Abstract
The present study was conducted to evaluate the effects of an experimental adhesive agent [methyl methacrylate-tributylborane liquid (MT)] and two adhesive agents containing silane on the bonding between a resin composite block of a computer-aided design and manufacturing (CAD/CAM) system and a light-curing resin composite veneering material. The surfaces of CAD/CAM resin composite specimens were ground with silicon-carbide paper, treated with phosphoric acid, and then primed with either one of the two silane agents [Scotchbond Universal Adhesive (SC) and GC Ceramic Primer II (GC)], no adhesive control (Cont), or one of three combinations (MT/SC, MT/GC, and MT/Cont). A light-curing resin composite was veneered on the primed CAD/CAM resin composite surface. The veneered specimens were subjected to thermocycling between 4 and 60 °C for 10,000 cycles, and the shear bond strengths were determined. All data were analyzed using analysis of variance and a post hoc Tukey–Kramer HSD test (α = 0.05, n = 8). MT/SC (38.7 MPa) exhibited the highest mean bond strengths, followed by MT/GC (30.4 MPa), SC (27.9 MPa), and MT/Cont (25.7 MPa), while Cont (12.9 MPa) and GC (12.3 MPa) resulted in the lowest bond strengths. The use of MT in conjunction with a silane agent significantly improved the bond strength. Surface treatment with appropriate adhesive agents was confirmed as a prerequisite for veneering CAD/CAM resin composite restorations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For over a quarter of a century, computer-aided design and manufacturing (CAD/CAM) systems have become widespread in prosthodontic treatment as an indirect method to fabricate inlays, onlays, crowns, and implant supported superstructures [1]. Among the restorative materials for CAD/CAM systems, such as machinable ceramics, metal alloys, and resin composites, the use of CAD/CAM resin composites has advantages of suitable mechanical properties, adequate wear characteristics, and non-metallic color [1–4].
Bonding of a resin-based material to a CAD/CAM resin composite is an important factor for the success of tooth colored restorations. Natural tooth color cannot be completely reproduced with only a monochromatic CAD/CAM resin composite block. Therefore, the machine-milled resin composites are occasionally veneered with light-curing resin composites using a layering technique. Composite–composite bonding is also required to adjust the contact point or repair minor defects of resin composite restorations [4–6]. Durable bonding between machine-milled resin composites and resin composite veneering materials is needed to prevent chipping or fracture of the resin composite restorations in oral cavity.
Bond strength results between CAD/CAM resin composites and additional light-curing resin composites that were obtained with several repair systems or with commercially available adhesive agents have been evaluated [7, 8]. A silica coating system (CoJet, 3M ESPE, 3M Japan Ltd., Tokyo, Japan) produced higher bond strengths than surface treatment with hydrofluoric acid or phosphoric acid [9]. Application of silane significantly improved the bond strengths, although it was dependent on the type of CAD/CAM resin composites [10–12]. The combined use of a silane primer and a light-curing adhesive agent was recommended for the bonding of prepolymerized resin composites [13, 14]. However, few studies have focused on the polymerization initiator as a component of the adhesive agents.
Methyl methacrylate (MMA)-tributylborane (TBB) based materials are essentially different from other resins with respect to two mechanisms, post-polymerization and the interfacial initiation of polymerization [15–18]. Although the TBB-initiated adhesives are advantageous for the bonding of tooth substances, metal alloys, and ceramics [19–21], the role of TBB for CAD/CAM resin composite bonding has not been investigated.
The purpose of the present study was to evaluate the effects of an experimental adhesive agent (MMA-TBB liquid, MT) and two adhesive agents containing silane on the bond strength between a CAD/CAM resin composite and a light-curing resin composite veneering material. The null hypothesis was that the application of MMA-TBB liquid on the substrate surface would not increase the bond strength between the CAD/CAM resin composite and the veneered resin composite.
Materials and methods
The materials used are listed in Table 1. A total of 96 rectangular specimens (8 × 10 × 3 mm) were cut from a CAD/CAM resin composite (Gradia Block, A3, GC Corp., Tokyo, Japan) and divided into 6 groups (Cont, MT/Cont, SC, MT/SC, GC, and MT/GC) of 16 specimens each.
Preparation of bonded specimen
All specimens were ground with No. 600 silicon-carbide paper and then rinsed with water spray. A 40 wt% phosphoric acid solution was applied to the specimen surfaces for 5 s, after which the surface was rinsed with water spray for 10 s and then air-dried (Fig. 1). A piece of masking tape with a 2 mm diameter circular hole was attached to the surface of each specimen to delineate the bonding area. Each adhesive agent (1 µL) was subsequently applied to the specimens with a micropipette (Eppendorf AG, Hamburg, Germany), except for the Cont group. The SC and MT/SC groups were light cured with a light emitting diode (LED) light unit (Pencure, J Morita Corp., Tokyo, Japan) for 10 s. An acrylic ring (4 mm internal diameter, 2.0 mm height) was placed to surround the bonding area, filled with the light-curing resin composite, and then light-cured for 40 s.
Shear bond test
After the specimens were stored at room temperature for 30 min, they were immersed in water at 37 °C for 24 h (designated thermocycle 0). Eight specimens for each test group were thermocycled for 10,000 cycles between water baths held at 4 and 60 °C, with a dwell time of 1 min in each bath. The bonded specimens were connected to a shear-testing jig (Device No. ISO/TR11405, Wago Industrial, Nagasaki, Japan). Shear bond strengths, the force at failure divided by the bonding area, were determined using a universal testing machine (AGS-10kNG, Shimadzu Corp., Kyoto, Japan) at a cross-head speed of 0.5 mm/min.
Statistical analysis
For each test group, the mean bond strength and standard deviation (SD) of 8 specimens were calculated. Homoscedasticity was analyzed by a Levene test. All data were analyzed using analysis of variance (ANOVA), and the mean values were compared by a post hoc Tukey–Kramer HSD test at a statistical significance of 0.05.
Failure mode
The debonded surfaces of all specimens were observed through an optical microscope (SMZ-10, Nikon Corp., Tokyo, Japan) at a magnification of 20× to assess bond failure. Failure modes were categorized as adhesive failure at the interface between the CAD/CAM resin composite and light-curing resin composite (A), cohesive failure within the CAD/CAM resin composite including crack propagation (C), cohesive failure within the light-curing resin composite (L), and mixed failures of these modes (AC and ACL).
Fourier transform infrared spectroscopy
Additional CAD/CAM resin composite (Gradia Block) was pulverized and mixed with KBr using an agate mortar, and then pressed to produce samples for Fourier transform infrared spectroscopy (FTIR) analysis. Absorption peaks were investigated using a spectrometer (FTIR-8400S, Shimadzu Corp.) within the spectral range of 700–2000 cm−1.
Results
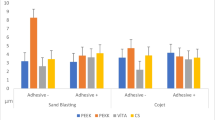
Two-way ANOVA (Table 2) indicates that the bond strength was significantly influenced individually by both thermocycling and the adhesive, and their interaction was not significant. The Levene test showed homoscedasticity among the test groups (P = 0.5458). Table 3 shows the bond strengths and failure modes. The mean bond strength varied from 12.3 to 42.6 MPa. At thermocycle 0, SC, MT/SC, and MT/GC exhibited the highest bond strengths. No-agent control (Cont) showed the lowest bond strength, and no significant differences were found among Cont, MT/Cont, and GC. P values for significantly different pairs were Cont and SC (P < 0.0001), Cont and MT/SC (P = 0.0059), and Cont and MT/GC (P = 0.0003).
At thermocycle 10,000, MT/SC exhibited the highest bond strengths, followed by MT/GC, SC, and MT/Cont. Cont and GC exhibited the lowest bond strengths, and significant differences were found between Cont and MT/SC (P = 0.0001), Cont and MT/GC (P = 0.0368), GC and MT/SC (P < 0.0001), and GC and MT/GC (P = 0.0266).
With regard to failure modes at thermocycle 0, 31 specimens (8 specimens of Cont, 8 of MT/Cont, 1 of SC, 6 of GC, and 8 of MT/GC) failed with complete adhesive failure (A), and 17 specimens (7 specimens of SC, 8 of MT/SC, and 2 of GC) exhibited mixed failure (AC or ACL). After 10,000 thermocycles, the numbers of specimens that exhibited A and AC were 15 (4 specimens of Cont, 8 of MT/Cont, 1 of MT/SC, and 2 of GC) and 33 (4 specimens of Cont, 8 of SC, 7 of MT/SC, 6 of GC, and 8 of MT/GC), respectively.
FTIR absorption peaks appeared in the spectra ranges of 1000–1100, 1600–1650, and 1650–1750 cm−1 (Fig. 2). The peaks detected at 1050, 1637, and 1731 cm−1 were assigned to the C–O stretching vibration of ester, the C=C stretching vibration of methacryloyl groups, and the C=O stretching vibration of ester, respectively.
Discussion
The present study revealed that the bond strength between the CAD/CAM resin composite and the veneered resin composite can be improved by the use of MMA-TBB liquid with silane rather than individual adhesive agents. Therefore, the null hypothesis was rejected.
Gradia Block was selected as a substrate material, because it was widely used for fabricating CAD/CAM resin composite restorations and the chemical compositions were published in relatively detail by the manufacturer (Table 1). 10,000 cycles of thermocycling was used to compare the bonding durability based on a report in the literature [8]. Repeated thermal stress induces expansion and contraction of the materials, which expedites the absorption of water into the bonded interface rather than long-term water immersion.
GC contains a silane and a phosphate monomer. Silanes that possess two different functional groups in a molecule generally react with SiO2 of the substrate material to form siloxane bonds, and also copolymerize with methacrylates [22, 23]. Acidic compounds accelerate the formation of siloxane bonds [24]. However, the low bond strength of GC suggests limitations in the role of silane for bonding the CAD/CAM resin composite. Taking into account that SiO2 is the main target of silane, organic components more than inorganic components may be exposed on the substrate surface.
On the other hand, SC contains a silane, a phosphate monomer, and a light-activated polymerization initiator. The values for SC were relatively higher than those for GC and Cont, which suggests that the light-activated polymerization initiator contributed to the bonding. These results are consistent with previous reports that recommended specific combinations of silane and a light-curing adhesive agent [13, 14].
FTIR was conducted to qualitatively analyze the polymerizable components of methacryloyl groups in the CAD/CAM resin composite. It is considered that the C=C double bonds were derived from pendant methacryloyl groups and residual monomers in the resin composite, because no elutable components were extracted from the resin composite specimen [25]. Accordingly, we speculate that pendant methacryloyl groups and/or residual monomers of methacrylates are present in the substrate material, and that they are bonded with the components of the adhesive agents by radical polymerization.
After thermocycling, MT/SC and MT/GC exhibited relatively higher bond strengths than SC and GC. The role of MMA-TBB liquid could be explained by the two mechanisms of post-polymerization and the interfacial initiation of polymerization [15–18]. When using TBB, the amount of residual monomer was decreased during post-polymerization in the long term compared with that when using a light-curing camphorquinone (CQ) system [15]. Furthermore, when the resin was polymerized by a combination of TBB and the CQ system, the monitored residual monomer was significantly decreased for up to 24 h [16].
Another mechanism is based on the concept of interfacial initiation of polymerization [17]. When using light-curing systems, light irradiation of the resin surface initiates polymerization from the surface, so that polymerization shrinkage acts from the bonded interface toward the outside and the contraction stress weakens adhesive bonding. In contrast, with the interfacial initiation of polymerization, polymerization shrinkage is directed toward the bonded interface. The polymerization of MMA by TBB is accelerated by adequate amount of oxygen, specific metal ions, or water (0.3–0.5 mol per mole of TBB) [17–19]. In the MT/Cont, MT/SC, and MT/GC groups, water molecules present on the substrate material would assist the initiation of polymerization by TBB at the bonded interface. Although further studies are required to verify these mechanisms, acceleration of polymerization at the bonded interface is considered to be an important factor in achieving durable bonding.
All of the specimens exhibited complete or partial adhesive failure. The adhesive failure indicates that the adhesive force is lower than the cohesive strengths of the resin composite materials. In addition, the numbers of specimens showing partial cohesive failure (AC) increased after thermocycling, which suggests that thermal stress weakens the cohesive strength of the CAD/CAM resin composite. Additional surface treatments to increase the actual bonding area, such as air abrasion, may be useful to maximize the bond strength in clinical applications.
Within the limits of the present study, it was concluded that the chemical composition of the adhesive agents affected the adhesive bonding between the CAD/CAM resin composite and the resin composite veneering material. The combined use of the MMA-TBB liquid and the silane agent (MT/SC or MT/GC) significantly improved the bond strength. Appropriate adhesive agents should, thus, be applied to CAD/CAM resin composite restorations when veneering with light-curing resin composites.
References
Miyazaki T, Hotta Y, Kunii J, Kuriyama S, Tamaki Y. A review of dental CAD/CAM: current status and future perspectives from 20 years of experience. Dent Mater J. 2009;28:44–56.
Lauvahutanon S, Takahashi H, Shiozawa M, Iwasaki N, Asakawa Y, Oki M, Finger WJ, Arksornnukit M. Mechanical properties of composite resin blocks for CAD/CAM. Dent Mater J. 2014;33:705–10.
Giordano R. Materials for chairside CAD/CAM-produced restorations. J Am Dent Assoc. 2006;137:14S–21S.
Ruse ND, Sadoun MJ. Resin-composite blocks for dental CAD/CAM applications. J Dent Res. 2014;93:1232–4.
LeSage B. Direct composite resin layering techniques for creating lifelike CAD/CAM-fabricated composite resin veneers and crowns. J Prosthet Dent. 2014;112:5–8.
Wiegand A, Stucki L, Hoffmann R, Attin T, Stawarczyk B. Repairability of CAD/CAM high-density PMMA- and composite-based polymers. Clin Oral Investig. 2015;19:2007–13.
Elsaka SE. Repair bond strength of resin composite to a novel CAD/CAM hybrid ceramic using different repair systems. Dent Mater J. 2015;34:161–7.
Stawarczyk B, Krawczuk A, Ilie N. Tensile bond strength of resin composite repair in vitro using different surface preparation conditionings to an aged CAD/CAM resin nanoceramic. Clin Oral Investig. 2015;19:299–308.
Elsaka SE. Influence of surface treatments on bond strength of metal and ceramic brackets to a novel CAD/CAM hybrid ceramic material. Odontology. 2016;104:68–76.
Zaghloul H, Elkassas DW, Haridy MF. Effect of incorporation of silane in the bonding agent on the repair potential of machinable esthetic blocks. Eur J Dent. 2014;8:44–52.
Elsaka SE. Bond strength of novel CAD/CAM restorative materials to self-adhesive resin cement: the effect of surface treatments. J Adhes Dent. 2014;16:531–40.
Stawarczyk B, Trottmann A, Hämmerle CHF, Özcan M. Adhesion of veneering resins to polymethylmethacrylate-based CAD/CAM polymers after various surface conditioning methods. Acta Odontol Scand. 2013;71:1142–8.
Hisamatsu N, Atsuta M, Matsumura H. Effect of silane primers and unfilled resin bonding agents on repair bond strength of a prosthodontic microfilled composite. J Oral Rehabil. 2002;29:644–8.
Hisamatsu N, Tanoue N, Yanagida H, Atsuta M, Matsumura H. Twenty-four hour bond strength between layers of a highly loaded indirect composite. Dent Mater J. 2005;24:440–6.
Hirabayashi C, Imai Y. Studies on MMA-TBB resin I. Comparison of TBB and other initiators in the polymerization of PMMA/MMA resin. Dent Mater J. 2002;21:314–21.
Hirabayashi C. Studies on MMA-TBB resin II. The effect of dual use of TBB and other initiators on polymerization of PMMA/MMA resin. Dent Mater J. 2003;22:48–55.
Imai Y, Kadoma Y, Kojima K, Akimoto T, Ikakura K, Ohta T. Importance of polymerization initiator systems and interfacial initiation of polymerization in adhesive bonding of resin to dentin. J Dent Res. 1991;70:1088–91.
Okamoto Y, Takahata K, Saeki K. Studies on the behavior of partially oxidized tributylborane as a radical initiator for methyl methacrylate (MMA) polymerization. Chem Lett. 1998;27:1247–8.
Taira Y, Imai Y. Review of methyl methacrylate (MMA)/tributylborane (TBB)-initiated resin adhesive to dentin. Dent Mater J. 2014;33:291–304.
Nakabayashi N, Masuhara E, Mochida E, Ohmori I. Development of adhesive pit and fissure sealants using a MMA resin initiated by a tri-n-butyl borane derivative. J Biomed Mater Res. 1978;12:149–65.
Chang JC, Hurst TL, Hart DA, Estey AW. 4-META use in dentistry: a literature review. J Prosthet Dent. 2002;87:216–24.
Bowen RL. Properties of a silica-reinforced polymer for dental restorations. J Am Dent Assoc. 1963;66:57–64.
Söderholm KJM, Shang SW. Molecular orientation of silane at the surface of colloidal silica. J Dent Res. 1993;72:1050–4.
Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89:268–74.
Nomoto R, Hirasawa T. Residual monomer and pendant methacryloyl group in light-cured composite resins. Dent Mater J. 1992;11:177–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shinohara, A., Taira, Y. & Sawase, T. Effects of tributylborane-activated adhesive and two silane agents on bonding computer-aided design and manufacturing (CAD/CAM) resin composite. Odontology 105, 437–442 (2017). https://doi.org/10.1007/s10266-016-0288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-016-0288-8