Abstract
The independent origin of roots in lycophytes and euphyllophytes has been proposed, mainly based on paleobotanical records. However, the question of how roots evolved within these lineages remains unresolved. Root apical meristem (RAM) organization in lycophytes would provide a clue toward understanding the early evolution of roots. Recently, we examined RAM organization in lycophytes (Lycopodiaceae, Isoetaceae, and Selaginellaceae) in terms of cell division activity and anatomy, comparing RAM among vascular plants. Lycophyte RAM exhibited four organization types (I, II, III, and apical); thus, RAM organization in extant lycophytes was more diverse than expected. Type I RAM contained a region with very low cell division frequency, reminiscent of the quiescent center (QC) in seed plant RAM. Although some euphyllophyte RAMs were structurally similar to types II and III and apical cell-type RAM, lycophyte RAM of types II and III had no QC-like area. These results support the paleobotanical predictions that roots evolved several times in lycophytes, as well as in euphyllophytes. In this review, we also introduce recent findings on RAM organization in extant lycophytes and discuss the origin of roots in vascular plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Roots are fundamental organs of vascular plants. The root performs essential functions necessary for the survival and development of plants, such as anchoring the plant body to a substrate, providing mechanical support, and absorbing water and nutrients. Despite functional similarities among all roots, phylogenetic analyses integrating key fossil taxa and extant lineages have suggested that roots evolved at least twice independently among vascular plants, once in lycophytes and once in euphyllophytes (monilophytes and seed plants) (Friedman et al. 2004; Kenrick and Crane 1997; Raven and Edwards 2001). Recent researches on Devonian fossil plants, especially lycophytes, have suggested that roots evolved several times independently from different source organs; underground shoots, rhizomorphs, basal swelling or protocorms (Doyle 2013; Hao et al. 2010; Hetherington and Dolan 2017; Matsunaga and Tomescu 2016). This hypothesis is consistent with the molecular clock-based estimate that three families of extant lycophytes (Selaginellaceae, Isoetaceae, and Lycopodiaceae) diverged in the Early Devonian (Kenrick and Strullu-Derrien 2014; Wikstrom and Kenrick 2001) before lycophyte ancestors acquired roots. However, it is not clear which organs gave rise to the roots of extant lycophytes; further neo- and palaeobotanical studies on lycophyte roots should therefore be conducted (Harrison 2017; Hetherington and Dolan 2017).
Root tissues consist of the epidermis, cortex, vascular cylinder, and root cap (Esau 1965; Evert 2006; Zhu et al. 1998). These tissues are entirely produced by the activity of the root apical meristem (RAM), a self-perpetuating tissue. RAM exhibits diversified structure and organization among the major taxa (Clowes 1961; Esau 1965), although research on these diversified structures has mainly been performed for euphyllophytes, especially for angiosperms. In contrast, information on lycophyte RAM structures is very limited. Very recently, we reported diverse root structures and cell division activities in extant lycophytes and provided evidence from these extant plants to support the multiple origin hypotheses. In the present article, we review recent discussions on the evolution and origin of roots in lycophytes, monilophytes, and seed plants, incorporating our previous results on lycophyte roots.
Comparative morphology of RAM of extant euphyllophytes and lycophytes
Euphyllophyte RAM organizations have been described separately for each major plant group. The RAM is similar across monilophytes in that it contains a single pyramidal initial cell, the apical cell (Gifford 1991, 1993; Ogura 1972). Gymnosperm RAMs have one to three initiating zones, depending on the taxon (von Guttenberg 1966; Pillai 1964, 1966). Angiosperm RAMs have two basic types of organization, open and closed, originally defined by von Guttenberg (1960). In the open RAM, all root tissues appear to arise from a common group of initial cells, whereas in the closed RAM, the epidermis, cortex, vascular tissue, and root cap are separately traceable to discrete tiers of initial cells, i.e., protoderm-, ground meristem-, procambium-, and root cap-initials (Esau 1965; Evert 2006). In addition, with respect to the origin of the epidermis, angiosperm RAMs are classified into monocot and dicot types; the epidermis has a common origin with the cortex in most monocots, but with the root cap in most dicots (Clowes 1994, 2000). Angiosperm RAMs were later further classified into 15 types (Heimsch and Seago 2008) based on differences in initial cell behavior and epidermis deviation.
Previous studies distinguished only two types of RAM organization in lycophytes. In Selaginellaceae, the RAM has an apical cell as the ultimate source of all tissues constituting roots (Imaichi and Kato 1989); Isoetaceae and Lycopodiaceae have a layered RAM structure, with multiple tiers of initial cells from which different tissues are derived (von Guttenberg 1966; Imaichi 2008; Ogura 1972; Yi and Kato 2001). Thus, RAM structural diversity has been underestimated in lycophytes, mainly due to a lack of studies on Lycopodiaceae (Bruchmann 1874; Campbell 1918; Stokey 1907). However, our recent work based on tissue organization has resulted in the recognition of three RAM types in addition to the apical cell type in Selaginella (Fig. 1, Fujinami et al. 2017). Type I RAM, which is found in some Lycopodium and Diphasiastrum species of Lycopodiaceae, has a non-layered structure with a mass of common initials, which are surrounded by procambium-, ground meristem-, protoderm-, and root cap-initials (Fig. 2a). This RAM type is reminiscent of open angiosperm RAM. Types II and III RAM similarly comprise multiple tiers of initial cells, but they are distinguished by the origin of the root epidermis. In type II RAM of Huperzia and Lycopodiella (Lycopodiaceae), initials of epidermis and of cortex, i.e. protoderm- and ground meristem-initials, form discrete cell layers that separate the root proper from root cap cells (Fig. 2b), whereas in type III RAM of Isoetaceae species, a common initial cell layer produces both the root cap and epidermis. The structure of types II and III RAM resembles that of some closed angiosperm RAM (Fujinami et al. 2017); however, these similarities evolved independently in lycophytes and euphyllophytes, as discussed below.
RAM structures mapped onto a phylogenetic tree of vascular plants. Character evolutions are based on Fujinami et al. (2017) and Hetherington and Dolan (2017, 2018, 2019). For seed plants, four RAM types are selected from Clowes (1981) and Heimsch and Seago (2008), which have similarities to those of lycophytes. Asteroxylon rooting axis is hypothesized as an ancestor of roots with type II RAM
Schematic illustrations and EdU staining images of type I and type II RAM. a, b Schematic illustrations of type I (a) and type II (b). The figures are based on Fujinami et al. (2017). c, d EdU image of five sections and a phase-contrast of a median longitudinal section. c EdU image of Lycopodium clavatum RAM (type I). EdU signals (green) are scarce in the center of RAM. Traced lines indicate the layers of the initial cells (the center of RAM) and the boundary of root tissues. d EdU image of Huperzia serrata RAM (type II). Scale bars, 100 µm. GMI ground meristem initials, PCI procambium initials, PDI protoderm initials, RCI root cap initials
Lycophyte RAM exhibits different cell division activity depending on RAM type
A quiescent center (QC) is generally observed at the heart of angiosperm and gymnosperm RAM (Clowes 1954, 1956, 1994; Peterson and Vermeer 1980). QC cells divide infrequently and serve as an organizing center to maintain the stem cell niche (van den Berg et al. 1997; Heyman et al. 2013). The presence of a QC in non-seed plants had been controversial. Some studies have argued that the root apical cell in monilophytes is quiescent, whereas others have considered the apical cell as mitotically active (Avanzi and D’Amato 1967; D’Amato and Avanzi 1965; Gifford 1991, 1993; Gifford and Kurth 1982). We analyzed the frequency and pattern of mitotic division in the four lycophyte and monilophyte RAM types using a thymidine analog, 5-ethuniyl-2′-deoxyuridine (EdU), as a proxy to identify actively dividing cells (Fujinami et al. 2017). We showed that type I RAM possesses a region with very low cell division frequency, reminiscent of the QC in angiosperm roots (Figs. 1, 2a, c). In contrast, type II and III layered RAM and apical cell-type RAM in Selaginellaceae do not show QC-like activity (Figs. 1, 2b, d, Fujinami et al. 2017). In the monilophyte Hypolepis punctata, the apical cell divided more frequently than the immediate derivative cells (Fig. 4d in Fujinami et al. 2017). Thus, a QC-like area and a QC likely evolved independently in lycophytes and seed plants. Nevertheless, it is interesting that RAM with a mass of initial cells shows quiescence in both lycophytes and seed plants. This commonality may indicate that quiescence facilitates maintenance of stem cell niche also in the type I RAM of lycophytes, although the function of the QC-like area in lycophytes remains unclear at present.
Root organization is superficially similar in lycophytes and euphyllophytes; however, the cell division dynamics observed in lycophyte RAM suggests a different mechanism of root growth from euphyllophyte RAM, supporting the independent evolution of roots in lycophytes and euphyllophytes (Doyle 2013; Friedman et al. 2004; Hetherington and Dolan 2019; Kenrick 2013; Raven and Edwards 2001).
Genes involved in establishing a QC-like area in lycophytes
In angiosperms, the proliferation rate of QC cells is controlled by the WUSCHEL-RELATED HOMEOBOX5 (WOX5) transcription factor, whose transcripts are exclusively found in QC cells (Haecker et al. 2004; Sarkar et al. 2007). Other key regulators of the QC include SCARECROW (SCR), which is required by QC for the maintenance of stem cell activity (Sabatini et al. 2003), and the ETHYLENE RESPONSEFACTOR family, a pacemaker of QC cell division (Heyman et al. 2013).
Transcriptomes for some lycophyte roots have recently been made available (Mello et al. 2019); however, very limited tissue-scale information is available on gene expression. WOX family genes have been isolated in Selaginella kraussiana (Selaginellaceae), but phylogenetic analyses showed that this species does not have a WOX5/WUS ortholog (Ge et al. 2016; Nardmann and Werr 2012, 2013; Nardmann et al. 2009). Ge et al. (2016) reported that SkWOX11C is expressed in microphylls, rhizophores, shoots, and stems, but not in roots. In our preliminary gene expression analyses, no WOX genes were expressed in the QC-like area of Lycopodium clavatum (R. Fujinami, unpublished observations). This finding may indicate that different molecular mechanisms may be responsible for the QC of seed plants and the QC-like area of lycophytes; further analyses are required to confirm this possibility.
Evolution of RAM organization and the origin of roots in vascular plants
Voronin (1969, as cited in Barlow 1995) proposed that the propensity to form centrally placed tetrahedral apical initials is primitive by assuming the apical initials of Selaginellaceae RAM as the archetype. This hypothesis further predicts that polyhedral apical initials with increased cutting faces were multiplied to form plural initials in seed plants. Such polyhedral apical initials are found in Osmunda and Angiopteris (Barlow 1995; Freeberg and Gifford 1984), which are phylogenetically basal monilophyte groups (Schuettpelz and Pryer 2008). However, the hypothesized evolutionary course of apical initials is unlikely because roots were acquired independently in lycophytes, monilophytes and seed plants (Friedman et al. 2004; Fujinami et al. 2017).
Fossil evidence rather suggests that, if the rooting axis of Early Devonian lycophyte Asteroxylon mackiei represents a primitive root without a root cap or root hairs, the ancestral organ of roots had RAM with multiple initials (Hetherington and Dolan 2018). These rooting axes form an epidermis over the surface of rooting axis meristems. This feature is similar to type II RAM in Lycopodiaceae in that epidermal (protoderm) initials and cortical (ground meristem) initials form discrete cell layers. Type II RAM also has discrete initials for the root cap. Therefore, the trajectory to type II RAM from rooting axis meristem can be explained by the addition of root cap initials outside the rooting axis proper (Fig. 1).
Many Devonian fossils suggest that roots originated at least twice within a relatively short period (Freidman et al. 2004; Kenrick and Crane 1997; Raven and Edwards 2001). A large land mass was present at high latitudes in the Southern Hemisphere prior to the Silurian; however, after it began to split, land pieces moved to mid-latitude and equatorial areas around the onset of the Devonian (Golonka 2000). This change in continent distribution triggered rapid rock weathering due to high precipitation in these areas (Le Hir et al. 2011). Consequently, rooting organs are likely to have become adaptive on the Early Devonian land. However, the primitive soil was still nutritionally poor, especially in phosphates and nitrates. Therefore, the functions of the earliest roots may have been limited to anchoring plants to pebbly substrates rather than absorbing nutrients, as in the rooting axes of Asteroxylon, which had no root hairs (Hetherington and Dolan 2018). The cultivation of land by pioneer plants would have led to accumulation of nutrients in the soil, offering an advantage to plants that could utilize these nutrients effectively. Interestingly, the earliest Devonian roots have mainly been reported in the lycophyte lineage (e.g., Hao et al. 2010; Hetherington and Dolan 2018, 2019; Matsunaga and Tomescu 2016), suggesting that euphyllophyte roots evolved later than lycophyte roots, allowing the exploitation of nutrients made available through lycophyte efforts. Such differences in the timing of root origins, as well as in their functional requirements, are likely related to multiple root origins in the evolution of vascular plants.
Conclusion and future perspectives
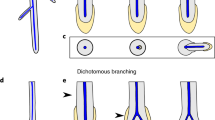
The diversity of RAM among extant lycophytes suggests that root evolution and diversification occurred through different evolutionary pathways, supporting paleobotanical hypothesis of the multiple origins of roots. The presence of a QC-like region in non-seed plants allowed us to compare the molecular mechanisms regulating root structure between lycophytes and seed plants, and it will contribute to the elucidation of root evolution. More importantly, lycophytes and euphyllophytes differ in the branching pattern of their roots. Lycophyte roots branch dichotomously from their apex, whereas euphyllophyte roots typically form lateral roots endogenously (Gifford and Foster 1989). This difference in root branching patterns could be the key to understanding how roots and shoots evolved from a common vascular plant ancestor that had no shoots and roots, but rather axial organs. The origin of roots will be further elucidated as we improve our understanding of branching patterns through molecular genetic analysis and further exploration of fossil evidence.
References
Avanzi S, D’Amato F (1967) New evidence on the organization of the root apex in Leptosporangiate ferns. Caryologia 20:257–264
Barlow PW (1995) Structure and function at the root apex-phylogenetic and ontogenetic perspectives on apical cells and quiescent centres. In: Baluska F, Ciamporova M, Gasparikova O, Barlow PW (eds) Structure and function of roots. Kluwer, Dordrecht, pp 3–18
Bruchmann H (1874) Ueber Anlage und Wachsthum der Wurzeln von Lycopodium und Isoetes. Jenaisch Zeitschrift für Naturwissenschaft 8:522–578
Campbell DH (1918) The structure and development of mosses and ferns (Archegoniatae), 3rd edn. Macmillan, New York
Clowes FAL (1954) The promeristem and the minimal constructional centre in grass root apices. New Phytol 53:108–116
Clowes FAL (1956) Localization of nucleic acid synthesis in root meristems. J Exp Bot 7:307–312
Clowes FAL (1961) Apical meristems. Blackwell, Oxford
Clowes FAL (1981) The difference between open and closed meristems. Ann Bot 48:761–767
Clowes FAL (1994) Origin of the epidermis in root meristems. New Phytol 127:335–347
Clowes FAL (2000) Pattern in root meristem development in angiosperms. New Phytol 146:83–94
D’Amato F, Avanzi S (1965) DNA content, DNA synthesis and mitosis in the root apical cell of Marsilea strigosa. Caryologia 18:383–394
Doyle JA (2013) Phylogenetic analyses and morphological innovations in land plants. Annu Plant Rev 45:1–50
Esau K (1965) Plant anatomy, 2nd edn. Wiley and Sons, New York
Evert RF (2006) Esau’s plant anatomy. Meristems, cells, and tissues of the plant body: their structure, function, and development, 3rd edn. John Wiley and Son, New Jersey
Freeberg JA, Gifford EM (1984) The root apical meristem of Osumunda regalis. Amer J Bot 71:558–563
Friedman WE, Moore RC, Purugganan MD (2004) The evolution of plant development. Am J Bot 91:1726–1741
Fujinami R, Yamada T, Nakajima A, Takagi S, Idogawa A, Kawakami E, Tsutsumi M, Imaichi R (2017) Root apical meristem diversity in extant lycophytes and implications for root organs. New Phytol 215:1210–1220
Ge Y, Liu J, Zeng M, He J, Qin P, Huanf H, Xu L (2016) Identification of WOX family genes in Selaginella kraussiana for studies on stem cells and regeneration in lycophytes. Front Plant Sci 7:93
Gifford EM (1991) The root apical meristem of Asplenium bulbiferum: structure and development. Am J Bot 78:370–376
Gifford EM (1993) The root apical meristem of Equissetum diffusum: structure and development. Am J Bot 80:468–473
Gifford EM, Foster AS (1989) Morphology and evolution of vascular plants, 3rd edn. W. Freeman, New York
Gifford EM, Kurth E (1982) Quantitative studies of the root meristem of Equisetum scirpoides. Am J Bot 69:464–473
Golonka J (2000) Cambrian-Neogene plate tectonic maps. Wydawnictwa Uniwersytetu Jagiellonskiego, Kraków
Haecker A, GroB-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131:657–669
Hao S, Xue J, Guo D, Wang D (2010) Earliest rooting system and root: shoot ratio from a new Zosterophyllum plant. New Phytol 185:217–225
Harrison CJ (2017) Development and genetics in the evolution of land plant body plans. Phil Trans R Soc B 372:20150490
Heimsch C, Seago JL (2008) Organization of the root apical meristem in angiosperms. Amer J Bot 95:1–21
Hetherington AJ, Dolan L (2017) The evolution of lycopsid rooting structures: conservatism and disparity. New Phytol 215:538–544
Hetherington AJ, Dolan L (2018) Stepwise and independent origins of roots among land plants. Nature 561:235–238
Hetherington AJ, Dolan L (2019) Rhynie chert fossils demonstrate the independent origin and gradual evolution of lycophyte roots. Curr Opin Plant Biol 47:119–126
Heyman J, Cool T, Vandenbussche F, Heybdrickx KS, Keene JV, Vercauteren I, Vanderauwera S, Vandepoele K, Jaeger GD, Straeten DVD, Veylder LD (2013) ERF115 controls root quiescent center cell division and stem cell replenishment. Science 342:860–863
Imaichi R (2008) Meristem organization and organ diversity. In: Ranker TA, Haufler CH (eds) Biology and evolution of ferns and lycophytes. Cambridge University Press, New York, pp 75–103
Imaichi R, Kato M (1989) Developmental anatomy of the shoot apical cell, rhizophore and root of Selaginella uncinata. Bot Mag Tokyo 102:369–380
Kenrick P (2013) The origin of roots. In: Eshel A, Beeckman T (eds) Plant roots: the hidden half, 4th edn. Taylor and Francis, London, pp 1–13
Kenrick P, Crane PR (1997) The origin and early diversification of land plants. Smithsonian Institution Press, Washington
Kenrick P, Strullu-Derrien C (2014) The origin and early evolution of roots. Plant Physiol 166:570–580
Le Hir G, Donnadieu Y, Goddéris Y, Meyer-Berthaud B, Ramstein G, Blakey RC (2011) The climate change caused by the land plant invasion in the Devonian. Earth Planet Sci Lett 310:203–212
Matsunaga KKS, Tomescu AMF (2016) Root evolution at the base of the lycophyte clade: insights from an early Devonian lycophyte. Ann Bot 117:585–598
Mello A, Efroni I, Rahni R, Birnbaum KD (2019) The Selaginella rhizophore has a unique transcriptional identity compared with root and shoot meristems. New Phytol 222:882–894
Nardmann J, Werr W (2012) The invention of WUS-like stem cell-promoting functions in plants predates leptosporangiate ferns. Plant Mol Biol 78:123–134
Nardmann J, Werr W (2013) Symplesiomorphies in the WUSCHEL clade suggest that the last common ancestor of seed plants contained at least four independent stem cell niches. New Phytol 199:1081–1092
Nardmann J, Reisewitz P, Werr W (2009) Discrete shoot and root stem cell-promoting WUS/WOX5 functions are an evolutionary innovation of angiosperms. Mol Biol Evol 26:1745–1755
Ogura Y (1972) Comparative anatomy of vegetative organs of the pteridophytes. Genrüder Borntraeger, Berlin
Peterson RL, Vermeer J (1980) Root apex structure in Ephedra monosperma and Ephedra chilensis (Ephedraceae). Am J Bot 67:815–823
Pillai A (1964) Root apical organization in Gymnosperms some conifers. Bull Torrey Bot Club 91:1–13
Pillai A (1966) Root apical organization in Gymnosperms. Planta 70:26–33
Raven JA, Edwards D (2001) Roots: evolutionary origins and biogeochemical significance. J Exp Bot 52:381–401
Sabatini S, Heidstra R, Wildwater M (2003) SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev 17:354–358
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hachimoto T, Nakajima K, Scheres BS, Heidstra R, Laux T (2007) Conserved factors regulate signaling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446:811–814
Schuettpelz E, Pryer KM (2008) Fern phylogeny. In: Ranker TA, Haufler CH (eds) Biology and evolution of ferns and lycophytes. Cambridge University Press, New York, pp 395–416
Stokey AG (1907) The roots of Lycopodium pithyoides. Bot Gaz 44:57–63
van den Berg C, Willemsen V, Hendriks G, Weisbeek W, Scheres B (1997) Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390:287–289
von Guttenberg H (1960) Grundzüge der Histonegese höherer Pelanzen. I. Die Angiospermen. Handbch der Pflanzenanatomie, Band 8, Teil 3. Genrüder Borntraeger, Berlin
von Guttenberg H (1966) Histogenese der Pteridophyten, 2nd edn. Handbch der Pflanzenanatomie, Band 7, Teil 2. Genrüder Borntraeger, Berlin
Wikström N, Kenrick P (2001) Evolution of Lycopodiaceae (Lycopsida): estimating divergence times from rbcL gene sequences by use of nonparametric rate smoothing. Mol Phylogenetics Evol 19:177–186
Yi S, Kato M (2001) Basal meristem and root development in Isoetes asiatica and Isoetes japonica. Int J Plant Sci 162:1225–1235
Zhu T, Lucas WJ, Rost TL (1998) Directional cell-to-cell communication in the Arabidopsis root apical meristem I An ultrastructural and functional analysis. Protoplasma 203:35–47
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (Grant nos. 25870088 and 18K06380 to RF, 24570098 and 18H02495 to TY, and 20570097 to RI) from the Japan Society for the Promotion of Science (JSPS) and a plant research grant from the New Technology Development Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Membership of the Botanical Society of Japan by any of the authors: Yes, Rieko Fujinami, Toshihiro Yamada, and Ryoko Imaichi.
Rights and permissions
About this article
Cite this article
Fujinami, R., Yamada, T. & Imaichi, R. Root apical meristem diversity and the origin of roots: insights from extant lycophytes. J Plant Res 133, 291–296 (2020). https://doi.org/10.1007/s10265-020-01167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-020-01167-2