Abstract
Roots are one of the three fundamental organ systems of vascular plants1, and have roles in anchorage, symbiosis, and nutrient and water uptake2,3,4. However, the fragmentary nature of the fossil record obscures the origins of roots and makes it difficult to identify when the sole defining characteristic of extant roots—the presence of self-renewing structures called root meristems that are covered by a root cap at their apex1,2,3,4,5,6,7,8,9—evolved. Here we report the discovery of what are—to our knowledge—the oldest meristems of rooting axes, found in the earliest-preserved terrestrial ecosystem10 (the 407-million-year-old Rhynie chert). These meristems, which belonged to the lycopsid Asteroxylon mackiei11,12,13,14, lacked root caps and instead developed a continuous epidermis over the surface of the meristem. The rooting axes and meristems of A. mackiei are unique among vascular plants. These data support the hypothesis that roots, as defined in extant vascular plants by the presence of a root cap7, were a late innovation in the vascular lineage. Roots therefore acquired traits in a stepwise fashion. The relatively late origin in lycophytes of roots with caps is consistent with the hypothesis that roots evolved multiple times2 rather than having a single origin1, and the extensive similarities between lycophyte and euphyllophyte roots15,16,17,18 therefore represent examples of convergent evolution. The key phylogenetic position of A. mackiei—with its transitional rooting organ—between early diverging land plants that lacked roots and derived plants that developed roots demonstrates how roots were ‘assembled’ during the course of plant evolution.
Similar content being viewed by others
Main
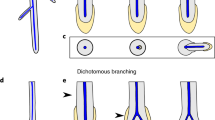
The body plan of A. mackiei comprised three types of axes; leafy shoot axes, rooting axes and the transition region between these axes11,12,13,19,20. To identify meristems at the apices of A. mackiei rooting axes, we visually inspected 641 thin sections prepared from the Rhynie chert. We discovered the apices of five rooting axes among three thin sections (Fig. 1a–e). These five apices were assigned to A. mackiei on the basis of two pieces of evidence. First, each of the five apices was discovered on thin sections in which A. mackiei was the only plant species present (Extended Data Fig. 1). Second, the morphology of the G-type tracheids in two of the rooting axes is diagnostic of A. mackiei21,22 (white arrowheads, Fig. 1f, g). These data indicate that the five apices are the tips of axes of A. mackiei.
a–e, The tips of five rooting axes of A. mackiei that terminate in apices. All apices are covered by a continuous layer of tissue and lack the tapered root caps that are characteristic of the roots of extant vascular plants. f, g, Confocal image of the tracheids in the axes shown in a (f) and e (g). The sheet perforations between thickening (arrowheads) are a characteristic of G-type tracheids that is diagnostic of A. mackiei. a, b, f, Specimen accession code: Natural History Museum, London (NHMUK) V.15642. c, e, g, Specimen accession code: The Hunterian, University of Glasgow (GLAHM) Kid 3080. d, Specimen accession code: Oxford University Herbaria (OXF) 108. Scale bars, 500 μm (a, b, e), 250 μm (c, d), 20 μm (f, g).
Three characteristics enabled us to identify the five apices as the rooting axes of A. mackiei. First, A. mackiei rooting axes lacked leaves and stomata11,12,13,19 (Fig. 1a). Stomata, leaf primordia and leaves were not present on either apical or subapical surfaces on any of the apices (Fig. 1a–e). Second, A. mackiei rooting axes grew in the direction of the gravity vector11,12,13,19. The positively gravitropic growth of two apices (Fig. 1a, b) was inferred from their orientation relative to sediment layers in both the growth substrate and a geopetally infilled void preserved in the thin section (Extended Data Fig. 2). The two axes that terminated in apices (Fig. 1a, b, Extended Data Fig. 2) were orientated perpendicular to the sediment layers; this indicated that the axes grew gravitropically into the sediment. Third, A. mackiei rooting axes were isotomously branching systems11,12,13,19. The organization of the pair of apices on the rooting axis in Fig. 1b indicates that they had recently branched isotomously. Taken together, the lack of leaves and stomata, the positively gravitropic growth and isotomous branching indicate that we identified five apices of A. mackiei rooting axes.
Having verified the identity of the A. mackiei apices, we next characterized the organization of tissues in these meristems and compared their organization with those of the roots of extant vascular plants. Three fundamental tissues were present in the apices of A. mackiei rooting axes (Fig. 1). Vascular tissue developed at the centre of the axis and was surrounded by layers of ground tissue; these two tissues were covered by a distinct epidermis that comprised cells that divided anticlinally (Fig. 1, Extended Data Fig. 3). None of the epidermal cells developed hair cells that resemble rhizoids or root hairs. The region in which these tissues converged on the tip constituted the promeristem23. The promeristem was multicellular, similar to the root meristems of many extant lycopsids17,24 and the shoot meristem of A. mackiei25. In all five cases the promeristem contained the apical-most cells of the axis; there was no tissue beyond the apical region of the promeristem, which is where a root cap would be located in roots of extant vascular plants17,23,24,26. The roots of extant vascular plants comprise four tissues—vascular, ground, epidermis and root cap—that are derived from the promeristem17,23,24,26. The vascular tissue, ground tissues and epidermis differentiate basally, as in A. mackiei. However, a root cap also develops apically and laterally in extant vascular plants17,23,24,26. The discovery that five apices of the rooting axes of A. mackiei lacked root caps suggests that the rooting axes of early diverging lycopsids did not develop root caps.
Given that all extant plant species develop root caps at their root apices7,23,24,26, we sought explanations that might account for the absence of the root cap in these fossils. We first considered that taphonomic processes could have led to the selective preservation of vascular, ground and dermal tissues without the preservation of root caps. Three of the apices (Fig. 1c–e) were preserved growing through a thin layer of degraded organic material called mulm27, which coats the apex and flanks. It is formally possible that the mulm around the apex of the rooting structure could represent a decayed root cap. However, mulm also coats the external surfaces of many plants preserved in the Rhynie chert27, including leafy shoots of A. mackiei preserved on the same thin sections as the three apices (Fig. 1c–e, Extended Data Fig. 4). The preservation of mulm around leafy shoots indicates that the presence of this substance at the apices of rooting axes does not represent the remains of root caps. Furthermore, given that all other tissues in the apices were well-preserved, it is likely that root caps would have been preserved if they were present (Fig. 1). Finally, root caps were readily preserved in permineralized plant deposits in the Carboniferous and Permian periods, which demonstrates that root caps can be fossilized28,29. We therefore rule out the possibility that root caps formed but were not preserved.
An alternative explanation for the lack of root caps in these apices is that the root cap may not have been present on apices that had stopped growing. We therefore established whether the apices were fossilized during active growth or after growth had ceased. During active growth, the root meristems of extant plants comprise large numbers of relatively small, dividing cells, and cells increase in size with distance from the apex as they elongate and differentiate23,28,30. In three of the five A. mackiei apices, large numbers of small cells were located at the apex and cell size gradually increased with distance from the tip (Fig. 1c–e, Extended Data Fig. 3). This cellular organization indicated that these three apices were actively growing when fossilized. In the two remaining apices, the differentiation of vascular tissue near the tip of the apex28,30 indicated that neither apex was active at the time of preservation (Fig. 1a, b, Extended Data Fig. 3). The absence of root caps from the three meristems that were actively growing when fossilized is consistent with the hypothesis that root caps did not develop on A. mackiei rooting axes.
If root caps developed in A. mackiei, there would be evidence in the preserved promeristems of the cell divisions from which the root caps developed. We characterized the cellular organization of the two well-preserved promeristems that were active when fossilized (Fig. 1c, d). We determined the orientations of cell walls at the apex to test whether these were consistent with the development of a root cap23. If a root cap was formed, periclinal divisions (in which the new wall is parallel to the surface) would occur near the apex of the promeristem23. Figure 2a, b shows an oblique section through the meristem that preserves the apical promeristem. There are no periclinal divisions in the outer layer of the apical promeristem (Fig. 2a, b). Instead, cells in the outermost layer of the apical region divided anticlinally (new cell walls are oriented perpendicular to the surface). This indicates that the cell-division pattern of the apical region of the meristem was inconsistent with the development of a root cap, and was instead consistent with the development of a continuous epidermis over the surface of the meristem. If a lateral root cap formed, it would develop from a combination of both anticlinal and periclinal cell divisions and produce layers of cells that taper with distance from the apex, as cells are sloughed off23. Figure 2c, d shows a longitudinal section through the meristem, in which the outer layer of the apex is preserved. Only anticlinal cell divisions occur in the outer layer and there is no evidence of periclinal divisions. The cell-division pattern of these meristems is inconsistent with the development of a lateral root cap, and is instead consistent with the development of a continuous epidermal surface. Together, the cellular organization of two promeristems enabled us to rule out the development of a root cap in A. mackiei; we predict that A. mackiei meristems were covered by a continuous epidermis.
a–d, Transmitted light (a, c) and confocal laser (b, d) microscopy images showing the cellular organization of two meristems of the rooting axes of A. mackiei. a, b, The absence of periclinal cell divisions in the apical promeristem is inconsistent with the formation of a apical root cap. c, d, The absence of periclinal cell divisions on the flanks of the meristem is inconsistent with the formation of a lateral root cap. Anticlinal cell divisions in both the promeristem (a, b) and lateral flanks (c, d) are consistent with the meristem being covered by a continuous epidermis. a, b, OXF 108 (Fig. 1d). c, d, GLAHM Kid 3080 (Fig. 1c). Scale bars, 100 μm.
To test the hypothesis that a continuous epidermal surface covered the meristems of rooting axes of A. mackiei, we reconstructed a three-dimensional model of the surface of the meristem from a z-stack of images captured on a confocal microscope (Fig. 3, Supplementary Videos 1, 2). A continuous and smooth layer of epidermis covered the meristem and there was no evidence of tapering or cells sloughing off. We conclude that meristems of the rooting axes of A. mackiei developed a continuous epidermis that covered the apex.
Three-dimensional model—from a z-stack of images captured on a confocal microscope—of the meristem of a rooting axis of A. mackiei, with the epidermal layer highlighted in blue. The cells in the epidermis form a smooth continuous surface over the meristem. Model based on images of GLAHM Kid 3080 (Figs. 1c, 2c, d). Scale bar, 50 μm.
The cellular organization of the five apices demonstrates that the rooting axes of A. mackiei developed from a previously unknown type of meristem, which lacked both root caps and root hairs. We conclude that the evolution of rooting axes in lycopsids occurred in a stepwise manner. There was a stage—represented by A. mackiei—that was characterized by the presence of radially symmetric, positively gravitropic, isotomously branching axes with vascular tissue organization that is distinct from that in the leafy shoot, as well as by a lack of leaves and stomata11,12,13,19 (Fig. 4). Subsequently, a root cap, root hairs, endogenous development and an endodermis all evolved16,17,18,24 (Fig. 4). This sequence of events is consistent with the hypothesis that the common ancestor of all extant vascular plants was rootless and that roots with caps had at least two separate origins, among lycophytes and euphyllophytes, respectively2 (Fig. 4). The similarities in root anatomy (the presence of a root cap, root hairs, an endodermis and quiescent centre16,17,18,24), development (endogenous origin)18 and gene expression15 shared by extant lycophytes and euphyllophytes therefore represent examples of convergent evolution. The simultaneous presence of rooting axes and leaves on shoots in A. mackiei suggests that the co-evolution of roots and leaf-bearing shoots may have contributed to the evolution of an efficient transpiration stream among early lycopsids.
The roots of extant lycophytes evolved in a stepwise manner. There was a stage—represented by A. mackiei— that was characterized by the presence of radially symmetric, positively gravitropic, isotomously branching axes. Subsequently, a root cap, root hairs, endogenous development and an endodermis all evolved. This sequence of events is consistent with the hypothesis that the common ancestor of all extant vascular plants was rootless, and roots with caps had at least two independent origins among lycophytes and euphyllophytes. The extensive similarities between roots of extant lycophytes and euphyllophytes therefore represent examples of convergent evolution.
Methods
Identifying apices of rooting axes of A. mackiei
To identify meristems at the apices of rooting axes of A. mackiei, we visually inspected 641 thin sections prepared from the Rhynie chert from the following collections: 11 from the collections of the School of Biology, University of St Andrews; 7 from the Oxford University Herbaria; 33 from the collections of the Manchester Museum, The University of Manchester; 299 from the Natural History Museum, London; 291 from The Hunterian, University of Glasgow.
Imaging of rooting apices
Photographs of thin sections were taken using a Nikon D80 with a 60-mm macro lens, set up on a copystand. Thin sections were lit from below with a lightbox and lit from above with aerial lights (Extended Data Figs. 1, 2b). High-resolution images of the apices were taken using Nikon Eclipse LV100ND (Figs. 1a–c, e, 2c, Extended Data Figs. 2a, c, 3a, c, 4a, b) and Olympus BX50 (Figs. 1d, 2a, Extended Data Figs. 3b, d, 4c) compound microscopes and a Leica M165 FC (Extended Data Fig. 4d) stereo microscope. To create high-definition images, multiple overlapping photographs were taken and combined to make a single image using AutoStitch31.
Confocal laser scanning microscopy was used to image meristems of rooting axes of A. mackiei. Confocal images were acquired with a Nikon A1-Si laser-scanning confocal microscope (Natural History Museum, London) (Figs. 1f, g, 2d) and a Leica SP5 confocal microscope (Department of Plant Sciences, University of Oxford) (Fig. 2b). The Nikon A1-Si laser-scanning confocal microscope was used with a 40× oil-immersion objective with a 1.3 numerical aperture and 29.37-μm pinhole. Autofluorescence of the sample was excited with a 561-nm and 640-nm laser and emission was collected with windows of 570–620 nm and 675–725 nm for each laser, respectively. The Leica SP5 confocal microscope was used with a 20× oil-immersion objective with a 0.7 numerical aperture and a 60.7-μm pinhole. Autofluorescence of the sample was excited with a 633-nm laser and emission was collected with a window of 645–800 nm.
Segmentation and visualization of A. mackiei meristem
Images were processed using FIJI32. Segmentation of the epidermal surface of the meristem was carried out in MorphoGraphX33 (Supplementary Video 1). The three-dimensional model was visualized in Blender34 (Fig. 3, Supplementary Video 2).
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The three thin sections analysed in this study are housed in publically available collections: GLAHM Kid 3080, OXF 108 and NHMUK V.15642. The confocal laser scanning microscopy datasets generated are available from the corresponding author upon reasonable request. All other data supporting the findings of this study are included in the paper and its Extended Data and Supplementary Information.
References
Schneider, H., Pryer, K. M., Cranfill, R., Smith, A. R. & Wolf, P. G. in Developmental Genetics and Plant Evolution (eds Cronk, Q. C. B. et al.) 330–364 (Taylor & Francis, London, 2002).
Raven, J. A. & Edwards, D. Roots: evolutionary origins and biogeochemical significance. J. Exp. Bot. 52, 381–401 (2001).
Kenrick, P. & Strullu-Derrien, C. The origin and early evolution of roots. Plant Physiol. 166, 570–580 (2014).
Kenrick, P. in Plant Roots: The Hidden Half (eds Eshel, A. & Beeckamn, T.) 1–14 (Taylor & Francis, Boca Raton, 2013).
Gensel, P. G., Kotyk, M. E. & Brasinger, J. F. in Plants Invade The Land: Evolutionary and Environmental Perspectives (eds Gensel, P. G. & Edwards, D.) 83–102 (Columbia Univ. Press, New York, 2001).
Matsunaga, K. K. S. & Tomescu, A. M. F. Root evolution at the base of the lycophyte clade: insights from an Early Devonian lycophyte. Ann. Bot. 117, 585–598 (2016).
Sachs, J. Text-book of Botany, Morphological and Physiological (translated and annotated by A. W. Bennett and W. T. Thiselton Dyer) (Clarendon, Oxford, 1875).
Hao, S., Xue, J., Guo, D. & Wang, D. Earliest rooting system and root:shoot ratio from a new Zosterophyllum plant. New Phytol. 185, 217–225 (2010).
Matsunaga, K. K. S. & Tomescu, A. M. F. An organismal concept for Sengelia radicans gen. et sp. nov. – morphology and natural history of an Early Devonian lycophyte. Ann. Bot. 119, 1097–1113 (2017).
Edwards, D., Kenrick, P. & Dolan, L. History and contemporary significance of the Rhynie cherts—our earliest preserved terrestrial ecosystem. Phil. Trans. R. Soc. B 373, 20160489 (2018).
Edwards, D. Embryophytic sporophytes in the Rhynie and Windyfield cherts. Trans. R. Soc. Edinb. Earth Sci. 94, 397–410 (2003).
Kidston, R. & Lang, W. H. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part III. Asteroxylon mackiei, Kidston and Lang. Trans. R. Soc. Edinb. Earth Sci. 52, 643–680 (1920).
Kidston, R. & Lang, W. H. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion of their bearing on the general morphology of the Pteridophyta and the origin of the organisation of land plants. Trans. R. Soc. Edinb. Earth Sci. 52, 831–854 (1921).
Kenrick, P. & Crane, P. R. The Origin and Early Diversification of Land Plants: A Cladistic Study (Smithsonian Series in Comparative Evolutionary Biology) (Smithsonian Institute, Washington DC, 1997).
Huang, L. & Schiefelbein, J. Conserved gene expression programs in developing roots from diverse plants. Plant Cell 27, 2119–2132 (2015).
Hetherington, A. J. & Dolan, L. The evolution of lycopsid rooting structures: conservatism and disparity. New Phytol. 215, 538–544 (2017).
Fujinami, R. et al. Root apical meristem diversity in extant lycophytes and implications for root origins. New Phytol. 215, 1210–1220 (2017).
Foster, A. S. & Gifford, E. M. Comparative Morphology of Vascular Plants (W. H. Freeman, San Francisco, 1959).
Bhutta, A. A. Studies on the Flora of the Rhynie Chert. PhD thesis, Univ. Wales, Cardiff (1969).
Kerp, H. Organs and tissues of Rhynie chert plants. Phil. Trans. R. Soc. B 373, 20160495 (2018).
Kenrick, P. & Crane, P. R. Water-conducting cells in early fossil land plants: implications for the early evolution of tracheophytes. Bot. Gaz. 152, 335–356 (1991).
Strullu-Derrien, C., Wawrzyniak, Z., Goral, T. & Kenrick, P. Fungal colonization of the rooting system of the early land plant Asteroxylon mackiei from the 407-Myr-old Rhynie Chert (Scotland, UK). Bot. J. Linn. Soc. 179, 201–213 (2015).
Clowes, F. A. L. Apical Meristems (Blackwell, Oxford, 1961).
Guttenberg, H. V. Histogenese der Pteridophyten. Handbuch der Pflanzenanatomie Vol. VII.2 (Gebrüder Borntraeger, Berlin, 1966).
Hueber, F. M. Thoughts on the early lycopsids and zosterophylls. Ann. Mo. Bot. Gard. 79, 474–499 (1992).
Heimsch, C. & Seago, J. L. Jr. Organization of the root apical meristem in angiosperms. Am. J. Bot. 95, 1–21 (2008).
Kelman, R., Feist, M., Trewin, N. H. & Hass, H. Charophyte algae from the Rhynie chert. Trans. R. Soc. Edinb. Earth Sci. 94, 445–455 (2003).
Hetherington, A. J., Dubrovsky, J. G. & Dolan, L. Unique cellular organization in the oldest root meristem. Curr. Biol. 26, 1629–1633 (2016).
Strullu-Derrien, C., McLoughlin, S., Philippe, M., Mørk, A. & Strullu, D. G. Arthropod interactions with bennettitalean roots in a Triassic permineralized peat from Hopen, Svalbard Archipelago (Arctic). Palaeogeogr. Palaeoclimatol. Palaeoecol. 348–349, 45–58 (2012).
Shishkova, S., Rost, T. L. & Dubrovsky, J. G. Determinate root growth and meristem maintenance in angiosperms. Ann. Bot. 101, 319–340 (2008).
Brown, M. & Lowe, D. G. Automatic panoramic image stitching using invariant features. Int. J. Comput. Vis. 74, 59–73 (2007).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Barbier de Reuille, P. et al. MorphoGraphX: A platform for quantifying morphogenesis in 4D. eLife 4, 05864 (2015).
Garwood, R. & Dunlop, J. The walking dead: Blender as a tool for paleontologists with a case study on extinct arachnids. J. Paleontol. 88, 735–746 (2014).
Acknowledgements
A.J.H. was funded by the George Grosvenor Freeman Fellowship by Examination in Sciences, Magdalen College (Oxford). L.D. was funded by a European Research Council Advanced Grant (EVO500, contract 250284) and a European Commission Framework 7 Initial Training Network (PLANTORIGINS, project identifier 238640). We are grateful to the Oxford University Herbaria, the University of Manchester, Manchester Museum, The Hunterian, University of Glasgow and the London Natural History Museum for access to fossil thin sections, and to the curators of these collections (S. Harris, K. Sherburn, N. Clark and P. Hayes) for their assistance. We thank T. Goral, C. Strullu-Derrien, C. Kirchhelle, I. Moore and I. Rahmen for assistance and advice for confocal imaging, segmentation and 3D reconstructions; J. Baker for photographic advice; and A. M. Hetherington, N. J. Hetherington and members of the Dolan laboratory for helpful comments and discussions.
Reviewer information
Nature thanks P. Crane and P. Kenrick for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.J.H. and L.D. designed the project. A.J.H. carried out the analyses. A.J.H. and L.D. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Five root apices were found on three thin sections in which A. mackiei was the only plant species present.
a–c, Diagnostic features of A. mackiei include the apices of leafy shoots (black arrowhead, a), star-shaped xylem (black arrowhead, b) and leaves (black arrowhead, c). a, GLAHM Kid 3080. b, NHMUK V.15642. c, OXF 108. Scale bars, 1 cm.
Extended Data Fig. 2 A. mackiei rooting axes grew in the direction of the gravity vector.
a–c, Positively gravitropic growth of two apices (a) was inferred from their orientation relative to sediment layers in both the growth substrate (dark brown and black bands at base of b) and a geopetally infilled void (c) preserved in the thin section. The position of both the apices and geopetally infilled void are highlighted with black boxes within the thin section (b). a–c, NHMUK V.15642. Scale bars, 1 mm (a, c), 1 cm (b).
Extended Data Fig. 3 Fundamental tissues present in an apex of a rooting axis preserved after growth had finished, and a meristem of a rooting axis preserved during active growth.
a, b, Root apices with fundamental tissue types colour-coded. Blue, epidermis; pink, promeristem; orange, cortex; and green, procambium. c, d, Magnified images of the apical regions of a (c) and b (d). The presence of differentiated vascular tissue (arrowhead, c) close to the tip of the apex indicates that this apex was not active at the time of preservation. By contrast, in d there is no differentiated vascular tissue. Instead, the apex is characterized by large numbers of cells and cell size gradually increases with distance from the tip, which indicates that the apex was active when fossilized. a, c NHMUK V.15642 (same specimen as illustrated in Fig. 1a), b, c, OXF 108 (same specimen as illustrated in Figs. 1d, 2a, b). Scale bars, 500 μm (a), 250 μm (b), 150 μm (c), 100 μm (d).
Extended Data Fig. 4 Mulm coats the rooting axes and leafy shoots of A. mackiei.
a–d, A thin layer of degraded organic material called mulm27 (highlighted with arrowheads, a–d) coats both the rooting axes (a, c) and leafy shoots of A. mackiei. a, b, GLAHM Kid 3080. c, d, OXF 108. Scale bars, 500 μm (a, c, d), 1 mm (b).
Supplementary information
Video 1
Segmentation of the epidermal surface of an Asteroxylon mackiei rooting axis meristem. MorphoGraphX was used to segment the epidermal surface from a z-stack of images captured on a confocal microscope. GLAHM Kid 3080. Scale bar 50 µm.
Video 2
Asteroxylon mackiei rooting axis meristems were covered by a continuous layer of epidermis and lacked a root cap. Animation showing the three-dimensional model produced of the rooting axis meristem of A. mackiei segmented using MorphoGraphX and animated using BlenderTM. GLAHM Kid 3080. Scale bar 50 µm.
Rights and permissions
About this article
Cite this article
Hetherington, A.J., Dolan, L. Stepwise and independent origins of roots among land plants. Nature 561, 235–238 (2018). https://doi.org/10.1038/s41586-018-0445-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0445-z
- Springer Nature Limited
Keywords
This article is cited by
-
A trait-based root acquisition-defence-decomposition framework in angiosperm tree species
Nature Communications (2024)
-
Composition of continental crust altered by the emergence of land plants
Nature Geoscience (2022)
-
The origin of a land flora
Nature Plants (2022)
-
Comparative transcriptomic analysis reveals conserved programmes underpinning organogenesis and reproduction in land plants
Nature Plants (2021)
-
Multiple origins of dichotomous and lateral branching during root evolution
Nature Plants (2020)