regrowth or bacterial inactivation inside tubules [15] and improve sealer retention [16]. Therefore, sealer penetration into dentinal tubules is considered clinically relevant [17]. Several studies evaluated the effect of different final irrigation solutions and procedures on dentinal tubule penetration by root canal sealers [16, 18, 19]. However, to the best of our knowledge, there have been no studies of the effect of chitosan irrigation solution on dentinal tubule penetration by root canal sealers. Thus, the aim of the present study was to investigate the effect of QMix, chitosan, and EDTA solutions on dentinal tubule penetration by TotalFill BC sealer.
Jeong et al. [20] reported that there was no significant difference between the single-cone and warm vertical-compaction technique in terms of bioceramic-based sealer penetration into dentinal tubules. Also, the application of excessive forces during obturation can cause unexpected root fractures. Thus, in the present study, to standardize the obturation pressure, the single-cone technique was used.
Light microscopy [21], scanning electron microscopy [22], and CLSM [17] have been used to investigate sealer penetration into dentinal tubules. CLSM has two main advantages: It does not require sample processing, and it significantly reduces technical artifacts [14]. In addition, using CLSM, the surface below the smear layer can be scanned and analyzed [23]. Thus, in the present study, CLSM was used.
In CLSM analyses of sealer penetration, the sealer is labeled with a specific fluorescent dye. Previous CLSM studies utilized rhodamine B as an indicator of sealer penetration [14]. Rhodamine B dye promotes fluorescence of the sealer because CLSM works with high contrast points to identify the sealers within dentinal tubules [24]. In the present study, for fluorescence of the bioceramic sealer, the sealer was mixed with Rhodamine B (Sigma-Aldrich), employing a similar method to that used by Wiesse et al. [19].
In this study, slices were obtained 3 and 5 mm from the root apex to minimize the inclusion of apical deltas and anatomical irregularities that could affect sealer penetration. In some of the slices scanned using CLSM, sealer penetration into the dentinal tubules was not homogenous. Also, the dentinal tubule directions can affect the results. Therefore, maximal penetration should not be the only parameter used to evaluate sealer penetration. In the present study, three parameters (penetration depth, area, and percentage) were used to calculate dentinal tubule penetration, employing a method similar to that of Gharib et al. [14].
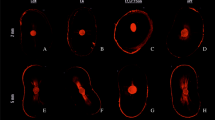
The mineral infiltration zone (MIZ) is a hybrid zone where hydroxyapatite recrystallization occurs in dentin when calcium silicate-based cement is applied [25]. In the present study, some images showed a similar zone when interfacial reactions and hydroxyapatite recrystallization occurred in dentinal tubules (Fig. 3) [20]. In the present study, the images were captured 14 days after obturation to allow for dynamic interactions between dentinal tissue and the calcium silicate-based sealer. The aforementioned may explain the presence of MIZ in the images.
According to the images obtained in the present study, significantly more tubule penetration occurred in the QMix and EDTA groups than in the chitosan group. Thus, the null hypothesis of the present study was rejected. The ability of sealers to penetrate into dentinal tubules depends on many factors such as the humidity, temperature, and contents of dentinal tubules, as well as physical and chemical properties of root canal sealers [15]. In addition, the presence of the smear layer can act as a barrier and prevent the penetration of sealers into dentinal tubules [26]. Additionally, no activation technique was used in the present study. Thus, the smear layer removal capacity of the irrigation solutions might be reduced.
Previous studies of the smear layer removal efficacy of QMix and EDTA solutions reported conflicting results [4, 27,28,29,30]. Some studies found that the smear layer removal efficacy of QMix was as effective as that of EDTA [4, 27, 28]. However, other studies reported that QMix was more successful than EDTA in terms of smear layer removal efficacy [29, 30]. Jardine et al. [31] compared the effect of QMix, BioPure MTAD (Dentsply Sirona), and EDTA final solutions on dentinal tubule penetration by AH Plus (Dentsply Sirona) sealer. Similar to the results of the present study, the authors reported that QMix and EDTA had similar effects on sealer penetration. Chaudhry et al. [32] reported that the performance of QMix and EDTA solutions as regards sealer penetration was the same. The increased tubule penetration obtained with the QMix and EDTA solutions in the present study might be explained by their high smear layer removal ability.
Chitosan nanoparticles are recommended for use in endodontic irrigation solutions due to their antimicrobial properties and smear removal abilities [33]. To the best of our knowledge, no previous studies have evaluated sealer penetration of chitosan nanoparticles. Thus, the results of the present study cannot be directly compared with those of other studies. Previous research reported that the irrigation of root canals with chitosan nanoparticle solution for 3 min effectively removed the smear layer from root canals [9]. According to the results of the current study, less tubule penetration occurred in the chitosan group than in the EDTA and QMix groups. This result might be explained by the reduced smear removal ability of chitosan nanoparticles as compared with that of QMix and EDTA.
Sealer penetration was found to be greater in the bucco-lingual direction compared with the mesio-distal direction, and this finding is in line with that of a previous study [21]. The reason may be related to a phenomenon called the ‘‘butterfly effect,’’ a butterfly like appearance seen on the root cross-sections that occurs as a result of increased sclerosis along the tubules located on the mesial and distal sides of the canal lumen. This effect is common in the single-rooted teeth of humans in a wide range of ages [34, 35].
In the present study, the slices in all groups showed lower penetration depths, areas, and percentages in the 3-mm regions than 5-mm regions. Previous studies also reported decreased tubule penetration values in coronal areas as compared with apical thirds [14, 36, 37]. Areas of sclerotic dentin are more common in the apical third [38]. In addition, the diameters of tubules in the apical third are smaller than those in the middle and coronal third, and the apical third has a lower number of tubules than the middle and coronal third [27]. Furthermore, it is more difficult to remove the smear layer from the apical third than middle and coronal third because of reduced irrigant delivery [29]. These factors might have influenced the findings of the present study.
The results of the current study are clinically relevant because both the area and depth of tubule penetration by sealers affect filling of the root canal system and prevent reinfection [15, 36]. On the other hand, a previous study showed that sealer penetration into dentinal tubules was not directly associated with the apical seal [39]. Root dentin may contain tubule areas across its entire circumference, in addition to areas of tubules with unipolar, bipolar, tripolar, and tetrapolar distributions, or even show a total absence of tubules. The major limitation of the present study was that it was impossible to standardize the amount and distribution of sclerotic dentin. Irregular secondary dentin may influence sealer penetration. As reported previously, careful sample selection does not guarantee a homogeneous dentin pattern among specimens [31]. One of the other limitations of present study was that both of the solutions contain EDTA. Thus, further researches might be beneficial to investigate the effect of different irrigation solutions on bioceramic-based sealers.
Conclusion
Within the limitations of the present study, it can be concluded that QMix and EDTA promoted sealer penetration and that the level of penetration was superior to that achieved by chitosan nanoparticles.
References
Bayram HM, Bayram E, Kanber M, Celikten B, Saklar F. Effect of different chelating solutions on the push-out bond strength of various root canal sealers. Biomed Res. 2017;1:401–6.
Prado M, Simão RA, Gomes BPFA. A microleakage study of gutta-percha/AH Plus and Resilon/Real self-etch systems after different irrigation protocols. J Appl Oral Sci. 2014;22:174–9.
Teixeira C, Felippe M, Felippe W. The effect of application time of EDTA and NaOCl on intracanal smear layer removal: an SEM analysis. Int Endod J. 2005;38:285–90.
Stojicic S, Shen Y, Qian W, Johnson B, Haapasalo M. Antibacterial and smear layer removal ability of a novel irrigant, QMiX. Int Endod J. 2012;45:363–71.
Baskar D, Kumar TS. Effect of deacetylation time on the preparation, properties and swelling behavior of chitosan films. Carbohydr Polym. 2009;78:767–72.
Kumar MR, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb A. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–84.
Ji X, Zhong Z, Chen X, et al. Preparation of 1, 3, 5-thiadiazine-2-thione derivatives of chitosan and their potential antioxidant activity in vitro. Bioorg Med Chem Lett Title. 2007;17:4275–9.
Ghadi A, Mahjoub S, Tabandeh F, Talebnia F. Synthesis and optimization of chitosan nanoparticles: potential applications in nanomedicine and biomedical engineering. Caspian J Intern Med. 2014;5:156–61.
Silva P, Guedes D, Nakadi F, Pécora J, Cruz-Filho A. Chitosan: a new solution for removal of smear layer after root canal instrumentation. Int Endod J. 2013;46:332–8.
Lee JK, Kwak SW, Ha J-H, Lee W, Kim H-C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg Chem Appl. 2017;1:1–8.
McMichael GE, Primus CM, Opperman LA. Dentinal tubule penetration of tricalcium silicate sealers. J Endod. 2016;42:632–6.
Vimal S, Taju G, Nambi KN, Majeed SA, Babu VS, Ravi M, Hameed AS. Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquaculture. 2012;15:14–22.
Barreto MS, do Amaral Moraes R, da Rosa RA, Moreira CHC, Só MVR, Bier CAS. Vertical root fractures and dentin defects: effects of root canal preparation, filling, and mechanical cycling. J Endod 2012;38:1135–9.
Gharib SR, Tordik PA, Imamura GM, Baginski TA, Goodell GG. A confocal laser scanning microscope investigation of the epiphany obturation system. J Endod. 2007;33:957–61.
Kokkas AB, Boutsioukis AC, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J Endod. 2004;30:100–2.
Akcay M, Arslan H, Durmus N, Mese M, Capar ID. Dentinal tubule penetration of AH Plus, iRoot SP, MTA Fillapex, and GuttaFlow Bioseal root canal sealers after different final irrigation procedures: a confocal microscopic study. Laser Surg Med. 2016;48:70–6.
Tuncer AK, Tuncer S. Effect of different final irrigation solutions on dentinal tubule penetration depth and percentage of root canal sealer. J Endod. 2012;38:860–3.
Generali L, Cavani F, Serena V, Pettenati C, Righi E, Bertoldi C. Effect of different irrigation systems on sealer penetration into dentinal tubules. J Endod. 2017;43:652–6.
Wiesse P, Pereira R, Silva-Sousa Y, Estrela C, Sousa-Neto M, Pécora J. Effect of ultrasonic and sonic activation of root canal sealers on the push-out bond strength and interfacial adaptation to root canal dentine. Int Endod J 2018;51:102–11.
Jeong JW, DeGraft-Johnson A, Dorn SO, Di Fiore PM. Dentinal tubule penetration of a calcium silicate-based root canal sealer with different obturation methods. J Endod. 2017;43:633–7.
Weis MV, Parashos P, Messer H. Effect of obturation technique on sealer cement thickness and dentinal tubule penetration. Int Endod J. 2004;37:653–63.
Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F. Sealer penetration and adaptation in the dentinal tubules: a scanning electron microscopic study. J Endod. 2011;37:1576–9.
Van Meerbeek B, Vargas M, Inoue S, et al. Microscopy investigations. Techniques, results, limitations. Am J Dent. 2000;13:3–18.
Baumgartner JC, Ibay AC. The chemical reactions of irrigants used for root canal debridement. J Endod. 1987;13:47–51.
Atmeh A, Chong E, Richard G, Festy F, Watson T. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res. 2012;91:454–9.
Okşan T, Aktener B, Şen B, Tezel H. The penetration of root canal sealers into dentinal tubules. A scanning electron microscopic study. Int Endod J. 1993;26:301–5.
Dai L, Khechen K, Khan S, et al. The effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod. 2011;37:80–4.
Aranda-Garcia AJ, Kuga MC, Vitorino KR et al. Effect of the root canal final rinse protocols on the debris and smear layer removal and on the push-out strength of an epoxy-based sealer. Microsc Res Tech 2013;76:533–7.
Eliot C, Hatton JF, Stewart GP, Hildebolt CF, Gillespie MJ, Gutmann JL. The effect of the irrigant QMix on removal of canal wall smear layer: an ex vivo study. Odontology. 2014;102:232–40.
Elnaghy A. Effect of QMix irrigant on bond strength of glass fibre posts to root dentine. Int Endod J. 2014;47:280–9.
Jardine AP, Da Rosa RA, Santini MF, et al. The effect of final irrigation on the penetrability of an epoxy resin-based sealer into dentinal tubules: a confocal microscopy study. Clin Oral Investig. 2016;20:117–23.
Chaudhry S, Yadav S, Talwar S, Verma M. Effect of EndoActivator and Er, Cr: YSGG laser activation of Qmix, as final endodontic irrigant, on sealer penetration: a confocal microscopic study. J Clin Exp Dent. 2017;9:e218–22.
del Carpio-Perochena A, Kishen A, Felitti R, et al. Antibacterial properties of chitosan nanoparticles and propolis associated with calcium hydroxide against single-and multispecies biofilms: an in vitro and in situ study. J Endod. 2017;43:1332–6.
Vasiliadis L, Darling AI, Levers BG. The amount and distribution of sclerotic human root dentine. Arch Oral Biol. 1983;28:645–9.
Russell AA, Chandler NP, Hauman C, et al. The butterfly effect: an investigation of sectioned roots. J Endod. 2013;39:208–10.
Moon Y-M, Kim H-C, Bae K-S, Baek S-H, Shon W-J, Lee W. Effect of laser-activated irrigation of 1320-nanometer Nd: YAG laser on sealer penetration in curved root canals. J Endod. 2012;38:531–5.
Kara TA. Effect of QMix 2in1 on sealer penetration into the dentinal tubules. J Endod. 2015;41:257–60.
Ribeiro RG, Marchesan MA, Silva RG, Sousa-Neto MD, Pécora JD. Dentin permeability of the apical third in different groups of teeth. Braz Dent J. 2010;21:216–9.
De-Deus G, Brandão M, Leal F, et al. Lack of correlation between sealer penetration into dentinal tubules and sealability in nonbonded root fillings. Int Endod J. 2012;45:642–51.
Acknowledgement
The authors deny any conflicts of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Aydın, Z.U., Özyürek, T., Keskin, B. et al. Effect of chitosan nanoparticle, QMix, and EDTA on TotalFill BC sealers’ dentinal tubule penetration: a confocal laser scanning microscopy study. Odontology 107, 64–71 (2019). https://doi.org/10.1007/s10266-018-0359-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-018-0359-0