Abstract
We isolated the TaMYBsm1 genes, encoding R2R3-type MYB proteins in common wheat, aimed to uncover the possible molecular mechanisms related to drought response. The TaMYBsm1 genes, TaMYBsm1-A, TaMYBsm1-B and TaMYBsm1-D, were isolated and analyzed from the common wheat cultivar Shimai 15. Their expression patterns under PEG 6000 and mannitol were monitored by semi-quantitative RT-PCR and β-glucuronidase (Gus) assay. The function of TaMYBsm1-D under drought stress in transgenic Arabidopsis plants was investigated, and the germination rate, water loss rate, as well as the proline and malondialdehyde (MDA) content were compared with that in wild type (WT) plants. The expression of three downstream genes (DREB2A, P5CS1 and RD29A) in TaMYBsm1-D transgenic plants was analyzed. The R2R3-MYB domains of the MYBsm1 proteins were highly conserved in plants. In addition, the TaMYBsm1 proteins were targeted to the nucleus and contained transcriptional activation domains (TADs). Gus assay and semi-quantitative RT-PCR analysis demonstrated that the TaMYBsm1 genes were up-regulated when the wheat was treated by PEG and mannitol. Compared with WT plants, the germination rates were much higher, but the water loss rates were much lower in TaMYBsm1-D overexpression plants. TaMYBsm1-D transgenic plants showed distinct higher proline contents but a lower MDA content than the WT plants. The three downstream genes were highly expressed in TaMYBsm1-D transgenic plants. We concluded from these results that TaMYBsm1 genes play an important role in plant drought stress tolerance through up-regulation of DREB2A, P5CS1 and RD29A. The increase of proline content and decrease of MDA content may also be involved in the drought response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The MYB proteins constitute a large family of transcription factors (TFs), which are functionally diverse and present in all eukaryotes (Dubos et al. 2010). The MYB proteins are characterized by a highly conserved MYB domain generally with 1–4 imperfect amino acid sequence repeats. According to the number of adjacent repeats of the DNA-binding domains SWI3, ADA2, N-CoR, and TFIIIB (SANT), MYB proteins are divided into four groups, R2R3-MYB, 3R-MYB, 4R-MYB and 1R-MYB (Dubos et al. 2010).
The R2R3-MYB proteins are the largest group of MYB TFs in plants (Feller et al. 2011). The large family of plant-specific R2R3-MYB has been proved to associate with evolution of plant resistance to environmental stress (Dubos et al. 2010). Many R2R3-MYB proteins are involved in drought responses in plants. Several R2R3-MYB TFs in response to drought stress have been isolated and studied. For example, AtMYB15 functions as a TF involved in improvement of drought tolerance in Arabidopsis (Manu et al. 2006). AtMYB41 is reported to negative regulate of transcriptional response to osmotic stress (Lippold et al. 2009), OsMYB2 encodes stress-responsive MYB TFs to regulate dehydration response (Yang et al. 2012). The expression of TaMYB1 in roots is strongly related to the wheat plant responses to oxygen concentration stresses (Lee et al. 2007). TaMYBsdu1 is markedly up-regulated in the leaf and root of wheat under long-term drought stress (Rahaie et al. 2010). TaMYB33 enhances salt and drought tolerance, partly through reconstruction of osmotic balance and detoxification of reactive oxygen species (Qin et al. 2012; Zhang et al. 2012b). TaMYB30-B and TaMYB19-B can improve drought stress tolerance of the Arabidopsis transgenic plants (Zhang et al. 2012b, 2014).
Wheat is one of the most important crops worldwide. Its production is severely affected by drought stress. A crop simulation model showed that the wheat production has reduced by 4.6 % during 1961 and 1980 in China (Song and Dong 2006), and the loss presented an upward tendency from 1962 to 2010 (Cao et al. 2014). Therefore, identification of drought response genes will help to elucidate the molecular mechanisms of plant drought response and tolerance. In this study, we firstly reported the isolation and characterization of three homeologous copies of the TaMYBsm1 genes, including TaMYBsm1-A, TaMYBsm1-B and TaMYBsm1-D. Correlation between the expression of the genes encoding wheat TaMYBsm1 and drought was evaluated,
Materials and methods
Plant material and wheat BAC library
T. aestivum cv. Shimai 15 was used to clone the genomic DNA and cDNA sequences of TaMYBsm1. The nullisomic/tetrasomic lines of the Chinese Spring (CS NT) lines were used to determine the chromosomal location of TaMYBsm1. Arabidopsis thaliana Columbia-0 was used to generate TaMYBsm1-promoter (TaMYBsm1-A, TaMYBsm1-B and TaMYBsm1-D) transgenic lines.
Isolation of full-length cDNAs and genomic DNAs of the TaMYBsm1 genes
Based on expressed sequence tag (EST, GenBank: CA653725) sequence, nested PCR primers (NP, Table 1) were designed to screen wheat cv. Shimai 15 BAC library. BAC pool plasmids were used as template and amplified for 35 cycles of 94 °C for 45 s, 55 °C for 45 s, 72 °C for 45 s.
Sequencing primers were designed based on known sequences of the TaMYBsm1 genes. BAC plasmids were used as template for sequencing on ABI 3730XL DNA Analyzer to acquire 5′ and 3′ ends of TaMYBsm1. These steps were repeated until the genomic DNA sequences of the TaMYBsm1 genes were obtained, including their 5′ flanking sequences.
The primers (TaMYBsm1FL, Table 1) used to isolate full-length cDNAs and genomic DNAs of the TaMYBsm1 genes were designed based on the predicted TaMYBsm1 genomic DNA sequences. The Premix PrimerSTAR HS (TaKaRa, Dalian, China) was used in the PCR amplification. The PCR program included 98 °C for 30 s, followed by 30 cycles of 98 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min, and then a final extension step at 72 °C for 7 min.
To obtain full length cDNA sequences of the TaMYBsm1 genes, total RNA was extracted from two-week-old wheat seedlings using RNAiso reagent (TaKaRa, Dalian, China). mRNA was isolated using polyATractmRNA isolation system III (Promega). One microgram of mRNA was reverse transcribed by using PrimerScript™ 1st Strand cDNA Synthesis Kit. The PCR products were cloned into the pMD18-T plasmid (TaKaRa, Dalian, China). The positive clones were picked randomly and sequenced with BigDye Terminator v3.1 Cycle Sequencing Kit (ABI) on ABI 3730XL DNA Analyzer. The 5′ UTR and 3′ UTR of the TaMYBsm1 genes were identified using the 5′/3′ RACE Kit (Roche) with oligo (dT) as primer.
Chromosomal location of the TaMYBsm1 genes
The primers were used to amplify TaMYBsm1 genes in the 42 CS NT lines in order to determine chromosomal location of TaMYBsm1. The primers, DW1 (Table 1) for TaMYBsm1-A (512 bp) and TaMYBsm1-D (717 bp) and DW2 (Table 1) for TaMYBsm1-B (573 bp), were designed based on the promoter sequences of TaMYBsm1 by using Primer premier 6.0 (Premier, Canada) software. The DNA of CS NT lines was used as template for 30 cycles of 94 °C for 45 s, 55 °C for 45 s, 72 °C for 45 s.
Sequence analysis
Sequence assembly and coding region prediction were performed using Lasergene SeqMan II Module (DNAStar; http://www.DNAStar.com). Multiple sequence alignments were analyzed by ClustalW 1.83 software (http://www.ch.embnet.org/software/ClustalW.html). Cluster analysis was conducted by MEGA4.1. A bootstrap analysis was carried out and the robustness of each cluster was verified in 1,000 replications. Sequences were shaded using the BoxShade program (http://www.ch.embnet.org/software/BOX_form.html ).
Transactivation assay of the TaMYBsm1 genes
The transactivation assay was performed according to literature previously described. The TaMYBsm1-A, TaMYBsm1-B, and MYBsm1-D constructs were produced via PCR reaction using full length TaMYBsm1 cDNA sequences. The primers, TRA1 for TaMYBsm1-A and TaMYBsm1-B and TRA2 for TaMYBsm1-D are listed in Table 1. The PCR products were cloned in pMD18-T plasmid (TaKaRa, Dalian, China) and were digested with EcoRI and BamH1. The digested constructs were cloned with the same restrict enzyme site of the pGBKT7 vector (Clontech). The constructs were transformed to yeast strain Y190 (MATa, HIS3, lacZ, rp1, leu2, cyhr2) and then the transformant was selected from a selection medium (SD/-Trp) including 25 mM 3-amino-1, 2, aminotriazole (3-AT). The selected transformant was cultured in the selection medium (SD/-Trp-His) including 25 mM 3-AT for 1 day, and a filter-lift assay was performed for blue color development.
Subcellular localization assay
The full-length cDNA sequences of the TaMYBsm1 genes were amplified by PCR using the primers TRA1 and TRA2 (Table 1), and were cloned in-frame into the binary vector pEGAD between the EcoRI and BamHI sites. The positive transformants were identified by enzyme cutting and sequencing. The vectors were introduced into Nicotiana benthamiana leaves through Agrobacterium tumefaciens mediated (strain EHA101) transformation. The epidermal cell layers of tobacco leaves expressing the green fluorescent protein (GFP)-TaMYBsm1 fusions were assayed for fluorescence using a confocal microscope (Leica SP8).
Semi-quantitative reverse transcription-PCR
The resulting cDNA was used as template for 30 cycles of 20 s at 95 °C, 35 s at 62 °C. Ten microliters of the PCR product was electrophoresed and visualized by ethidium bromide staining. Wheat tubulin was used as a loading control. The primers, WT1 for wheat tubulin, SQA for TaMYBsm1-A, SQB for TaMYBsm1-B and SQD for TaMYBsm1-D, are listed in Table 1.
Construction of TaMYBsm1pro: Gus and Gus assay
The promoter sequences of the TaMYBsm1 genes were amplified using BAC clone plasmids as templates. The primers, TaMYBsm1pro1 for amplification of the promoter regions of TaMYBsm1-A, TaMYBsm1-D and TaMYBsm1pro2 for amplification of the promoter region of TaMYBsm1-B, are listed in Table 1. PCR products were firstly cloned in pMD18-T plasmids (TaKaRa, Dalian, China) confirmed by sequencing, then the positive transformants were digested with BamHI and Hind III and cloned into pCAMBIA1381Z to generate TaMYBsm1pro:Gus. The vectors were transformed into Arabidopsis mediated by A. tumefaciens strain GV3101 by the floral dipping method (Clough and Bent 1998). Then the expression patterns of TaMYBsm1 genes under 16 % PEG 6000 and 400 mM dehydrant mannitol treatments were monitored by semi-quantitative RT-PCR and Gus staining. Briefly, the 12-day seedlings tested were infiltrated in Gus staining solutions and incubated at 37 °C for 4–8 h, the stained seedlings were then cleared in 70 % ethanol and photographed.
Construction of CaMV35S:TaMYBsm1-D vector and plant transformation
The open reading frame (ORF) of the TaMYBsm1-D was amplified using primers TaMYBsm1TR (Table 1). The amplified products were digested by KpnI and BamHI and then cloned into the vector pCAMBIA2300, in which TaMYBsm1-D was driven by the CaMV35S promoter; the resultant vector was named as CaMV35 s:TaMYBsm1-D. Then the vector was transformed into Arabidopsis mediated by A. tumefaciens strain GV3101 using the floral dipping method (Clough and Bent 1998). Three homozygous transgenic Arabidopsis lines were confirmed by PCR and RT-PCR, and were selected for further study.
Drought tolerance analysis of the TaMYBsm1-D transgenic Arabidopsis plants
For drought stress assay, the CaMV35 s:TaMYBsm1-D transgenic Arabidopsis plants together with the wild type (WT) control were grown under normal conditions for 4 weeks and stopped watering for another 2 weeks. To observe the adaptability of TaMYBsm1-D transgenic plants against to drought, and to detect if the response is reversible, we re-watered the plants for 2 days for observation. All the tests were photographed to record their phenotypic changes.
Physiological characteristics of the TaMYBsm1-D transgenic Arabidopsis plants
Arabidopsis seeds of the transgenic Arabidopsis plants and the WT plants used in the germination assays were planted in MS medium containing 15 % PEG 6000. Seeds with radicle tip fully expanding the seed coat were regarded as germination. The percentage of germinated seeds was scored as the germination rate.
The transgenic Arabidopsis plants and the WT seedlings grown for 4 weeks under normal conditions were used for water-loss assay. Their aerial parts were collected to record their fresh weight at room temperature (40 % relative humidity), afterwards, they were weighed per hour until no obvious differences were detected between two adjacent time points. To record their final dry weight, they were dried in an oven at 85 °C for 24 h. The water-loss rate was calculated on the basis of the initial weight of the plants. Sixteen plants in each line were pooled for measurement.
The transgenic Arabidopsis plants and the WT seedlings were cultured for 4 weeks at normal conditions, then they were drought-stressed for 2 weeks, their rosette leaves were collected for free proline and malondialdehyde (MDA) measurement. The proline content (mg/g) was quantified by the ninhydrin acid reagent method using proline as the standard (Bates et al. 1973). MDA content was determined as described previously (Draper and Hadley 1990). For statistical analysis, thirty seedlings were measured for individual WT and transgenic Arabidopsis lines, and Student’s t test was performed.
Real-time quantitative PCR assay of the drought-stress-related genes in the TaMYBsm1-D transgenic Arabidopsis plants
Two-week-old seedlings of CaMV35 s:TaMYBsm1-D transgenic Arabidopsis plants together with the WT control were treated with 16 % PEG 6000 for 24 h. Three downstream drought-stress-related genes (DREB2A, P5CS1 and RD29A) were selected for real-time quantitative PCR assay. The primers used to amplify these genes in real-time quantitative PCR analysis are listed in Table 1. The Arabidopsis β-actin gene was amplified as internal control. The cDNA product was used as template for 40 cycles of 30 s at 95 °C, 5 s at 95 °C, 34 s at 60 °C. The quantitative analysis was performed using the 2−ΔΔCt method.
Results
Cloning of the TaMYBsm1 genes
The dehydration-induced EST-CA653725, annotated as TaMYBsm1, was identified in wheat expression profile. To further determine the function of the TaMYBsm1 genes in the regulation of drought tolerance in common wheat, we screened the hexaploid wheat genomic DNA library. Eight genomic BAC clones were isolated and three highly homologous genomic sequences (TaMYBsm1-A, TaMYBsm1-B and TaMYBsm1-D) were identified, then their corresponding DNA and cDNA sequences of the TaMYBsm1 genes were confirmed by PCR amplification.
The TaMYBsm1 genes were located on wheat chromosomes 7A, 7B and 7D, respectively (Fig. 1). The three promoter sequences of each of the TaMYBsm1 genes were highly identical to sequences on chromosomes 7A, 7B and 7D, respectively. All these results indicated that TaMYBsm1 was located on the short arm of chromosome 7.
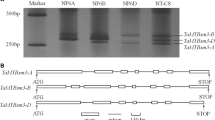
All the TaMYBsm1 genes had ORFs of 975 bp, each of which encoded an R2R3-MYB of 324 amino acids. The three genes share 95.0–96.1 % identity at the nucleotide level and 93.2–94.8 % identity at the amino acid level. The TaMYBsm1 genes had identical structure, containing two introns and three exons.
Cluster analysis of plant MYBsm1 proteins
Using TaMYBsm1 as the query sequences, sixteen TaMYBsm1 homologs were identified. Cluster tree showed that plant MYBsm1 proteins were divided into two divergent branches, corresponding to monocot and dicot plant sequences (Fig. S1a). The AeMYBsm1 of Aegilops tauschii is identical to TaMYBsm1-D. Only 2 of 324 amino acids in the TuMYBsm1 of Triticum urartu were different from TaMYBsm1-A. The results were in consistent with chromosomal locations of the TaMYBsm1 genes.
Multiple alignment revealed that 35 (76 %) and 32 (73 %) of amino acids were identical in the R2 and R3 MYB repeat regions, respectively, among all the plant MYBsm1 proteins (Fig. S1b). These results indicating that the R2R3-MYB domains were highly conserved in plant MYBsm1 proteins.
Autoactivation in the heterologous yeast system
The transcriptional activations of the TaMYBsm1 proteins were investigated via yeast-based transactivation assay. The full length of the TaMYBsm1 genes were fused to the GAL4 DNA-binding domain in the pGBKT7 plasmids. When the constructed plasmids were induced in yeast Y190 strain containing GAL1 promoter and HIS3 and lacZ reporter genes, β-galactosidase activity was observed in yeast cells harboring TaMYBsm1-A, TaMYBsm1-B, TaMYBsm1-D, and TaNAC6, but not in yeast cells containing pGBKT7 plasmids (Fig. 2). The results indicated that each of the TaMYBsm1 proteins may contain a transcriptional activation domain.
Transactivation assay of TaMYBsm1 proteins in yeast cells. The transformed yeast cells were grown on selective medium SD/-Trp (left) and SD/-Trp-His-Ade (middle). An X-gal lift assay was carried out using SD/-Trp-His plate (right). V yeast transformed with the pGBKT7 vector, F yeast transformed with the TaMYBsm1 genes, N yeast transformed with T. aestivum NAC6 (TaNAC6), A TaMYBsm1-A, B TaMYBsm1-B, D TaMYBsm1-D
Subcellular localization of the TaMYBsm1 proteins
To investigate the subcellular location of the TaMYBsm1 proteins, we made three constructs harboring a GFP-TaMYBsm1 fusion protein under the control of the constitutive CaMV35S promoter, respectively. The generated constructs were introduced into N. benthamiana leaf epidermal cells via A. tumefaciens-mediated infiltration (Sparkes et al. 2006). The fluorescent signals from the constructs of GFP:TaMYBsm1-A, GFP:TaMYBsm1-B and GFP:TaMYBsm1-D were all observed in the nucleus of N. benthamiana mesophyll cells, indicating that all the TaMYBsm1 genes have localized in the nucleus (Fig. 3).
Expression analysis of the TaMYBsm1 genes in common wheat
The expression patterns of the TaMYBsm1 genes under 16 % PEG 6000 were monitored by semi-quantitative RT-PCR (Fig. 4a–c). The results showed that the TaMYBsm1 genes were induced by 16 % PEG 6000 treatment in the root and leaf of wheat. TaMYBsm1 transcripts accumulated at the late stage of PEG treatment in root (Fig. 4a) and at the early stage in leaf (Fig. 4b). The TaMYBsm1 genes were mainly expressed in roots, leaves and stamens of wheat under normal conditions. The expression of The TaMYBsm1 genes in roots is stronger than those in other organs (Fig. 4c), indicating that the TaMYBsm1 genes mainly functioned in wheat roots.
Using PCR amplification, the 1,709–2,144 bp sequences upstream of the start code of the TaMYBsm1 genes were amplified. Many motifs related to dehydration stress were predicted on PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html). The promoter region of TaMYBsm1-B is distinct from that of TaMYBsm1-A and TaMYBsm1-D. Compared to TaMYBsm1-A, two dehydration stress related motifs were deleted at the same position on the promoter region of TaMYBsm1-D (Fig. 5). Gus assay was performed in transgenic Arabidopsis plants expressing Gus gene under the control of the TaMYBsm1 promoters (TaMYBsm1-A promoter:Gus, TaMYBsm1-B promoter:Gus and TaMYBsm1-D promoter:Gus) to examine the expression of the TaMYBsm1 genes under 16 % PEG 6000 and 400 mM mannitol treatment (Fig. 4d). Results showed that Gus proteins accumulated when the Arabidopsis plants expressing three TaMYBsm1 promoter:Gus were treated by PEG and mannitol.
Overexpression of TaMYBsm1-D improves drought tolerance of transgenic Arabidopsis plants
The function of TaMYBsm1-D in drought stress tolerance was investigated in Arabidopsis plants. Three transgenic lines were selected for further study. All the transgenic plants together with the WT control grown for 3 weeks under normal conditions, all the plants grown well and no obvious phenotypic changes were observed.
The drought tolerance of TaMYBsm1-D transgenic Arabidopsis plants was investigated in soil under water deprivation conditions during the seedling stage. After stopping watering for 2 weeks, all WT plants (n = 16, 100 %) exhibited severe symptoms of water loss and wilting. On the contrary, the wilting symptoms in the TaMYBsm1 overexpression Arabidopsis transgenic lines were reduced and the majority of leaves were green at this time point. After re-watering, most of the WT plants died (n = 12.75 %), while a number (n = 4.92 %) of TaMYBsm1-D transgenic plants exhibited normal growth after 2 days of re-watering (Fig. 6a).
Transgene identification and drought stress response analysis in WT and transgenic plants. a Phenotypic changes of transgenic Arabidopsis plants before/after drought stress. b Effect of drought stress on the germination of seeds. c water-loss assay of all the transgenic Arabidopsis plants and WT control. d proline content. e MDA contents were measured before/after drought stress. WT wild type Columbia-0; L3, L4, and L9, TaMYBsm1-D overexpression transgenic lines. CK normal condition. The asterisks indicate the statistically significant differences between the WT control and the transgenic Arabidopsis lines determined through student’s t-tests (* P < 0.05, ** P < 0.001)
When growing in MS medium containing 16 % PEG 6000, the germination rates of TaMYBsm1-D overexpression plants were much higher than those of WT plants (Fig. 6b). Compared with the WT plants, the TaMYBsm1-D transgenic plants displayed lower rates of water loss at all the time points (1, 2, 3, 5, 7 h) after 4 weeks under normal conditions (Fig. 6c). When exposed to mannitol stress, the TaMYBsm1-D transgenic plants under dehydration showed distinct higher proline contents but lower MDA content than the WT plants (Fig. 6d, e). Taken together, these results suggested that there might be an association between the overexpression of TaMYBsm1-D and drought tolerance in transgenic Arabidopsis plants.
Expression of downstream genes in TaMYBsm1-D transgenic plants
In order to investigate whether the expression levels of drought stress-related genes were altered or not in TaMYBsm1-D transgenic plants, three drought stress-related genes (DREB2A, P5CS1 and RD29A) were chosen for analysis by real-time quantitative PCR. As a result, these genes exhibited a significantly higher expression level in the TaMYBsm1-D transgenic plants than WT (Fig. 7). These changes suggested that TaMYBsm1-D functioned in drought response through up-regulating the downstream drought stress-related genes.
Expression levels of three genes related to drought stress in transgenic TaMYBsm1-D Arabidopsis plants. Gene-specific primers were used for the detection of the relative transcript levels of the drought stress-responsive genes. The data represent the means of three replicates. WT Columbia-0, L4 TaMYBsm1-D overexpression transgenic line. CK normal condition. The asterisks indicate the statistically significant differences in the comparisons between the WT control and the transgenic Arabidopsis lines which were determined through student’s t-tests (* P < 0.05, ** P < 0.001)
Discussion
Drought stress is a major limited factor that influences the growth and development of plants. Therefore, isolating the genes involved in drought stress tolerance and cultivating plant varieties with enhanced drought stress tolerance are important for plant breeding. MYB gene family is one of the largest families encoding regulatory proteins with multiple functions (Dubos et al. 2010). In recent years, some R2R3-MYB genes have been described to be associated with drought responses and tolerance in some species, especially in rice and Arabidopsis, but only a few works have been reported in common wheat (Lee et al. 2007; Liu et al. 2011; Mao et al. 2011; Rahaie et al. 2010; Zhang et al. 2012a, b, 2014).
In this study, the function of the TaMYBsm1 genes was analyzed by screening the wheat genomic DNA library in transgenic Arabidopsis. The TaMYBsm1 genes (TaMYBsm1-A, TaMYBsm1-B and TaMYBsm1-D) were successfully isolated, and the potential function of these genes is closely related to plant drought tolerance. Based on variation of the TaMYBsm1 promoter sequences, the TaMYBsm1 genes were mapped on wheat chromosomes 7A, 7B, and 7D, named as TaMYBsm1-A, TaMYBsm1-B and TaMYBsm1-D, respectively. Gus staining indicated that the expressions of the TaMYBsm1 genes were up-regulated by dehydration stress in transgenic Arabidopsis. Semi-quantitative RT-PCR experiments showed that the TaMYBsm1 genes were induced by 16 % PEG 6000, but showed different expression profiles in roots and leaves of common wheat. The results indicated the TaMYBsm1 genes were the drought stress response genes, and differently expressed in different plant organs.
TaMYBsm1 N-terminal regions contained R2 and R3 domains with the conserved amino acid motifs R2 [-W-(X19)-W-(X19)-W-] and R3 [-F-(X18)-W-(X18)-W-] that are known to be essential for DNA binding (Dubos et al. 2010). In this study, the R2R3-MYB domains of plant MYBsm1 homologs exhibited significant sequence conservation and showed no difference between monocot and dicot plants. These results indicated that plant R2R3-MYB domains of the MYBsm1 proteins played important roles in their functions. Many R2R3-MYB proteins have transcriptional activation domains (TADs), that has been firstly identified in ZmC1 (Goff et al. 1991). Transcriptional activity was identified in all TaMYBsm1 proteins by yeast-based transactivation assay in our study. The promoter region of TaMYBsm1-B is distinct from that of TaMYBsm1-A and TaMYBsm1-D. Compared to TaMYBsm1-7A, two dehydration stress related motifs (MYBCORE) were deleted at the same position on the promoter region of TaMYBsm1-D. Under the dehydration stress, the TFs activate RNA polymerase II through targeting on the cis-element, induce expression of target genes to resist against the stress. However, this regulation depends on an interaction of a series IFs. For example, MYB IFs were reported to interact with basic/helix-loop-helix (bHLH) for environmental stress response (Singh 1999). Though the promoter elements of the three TaMYBsm1 genes were different as reported in our study, these elements may play a key role in co-regulation of drought stress. The plant TaMYBsm1 proteins branched into two distinct groups on the cluster tree corresponding to monocot and dicot plants, because of the difference of the C-terminal regions which drastically changed after the divergence of dicots and monocots from their common ancestor. Therefore, the MYBsm1 proteins may exhibit different transcriptional activation in monocot and dicot plants.
Arabidopsis seedlings of overexpressing TaMYBsm1-D exhibited enhanced tolerance to drought stress. Under drought stress, the transgenic plants had higher seed germination rate and lower water loss rate than the non-transgenic plants. In order to uncover the related molecular factors involved in the drought tolerance of TaMYBsm1-D in transgenic Arabidopsis, we detected physiological indicators of stress tolerance. Physiological indicators, such as MDA and proline, could be used as a reference for evaluation of plant stress tolerance. It has been reported that rapid damage, such as drought stress will damage the membrane of plant cells (Liu et al. 2016; Wang et al. 2016). The leakage of membrane is caused by the enhancement of free radical, which will result in lipid peroxidation (Mirzaee et al. 2013). MDA would produce during this process. Therefore, MDA is widely recognized as a marker for lipid peroxidation (RoyChoudhury et al. 2007). At the early period of drought, MDA substantially produced for response until the plant could not undergo biosynthesis. Then the content of MDA is decreased. Proline is compatible solutes that accumulate in plants in response to abiotic stress (Armengaud et al. 2004; Chyzhykova and Palladina 2005; Zhang et al. 2016). Under drought stress, transgenic Arabidopsis plants accumulated more proline but less MDA than that in the WT plants. It may be that proline is a factor that contributes to drought tolerance capabilities of the TaMYBsm1-D transgenic plants. Low MDA content provides the transgenic plants with more growth advantages.
Stress-responsive genes play important roles in regulating the defense response pathways. Some major stress-responsive genes in transcriptional regulatory networks in response to abiotic stresses have been identified in Arabidopsis. The expression profiles of these genes could provide important information for uncovering the mechanisms of TaMYBsm1-D in drought stress response and tolerance. In this study, the three downstream genes DREB2A, P5CS1 and RD29A were significantly up-regulated in transgenic Arabidopsis plants under drought stress. In Arabidopsis, DREB2A was induced by dehydration and high-salt stress (Liu et al. 1998; Nakashima et al. 2000; Sakuma et al. 2002). Overexpression of DREB2A activated the expression of many stress-inducible genes and resulted in significant drought stress tolerance (Sakuma et al. 2006). Under drought stress, TaMYBsm1 overexpression increased DREB2A expression level and the capabilities of transgenic Arabidopsis drought stress tolerance were improved by the activation of many stress-inducible genes. P5CS catalyzes the rate-limiting step of proline biosynthesis. P5CS1 is necessary for proline accumulation under osmotic stress (Székely et al. 2008). We also found the proline was significantly increased in transgenic plant in our study. Therefore, overexpression of P5CS improved proline level and increased tolerance to osmotic stress (Kishor et al. 1995). Due to up-regulation of P5CS1, a large amount of proline accumulated in TaMYBsm1 overexpression Arabidopsis plants which enhanced the drought stress tolerance of the transgenic plants. RD29A is induced by desiccation and has at least two cis-acting elements, one involved in the ABA-associated response to desiccation and the other induced by changes in osmotic potential (Shinozaki and Yamaguchi-Shinozaki 2007). The results above suggested that the enhanced drought tolerance of the TaMYBsm1-D transgenic Arabidopsis plants is at least partially due to up-regulating expression of downstream genes related to drought stress including DREB2A, P5CS1 and RD29A. We thought that the drought stress stimulates overexpression of DREB2A, which significantly activated the expression of many stress-inducible genes, such as P5CS1 and RD29A. However, this study limited to analyze the the transcriptional regulation of these genes to uncover the stress response at the protein level. We will perform a further study to explain the regulation mechanism in translational level.
In conclusion, overexpression of TaMYBsm1 enhances the drought tolerance of transgenic plants. Up-regulation of DREB2A, P5CS1 and RD29A genes play a key role in drought tolerance.
References
Armengaud P, Thiery L, Buhot N, Grenier-de March G, Savouré A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120:442–450
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Cao Y, Yang J, Xiong W, Wu Y, Feng L, Yang X (2014) Simulation of winter wheat yield influenced by potential drought in China during 1962–2010. Trans Chinese Soc Agric Eng 30:128–139
Chyzhykova O, Palladina T (2005) The role of amino acids and sugars in supporting of osmotic homeostasis in maize seedlings under salinization conditions and treatment with synthetic growth regulators. Ukr Biokhim Zh 78:124–129
Clough SJ, Bent AF (1998) Floral dip: a simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. Plant J 16:735–743
Draper H, Hadley M (1990) [43] Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581
Feller A, Machemer K, Braun EL, Grotewold E (2011) Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J 66:94–116
Goff S, Cone K, Fromm M (1991) Identification of functional domains in the maize transcriptional activator C1: comparison of wild-type and dominant inhibitor proteins. Genes Dev 5:298–309
Kishor PK, Hong Z, Miao G-H, Hu C-AA, Verma DPS (1995) Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Lee TG, Jang CS, Kim JY, Kim DS, Park JH, Kim DY, Seo YW (2007) A Myb transcription factor (TaMyb1) from wheat roots is expressed during hypoxia: roles in response to the oxygen concentration in root environment and abiotic stresses. Physiol Plant 129:375–385
Lippold F, Sanchez DH, Musialak M, Schlereth A, Scheible W-R, Hincha DK, Udvardi MK (2009) AtMyb41 regulates transcriptional and metabolic responses to osmotic stress in Arabidopsis. Plant Physiol 149:1761–1772
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant Cell Online 10:1391–1406
Liu H, Zhou X, Dong N, Liu X, Zhang H, Zhang Z (2011) Expression of a wheat MYB gene in transgenic tobacco enhances resistance to Ralstonia solanacearum, and to drought and salt stresses. Funct Integr Genomics 11:431–443
Liu SC, Jin JQ, Ma JQ et al (2016) Transcriptomic analysis of tea plant responding to drought stress and recovery. Plos One 11
Manu A, Yujin H, Avnish K, Chun-Hai D, Hiroaki F, Xianwu Z, Jian-Kang Z (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281:37636–37645
Mao X, Jia D, Li A et al (2011) Transgenic expression of TaMYB2A confers enhanced tolerance to multiple abiotic stresses in Arabidopsis. Funct Integr Genomics 11:445–465
Mirzaee M, Moieni A, Ghanati F (2013) Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cultivars. J Agri Sci Technol 15:593–602
Nakashima K, Shinwari ZK, Sakuma Y, Seki M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2000) Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration-and high-salinity-responsive gene expression. Plant Mol Biol 42:657–665
Qin Y, Wang M, Tian Y, He W, Han L, Xia G (2012) Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. Mol Biol Rep 39:7183–7192
Rahaie M, Xue G-P, Naghavi MR, Alizadeh H, Schenk PM (2010) A MYB gene from wheat (Triticum aestivum L.) is up-regulated during salt and drought stresses and differentially regulated between salt-tolerant and sensitive genotypes. Plant Cell Rep 29:835–844
RoyChoudhury A, Roy C, Sengupta DN (2007) Transgenic tobacco plants overexpressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep 26:1839–1859
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration-and cold-inducible gene expression. Biochemical and biophysical research communications 290:998–1009
Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell Online 18:1292–1309
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Singh KB (1999) Transcriptional regulation in plants: the importance of combinatorial control. Plant Physiol 118:1111–1120
Song YL, Dong WJ (2006) Influence of drought on winter wheat yield in China during 1961–2000. J Nat Disasters
Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1:2019–2025
Székely G, Ábrahám E, Cséplő Á et al (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Wang J, Li Q, Mao X, Li A, Jing R (2016) Wheat transcription factor TaAREB3 participates in drought and freezing tolerances in Arabidopsis. Int J Biol Sci 12:257–269
Yang A, Dai X, Zhang W-H (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. Journal of experimental botany:err431
Zhang L, Zhao G, Xia C, Jia J, Liu X, Kong X (2012a) A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J Exp Bot 63:5873–5885
Zhang Z, Liu X, Wang X, Zhou M, Zhou X, Ye X, Wei X (2012b) An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense-and stress-related genes. New Phytol 196:1155–1170
Zhang L, Liu G, Zhao G, Xia C, Jia J, Liu X, Kong X (2014) Characterization of a wheat R2R3-MYB transcription factor gene, TaMYB19, involved in enhanced abiotic stresses in Arabidopsis. Plant and Cell Physiology:pcu109
Zhang L, Zhang L, Xia C, Zhao G, Jia J, Kong X (2016) The Novel Wheat Transcription Factor TaNAC47 Enhances multiple abiotic stress tolerances in transgenic plants. Front Plant Sci 6
Acknowledgments
This research was supported by Shijiazhuang Science and Technology Pillar Program (No. 12149402A) and Natural Science Foundation of Hebei Province (No. C2014106075).
Author information
Authors and Affiliations
Corresponding author
Additional information
Meng-jun Li and Yu Qiao are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Mj., Qiao, Y., Li, Yq. et al. A R2R3-MYB transcription factor gene in common wheat (namely TaMYBsm1) involved in enhancement of drought tolerance in transgenic Arabidopsis . J Plant Res 129, 1097–1107 (2016). https://doi.org/10.1007/s10265-016-0857-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0857-5