Abstract
Phyllonoma, the sole genus of Phyllonomaceae (Aquifoliales) consisting of four Central American species, has not been well-characterized morphologically. Following a previous study of flower and inflorescence morphology, I here report the embryology of the genus based on P. tenuidens and compare its characteristics with those of other aquifolialean families, namely, Aquifoliaceae, Cardiopteridaceae, Helwingiaceae, and Stemonuraceae. Comparisons indicate that although Phyllonoma resembles all the other families embryologically, it more closely resembles Aquifoliaceae and Helwingiaceae in lacking a vascular bundle in its integument and bearing ab initio Cellular endosperm. The genus especially resembles Helwingiaceae by possessing a tenuinucellate ovule. This result corroborates molecular and floral morphological evidence, supporting the distinctness of Phyllonoma as a family and its sister-group relationship to East‒Asian Helwingiaceae. However, Phyllonoma is clearly distinguished from Helwingiaceae by seed coat structure. In Phyllonoma, the seeds (dispersed in berries) have a thick seed coat composed of irregularly enlarged, thick-walled exotestal cells, whereas the seeds (dispersed in drupes) have a thin membranous seed coat in Helwingiaceae. Taken together with earlier information on pollination (entomophily in Phyllonoma versus ambophily in Helwingiaceae), embryological evidence shows that distinct evolution has occurred in reproductive characters relating to pollination and seed dispersal in Phyllonoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phyllonoma, a genus consisting of four tree or shrub species, occurs from Mexico to Peru (Mori and Kallunki 1977). In the past, it had been placed in various families such as Saxifragaceae (Bentham and Hooker 1865; de Candolle 1873; Engler 1891, 1930; Schulze–Menz 1964), Grossulariaceae (Cronquist 1968, 1981; Mori and Kallunki 1977), Escalloniaceae (Hutchinson 1967, 1973), and Phyllonomaceae (Rusby 1905; Takhtajan 1969, 1997, 2009). However, based on molecular analyses (e.g., Bremer et al. 2002; Kårehed 2001; Olmstead et al. 2000), the Angiosperm Phylogeny Group (2003, 2009) has consistently accepted its own family Phyllonomaceae in the asterid order Aquifoliales (see also Reveal 2012; Stevens 2001 onwards). Within Aquifoliales, Phyllonomaceae are sister to East-Asian Helwingiaceae (Helwingia only); a clade of Phyllonomaceae and Helwingiaceae is sister to Aquifoliaceae (Ilex only); and a clade consisting of the three families is sister to a clade of Cardiopteridaceae and Stemonuraceae (Soltis et al. 2011; Tank and Donoghue 2010). Until I recently presented a study of its flower and inflorescence morphology (Tobe 2013, 2014), Phyllonoma had been poorly understood morphologically (Stevens 2001 onwards). The analyses of flower and inflorescence morphology showed that Phyllonoma shares an epiphyllous cymose inflorescence, inferior ovary, and epigynous disc nectary with Helwingiaceae, but differs clearly from Helwingiaceae in having glandular trichomes on its sepal margins and a bicarpellate, unilocular gynoecium bearing many ovules on parietal placentae (for more detail see Tobe 2013, 2014).
While examining the flower and inflorescence morphology, I investigated the embryology of Phyllonoma to explore evolution in other morphological characters. Embryology has previously provided data for more than 50 characters associated with anther, ovule, and seed development, and thus contributes to a better understanding of the relationships of families (Tobe 1989; for most recent examples, see Yamamoto et al. 2014: Biebersteiniaceae, Sapindales; Ao and Tobe 2015: Helwingiaceae, Aquifoliales). Among the embryological characters, those of seed coat development and structure have often provided evidence for the adaptive evolution of a species or species group (e.g., Tobe et al. 1987: Oenothera, Onagraceae). Earlier descriptions of embryological features of Phyllonoma are limited to brief descriptions of the ovules and seeds published by Krach (1976), Mauritzon (1933), Takhtajan (2000), and van Tieghem (1898). Van Tieghem (1898, p. 207) described the ovule of Phyllonoma as having a thin and ephemeral nucellus with a single integument. Mauritzon (1933, p. 126‒127) confirmed this observation in P. ruscifolia Willd. ex Schult. Krach (1976) examined mature seed structures of P. ruscifolia, describing that the seed coat is composed of a multi-layered outer integument and a two-layered inner integument. However, as I show later, the ovule of Phyllonoma is unitegmic and not bitegmic as Krach (1976) observed. Takhtajan (2000), without referring to its development, described the mature seed coat of P. laticuspis (Turcz.) Engl. and P. ruscifolia as consisting of the exotesta and endotesta with negligible remnants of mesotestal layers. Except for these data, no information is available for embryological characters.
In this paper I present the first description of many embryological characters for Phyllonoma or Phyllonomaceae. Based on results obtained, I will compare Phyllonoma (Phyllonomaceae) with four other families of Aquifoliales, particularly with Helwingiaceae. Although available information on the other Aquifoliales except for Helwingiaceae is currently insufficient (Ao and Tobe 2015; see also Stevens 2001 onwards), the comparisons seem useful to clarify how Phyllonoma is likely related to the four other families embryologically, and to show which characters we should evaluate in studies of the poorly known families (i.e., Aquifoliaceae, Cardiopteridaceae and Stemonuraceae) for a more critical comparison.
Materials and methods
Flower buds and fruits of Phyllonoma tenuidens Pittier in various stages of development were collected from Provincia Puntarenas, Costa Rica (voucher: William A. Haber 12765, MO). They were fixed in FAA (5 parts stock formalin, 5 parts glacial acetic acid, 90 parts 70 % ethanol). Flower buds and seeds were dehydrated via an ethanol series, and then embedded in Technovit 7100 (Kulzer, Wehrheim, Germany) for microtoming. Serial resin sections cut at a thickness of 5–7 µm were stained with Heidenhain’s hematoxylin and mounted in Entellan. All the microtome sections and acetocarmine-stained pollen grains were observed with an Olympus BX-51 microscope (Tokyo, Japan).
Results
Anthers and microspores
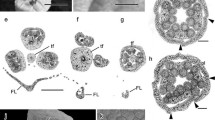
Flowers are bisexual and borne on a small epiphyllous cymose inflorescence (Fig. 1a). Each flower bears five sepals, five petals, five stamens, and a bicarpellate gynoecium with an inferior ovary and two short styles (Fig. 1b, c). The anther of a stamen is tetrasporangiate (Fig. 1b, c). Prior to maturation, its wall is composed of four cell-layers: an epidermis, an endothecium, one middle layer, and a tapetum (Fig. 1d). The middle layer has a common histogenetic origin with the endothecium (Fig. 1d). Therefore, wall formation conforms to the “Dicotyledonous” type (Davis 1966, p. 10). The tapetum is glandular (Fig. 1e, f). Its cells are initially uninucleate and later become binucleate (Fig. 1e). During maturation, the middle layer degenerates, and cells of both the epidermis and endothecium become enlarged (Fig. 1e). By the time of anther wall dehiscence, the cells of the endothecium develop fibrous thickenings (Fig. 1g, h). Although the cells of the epidermis are unspecialized, they are persistent. Anther dehiscence takes place by longitudinal slits, with each slit common to two microsporangia of the theca (Fig. 2g).
Development of anthers and microspores in Phyllonoma tenuidens. a Flowers on leaf. b Longitudinal section of flower bud. c Transverse section (TS) of anther in flower bud. d TS of young anther wall. Note that the middle layer has a common histogenetic origin with the endothecium. e TS of young anther wall showing binucleate tapetal cells and simultaneous cytokinesis in meiosis of microspore mother cells. f TS of older anther showing microspores arranged tetrahedrally. g TS of mature anther. Arrowheads indicate positions of longitudinal slit. h TS of mature anther wall enlarged. i Two-celled mature pollen grains. ent endothecium, ep epidermis, gc generative cell, ml middle layer, mmc pollen mother cell, ov ovule, pe petal, se sepal, st stamen, t tapetum, v nucleus of vegetative cell. Asterisks in c indicate two styles. Scale bars are 1 mm in a, 200 µm in b and c, 100 µm in g, 20 µm in e, f and h, and 10 µm in d and i
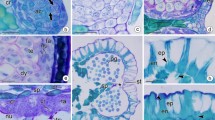
Development of ovules and female gametophytes in Phyllonoma tenuidens. a Transverse section (TS) of gynoecium with ovule primordia. b Longitudinal section (LS) of ovule primordium with an archesporial cell. c LS of ovule with megaspore mother cell. d LS of ovule with functioning megaspore on chalazal side. Note two degenerating megaspores (arrowheads) on the micropylar side. e LS of ovule with two-nucleate female gametophyte and two degenerating megaspores (arrowheads). f LS of ovule with mature female gametophyte. g LS of mature female gametophyte, showing an egg cell and one polar nucleus (shortly moving to the middle of the central cell) on the micropylar side. Two synergids appear in the adjacent sections. h LS of mature female gametophyte, showing two polar nuclei in the middle of the central cell. i LS of mature female gametophyte, showing two antipodal cells. Another antipodal appears in adjacent section. j TS of mature ovule. ant antipodal cell, arc archesporial cell, eg egg cell, fg female gametophyte, it integument, fm functioning megaspore, mc megaspore mother cell, n nucleus in female gametophyte, nc nucellar tissue, nep nucellar epidermis, ov ovule primordium, po polar nucleus. Scale bars are 100 µm in a, 50 µm in c‒f and j, 20 µm in b, and 10 µm in g‒i
Meiosis in a microspore mother cell is accompanied by simultaneous cytokinesis (Fig. 1e), and the resultant microspore tetrads are predominantly tetrahedral (Fig. 1f). Pollen grains are two-celled when shed (Fig. 1i).
Ovules and female gametophytes
Approximately 13‒14 ovules are borne from two parietal placentae in the single locule of the inferior ovary (Fig. 2a). The ovules are oriented variously, depending on their position in the locule as I documented previously (Tobe 2013). They tend to be erect with a micropyle oriented downward in the upper part of the locule, horizontal in the middle part of the locule, and erect with a micropyle oriented upward in the lower part of the locule. Irrespective of its position, the ovule becomes anatropous at maturity.
Early in development, the ovule has a one-celled archesporium differentiated beneath the apical dermal layer of the nucellus (Fig. 2b). The archesporial cell differentiates directly into a megaspore mother cell without producing a parietal cell (Fig. 2c). Thus, the ovule is tenuinucellate. Meiosis in the megaspore mother cell results in a linear triad of megaspores. Although I did not observe the first division, the second division occurs only in a lower cell of a dyad of megaspores. While the megaspore at the chalazal side is functional, the two remaining megaspores degenerate (Fig. 2d), so that two degenerating megaspores are always observed above a developing female gametophyte. Indeed, in each of the 13 ovules at the two-nucleate female gametophyte stage, I observed two degenerating megaspores (Fig. 2e). One might consider the possibility of a bisporic development of the female gametophyte. However, since no case has ever been reported in which the second division occurs only in an upper cell of a dyad of megaspores resulting in a triad of megaspores as in Fig. 2d, the female gametophyte of Phyllonoma is monosporic (for review of suppression of the second division in the upper cell that results in the triad, see Bouman 1984, p 132). A functional megaspore successively develops into the two-nucleate (Fig. 2e), four-nucleate, and eventually eight-nucleate female gametophyte. A mature female gametophyte is ellipsoidal in shape (Fig. 2f). However, because the female gametophyte, such as the ovule itself, is slightly curved, it is often sectioned obliquely. Based on such oblique-longitudinal sections, I confirmed that the mature female gametophyte has an egg cell, two synergids, two polar nuclei, and three antipodal cells (Fig. 2g‒i). Thus, the mode of development of the female gametophyte conforms to the Polygonum type. The antipodal cells are ephemeral.
Throughout the development of the female gametophyte, the apical dermal cells of the nucellus do not divide periclinally to form a nucellar cap (Fig. 2d, e). The apical dermal cells of the nucellus are destroyed by the developing female gametophyte and disappear, so that the upper half of the mature female gametophyte directly borders the integument (Fig. 2f, g, j).
The nucellus is small in the early stages of development. At the megaspore mother cell stage, the nucellus has only a few cells below the archesporial cell (Fig. 2b). However, as the ovule develops, cell divisions rapidly proceed below the megaspore mother cell or developing female gametophyte, forming a long, narrow cylindrical nucellar tissue on the chalazal side (Fig. 2c, f). No hypostase is formed (see chalazal region of a seed in Fig. 2f). The funicle is thick; however, no obturator develops from the funicle.
The ovule is unitegmic (Fig. 2b, c, f, j), as described by van Tieghem (1898). The integument is multiplicative and is initially four cell layers thick. As the ovule develops, cells other than those of the outer epidermis further divide periclinally, so that the integument becomes more than eight cell layers thick in the nearly mature ovule (Fig. 2f, j). A median longitudinal section of the young ovule shows that the integument develops not only on the antiraphal (dorsal) side but also on the raphal (ventral) side (Fig. 2c). The thick integument forms a micropyle above the female gametophyte. No vascular bundles differentiate in the integument (Fig. 2j). Cells of the inner epidermis are not specialized; thus, they do not develop into an integumentary tapetum or endothelium (Fig. 2f, j).
Endosperm and embryo
By the time of fertilization, the ovules grow and increase their sizes within the locule (Fig. 3a). Fertilization is porogamous. The pollen tube enters from the micropyle above the nucellar apex (Fig. 3b). Division of the primary endosperm nucleus occurs at nearly the middle of the central cell, and is accompanied by cell wall formation. Figure 3c shows a longitudinal section of a seed in the earliest stage of endosperm development. Thus, endosperm formation is of ab initio Cellular type. The seed shown in Fig. 3c appears to have a two-celled endosperm plus a zygote. At this developmental stage, the nucellar tissue still remains in the lower half. However, as the seed develops, the whole tissue of the nucellus is replaced by a developing cellular endosperm (Fig. 3d). No prominent structure of endosperm like a micropylar or chalazal haustorium is observed. The mature seed has a copious endosperm, whose cells appear to have accumulated abundant lipids (Fig. 3f).
Development of endosperm and embryo in Phyllonoma tenuidens. a Transverse sections of young fruit. b–f Longitudinal sections of seeds. a and b Young seed with the primary endosperm nucleus. b Young seed with a zygote and a primary endosperm nucleus. c Young seed with a zygote and two endosperm cells. d Young seed with a few endosperm cells. e Older seed with many endosperm cells. f Endosperm in mature seed. g Embryo in mature seed. ec endosperm cell, em embryo, en endosperm, nc nucellar tissue, pen primary endosperm cell, pt pollen tube, ts testa, z zygote. Scale bars are 200 µm in a and d, 100 µm in e and f, 50 µm in f, and 20 µm in b and c
Embryogenesis proceeds very slowly compared to the development of endosperm (Fig. 3e). I did not examine embryogenesis in detail; however, fragmentary data on early and late embryogenesis indicated that it proceeds normally to form a globular embryo (Fig. 3g). The embryo in mature seeds is small, approximately 1/17‒1/18 of the length of the endosperm. It shows the initiation of two cotyledons with a very short suspensor. Such a small embryo in mature seeds is also observed in P. laticuspis and P. ruscifolia (Krach 1976; Takhtajan 2000).
Seed and seed coat
When ripened, fruits become white spherical berries approximately 8.0‒8.4 mm in diameter (Fig. 4a). The endocarp is collapsed without being specialized (see Fig. 4e). All the ovules in the ovary are fertilized and develop into mature seeds (Fig. 4b). Mature seeds are exarillate, obovoid, but slightly curved, 2.6‒3.2 mm long and 1.1‒1.3 mm wide (measured from raphe to antiraphe; Fig. 4c, see also the longitudinal section of the mature seed at lower left). Each seed has a hilum at the position approximately 1/3 from the micropylar top of the seed (Fig. 4c). The surface is rugged with many irregularly shaped and sized warts (Fig. 4c).
Development of seeds, seed coats, and fruits in Phyllonoma tenuidens. a Mature fruit. b Mature seeds obtained from a. c Scanning electron micrograph of mature seed. Its longitudinal section is presented at lower left. Arrowhead indicates the position of a hilum. d Longitudinal section (LS) of young seed. e Magnified view of the seed enclosed by a rectangle in d. f LS of immature seed coat. An arrow indicates degenerating cells of testa. g LS of mature seed coat. h Transverse section (TS) of mature seed. i TS of mature seed coat. en endosperm, exts exotesta, fw fruit wall, rap raphe, ts testa. Scale bars are 6 mm in a, 2 mm in b, 500 µm in c, d, lower left in c, and h, 200 µm in e, 100 µm in f, and 50 µm in g and i
The seed coat is not multiplicative, and is approximately eight to ten cell layers thick in early stages of development (Fig. 4d, e). During seed development, cells of an outer epidermis (exotesta) enlarge, particularly to the radial direction (Fig. 4e) (terminology of the unitegmic seeds of Phyllonoma followed Schmid [1986]). In immature seeds, cells of the seed coat are mostly collapsed, except for the exotestal cells which enlarge further into irregular shaped cells (Fig. 4f). The seed coat is hard and thick at maturity, although it only consists of thick-walled exotestal cells. Many (but not all) of the exotestal cells are enlarged and 150‒200 μm high; however, the exotesta does not show a palisadal layer as Takhtajan (2000) described for seeds of P. laticuspis and P. ruscifolia. A group of approximately 10 or more exotestal cells form individual warts on the seed surface (Fig. 4c). This structure looks similar to the seed coat structures in P. laticuspis and P. ruscifolia which are illustrated by Takhtajan (2000: Figs. 4a, 5a, b, 9a‒c).
Discussion
Summary of embryological features of Phyllonoma
Prior to my analyses, information on the development of the anther, ovule, and seed of Phyllonoma was fragmentary, although a few embryological characters were described by Krach (1976), Mauritzon (1933), Takhtajan (2000), and van Tieghem (1898). My work based on P. tenuidens provides most of the missing information and updates current concepts for a few characters. The overall information on the embryological features of Phyllonoma can be summarized as follows.
Anther tetrasporangiate; anther wall four cell layers thick, its formation of the Dicotyledonous type; anther epidermis persistent, and its cells enlarged; endothecium fibrous; middle layer degenerating; and tapetum glandular and its cells binucleate. Cytokinesis in the microspore mother cell simultaneous; microspore tetrads predominantly tetrahedral; and pollen grains two-celled when shed.
Ovule borne from parietal placenta, anatropous, and tenuinucellate; archesporium hypodermal and one-celled. An archesporial cell differentiating directly into a megaspore mother cell, which undergoes meiosis resulting in a linear triad of megaspores (tetrad not formed due to the failure of the second division in the upper cell of a dyad of megaspores); chalazal megaspore functional, developing into an eight-nucleate Polygonum type female gametophyte; and antipodal cells ephemeral. Apical nucellar epidermal cells degenerating, and thus the upper half of mature female gametophyte directly bordering on the integument; hypostase not differentiating; and obturator absent.
Ovule unitegmic; integument multiplicative, thickening to more than eight cell layers thick, having no vascular bundles; micropyle formed by thick integument; and cells of inner epidermis unspecialized (not differentiating into endothelium).
Fertilization porogamous; endosperm formation of ab initio Cellular type; and mature seeds having lipids in copious endosperm. Embryogenesis uncertain and embryo in mature seed small, approximately 1/16‒1/18 in endosperm length.
Seed obovoidal, but slightly curved, exarillate, with a hilum at the position 1/3 from the top and its surface rugged with many irregularly shaped and sized warts. Seed coat not multiplicative; mature seed coat exotestal, consisting only of irregularly enlarged, thick-walled exotestal cells; and a group of approximately10 or more exotestal cells forming individual warts.
Comparisons with other Aquifoliales
Embryological features of Phyllonoma are compared with those available for the four other families of Aquifoliales (Aquifoliaceae, Cardiopteridaceae, Helwingiaceae, and Stemonuraceae) in Table S1 (supplementary Table). Except for Helwingiaceae whose embryological features have just been published by Ao and Tobe (2015), the embryology of the three other families remain poorly understood. As previously described (Ao and Tobe 2015), I obtained data from Brewbaker (1967), Copeland (1963), Corner (1976), Herr (1959, 1961), Schürhoff (1921), and van Tieghem (1898) for Aquifoliaceae; from Fagerlind (1945) and Mauritzon (1936) for Cardiopteridaceae; and from Fagerlind (1945), Mauritzon (1936), and Padmanabhan (1961) for Stemonuraceae. Some data are published for four [Cardiopteris, Citronella (=Villarestia), Gonocaryum, and Leptaulus] of the five genera constituting Cardiopteridaceae. As performed in a previous paper (Ao and Tobe 2015), I removed data on Cardiopteris reported by Kong et al. (2002) from Table S1. According to Kong et al. (2002), C. platycarya Gagnep. has orthotropous, ategmic ovules with a monosporic eight-nucleate female gametophyte, in which an egg apparatus is positioned on the chalazal side (see also Kong et al. 2014). These traits are very likely autapomorphies for the genus; therefore, they are not useful for extensive comparisons among the families.
Comparisons based on the available information showed that Phyllonoma has many embryological features in common with the other Aquifoliales. Features shared by all the five families of Aquifoliales include the following: ovule anatropous, unitegmic; arrangement of megaspores linear; female gametophyte formation conforming to Polygonum type; antipodals ephemeral; nucellar cap not formed; nucellar tissue in mature ovule lacking in the upper half of the nucellus; and chalaza not specialized. Furthermore, most features relating to the development of anthers and microspores are common to Phyllonomaceae, Helwingiaceae, and Stemonuraceae (data not available for Aquifoliaceae and Cardiopteridaceae), including the following: anther tetrasporangiate; anther wall four to five cell layers thick; endothecium fibrous; middle layers ephemeral; tapetum glandular; cytokinesis in the microspore mother cell simultaneous; and microspore tetrads predominantly tetrahedral. However, most of these common embryological features can be found in many other families of asterids. To discuss more critically how Aquifoliales are characterized by one or more embryological synapomorphies, we must know missing information on Aquifoliaceae, Cardiopteridaceae, and Stemonuraceae, which remain poorly known embryologically (see Table S1).
Despite the inadequate level of our knowledge on the embryology of Aquifoliales, Phyllonoma (Phyllonomaceae) likely conforms with Aquifoliaceae and Helwingiaceae in lacking a vascular bundle in the integument (present in Cardiopteridaceae and Stemonuraceae) and having ab initio Cellular endosperm (not Nuclear type endosperm as in Cardiopteridaceae and Stemonuraceae). It is uncertain whether either one or both of these features are apomorphic or not; however, the lack of a vascular bundle in the integument and/or the Cellular type endosperm supports either a clade of Aquifoliaceae, Helwingiaceae, and Phyllonomaceae or a clade of Cardiopteridaceae and Stemonuraceae. Within a clade of Aquifoliaceae, Helwingiaceae and Phyllonomaceae, Helwingiaceae and Phyllonomaceae likely share the tenuinucellate ovule as a synapomorphy (for discussion of this character in Aquifoliaceae see Ao and Tobe 2015, pp. 173‒174). Therefore, in agreement with evidence from molecular analyses (Soltis et al. 2011; Tank and Donoghue 2010) as well as from flower and inflorescence morphology (i.e., an inferior ovary and epiphyllous inflorescence; Tobe 2013), embryological evidence supports a sister-group relationship between Phyllonomaceae and Helwingiaceae. A few differences exist between Phyllonomaceae and Helwingiaceae. For instance, the endothelium is not differentiated in Phyllonoma but is differentiated in Helwingiaceae as in Aquifoliaceae; the testa is not multiplicative in Phyllonomaceae as in Aquifoliaceae, but is multiplicative in Helwingiaceae; and the mature seed coat is exotestal in Phyllonomaceae (as in Aquifoliaceae), but no particular cell layers develop to form a permanent mechanical structure in Helwingiaceae. Among these differences, the lack of endothelium is likely an autapomorphy of Phyllonomaceae, whereas both the non-multiplicative testa and the lack of a permanent cell layer(s) in the mature seed coat are likely autapomorphies of Helwingiaceae. However, this must be confirmed by additional studies of Aquifoliaceae, Cardiopteridaceae, and Stemonuraceae.
Thus, like molecular and floral-morphological evidence, the available embryological evidence supports the distinctness of Phyllonoma as a family and its sister-group relationship to Helwingiaceae in the Aquifoliales. Differences between Phyllonoma and Helwingiaceae are found mainly in seed coat structure. The seeds of Phyllonoma have a thick seed coat and are dispersed in berries, whereas those of Helwingiaceae have a thin membranous seed coat and are dispersed in drupes as in Aquifoliaceae (for data of seed coat in Helwingiaceae and Aquifoliaceae see Ao and Tobe [2015] and Corner [1976] ). Birds are most likely to play a role in seed dispersal in both families. In their digestive system, the seed entity must be protected by the thick seed coat in Phyllonoma and by the thick endocarp or pyrene in Helwingiaceae. Ao and Tobe (2015) showed that, in contrast to anemophily (insect-pollination) in Phyllonoma, ambophily (pollination by insect and wind) is characteristic of Helwingiaceae. A series of morphological and developmental analyses (Ao and Tobe 2015; Tobe 2013, 2014; present study) have shown the morphological diversification of flowers and seeds in Central-American Phyllonoma and East-Asian Helwingiaceae. Embryological information thus shows that in Phyllonoma, distinct character evolution has occurred in reproductive characters relating to pollination and seed dispersal.
The present study has further shown that embryological studies in the three other families Aquifoliaceae (Ilex only), Cardiopteridaceae (five genera), and Stemonuraceae (12 genera) are required for critical comparison. In particular, the unique development and structure of the female gametophyte in Cardiopteris, which was documented by Kong et al. (2002, 2014), must be confirmed. Likewise, an intensive developmental study of the female gametophyte is required in each of the four other genera (Citronella, Gonocaryum, Leptaulus, and Pseudobotrys) of Cardiopteridaceae.
References
Angiosperm Phylogeny Group (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APGIII. Bot J Linn Soc 161:105–121
Ao C, Tobe H (2015) Floral morphology and embryology of Helwingia (Helwingiaceae, Aquifoliales): systematic and evolutionary implications. J Plant Res 128:161–175
Bentham G, Hooker JD (1865) Genera Plantarum, vol 1. Reeve Co., London
Bouman F (1984) The ovule. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 123–157
Bremer B, Bremer K, Heidari N, Erixon P, Olmstead RG, Anderberg AA, Källersjö M, Barkhordarian E (2002) Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Mol Phyl Evol 24:274–301
Brewbaker JL (1967) The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in angiosperms. Am J Bot 54:1069–1083
Candolle A de (1873) Prodromus systematis naturalis regni vegetabilis. Vol. 17. Paris
Copeland HF (1963) Structural notes on hollies (Ilex aquifolium and I. cornuta, family Aquifoliaceae). Phytomorphology 13:455–464
Corner EJH (1976) The seeds of dicotyledons, vols. 1, 2. Cambridge University Press, Cambridge
Cronquist S (1968) The evolution and classification of flowering plants. Houghton Mifflin Co., Boston
Cronquist S (1981) An integrated system of classification of flowering plants. Columbia University Press, New York
Davis GL (1966) Systematic embryology of flowering plants. Wiley, New York
Engler A (1891) Saxifragaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, Teil 3 (2a). Wilhelm Engelmann, Leipzig, pp 41–93
Engler A (1930) Saxifragaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, Teil 18a. Wilhelm Engelmann, Leipzig, pp 74–226
Fagerlind F (1945) Bau des Gynöceums, der Samenanlage und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. Svensk Bot Tidskr 1945:346–364
Herr JM Jr (1959) The development of the ovule and the megagametophyte in the genus Ilex. J Elisha Mitchell Sci Soc 75:107–128
Herr JM Jr (1961) Endosperm development and associated ovule modifications in the genus Ilex. J Elisha Mitchell Sci Soc 77:26–32
Hutchinson J (1967) The genera of flowering plants, vol 2. Clarendon Press, Oxford
Hutchinson J (1973) The families of flowering plants: arranged according to a new system based on their probable phylogeny, 3rd edn. Clarendon Press, Oxford
Kårehed J (2001) Multiple origin of the tropical forest tree family Icacinaceae. Am J Bot 88:2259–2274
Kong DR, Peng H, Liang HX (2002) A new type of embryo sac in Cardiopteris and its systematic implication. Acta Bot Sinica 44:496–498
Kong DR, Schori M, Lu SG, Li L, Peng H (2014) Floral development of Cardiopteris, with emphasis on gynoecial structure and ovular morphology. J Syst Evol 52:629–642
Krach JE (1976) Samenanatomie der Rosifloren I. Die Samen der Saxifragaceae. Bot Jahrb Syst 97:1–60
Mauritzon J (1933) Studien über die Embryologie der Familien Crassulaceae und Saxifragaceae. Hakans Ohlsson, Lund
Mauritzon J (1936) Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. Svensk Bot Tidskr 30:541–550
Mori SA, Kallunki JA (1977) A revision of the genus Phyllonoma (Grossulariaceae). Brittonia 29:69–84
Olmstead RG, Kim KJ, Jansen RK, Wagstaff SJ (2000) The phylogeny of the Asteridae sensu lato based on chloroplast ndhF gene sequences. Mol Phyl Evol 16:96–112
Padmanabhan D (1961) A contribution to the embryology of Gomphandra polymorpha. Proc Natl Inst Sci India B27:389–398
Reveal JL (2012) An outline of a classification scheme for extant flowering plants. Phytoneuron 37:1–221
Rusby HH (1905) Phyllonomaceae. In: North American Flora. vol. 22, Part 2. New York Botanical Garden, p 191
Schultze-Menz GK (1964) Rosales. In: Melchior H (ed) A Engler’s Syllabus der Pflanzenfamilien. II. Gebrüder Borntraeg Erbarlin, pp 193‒242
Schürhoff PN (1921) Die Entwicklungsgeschichte von Ilex aquifolium. Ber Dtsch Bot Ges 39:377–379
Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Matvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu Y-L, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS (2011) Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot 98:704–730
Stevens PF (2001 onwards) Angiosperm phylogeny website. Version 12, July 2012. http://www.mobot.org/MOBOT/research/APweb. Accessed February 27, 2015
Takhtajan A (1969) Flowering plants. Origin and dispersal. Oliver & Boyd, Edinburgh
Takhtajan A (2000) Family Dulongiaceae. In: Anatomia seminum comparativa. Tomus 6. Dicotyledones. Rosidae II. NAUKA, Leningrad, pp 270‒274 (In Russian)
Takhtajan A (2009) Flowering plants, 2nd edn. Springer, New York
Tank DC, Donoghue MJ (2010) Phylogeny and phylogenetic nomenclature of the Campanulidae based on an expanded sample of genes and taxa. Sys Bot 35:425–441
Tobe H (1989) The embryology of angiosperms: its broad application to the systematic and evolutionary study. Bot Mag (Tokyo) 102:351–367
Tobe H (2013) Floral morphology and structure o Phyllonoma (Phyllonomaceae): systematic and evolutionary implications. J Plant Res 126:709–718
Tobe H (2014) Inflorescences of Phyllonoma (Phyllonomaceae, Aquifoliales): anatomical observations. Acta Phytotax Geobot 65:117–126
Tobe H, Wagner WL, Chin H-C (1987) A systematic and evolutionary study of Oenothera: seed coat anatomy. Bot Gaz 148:235–257
van Tieghem P (1898) Structure de quelques ovules et patti qu’on en pent tirer am liorer la classification. J Bot (Morot) 12:197–220
Yamamoto T, Vassiliades DD, Tobe H (2014) Embryology of Biebersteinia (Biebersteiniaceae, Sapindales): characteristics and comparisons with related families. J Plant Res 127:599–615
Acknowledgments
The study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 25440208). I am grateful to Peter H. Raven and William A. Haber for their assistance in obtaining the materials used in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tobe, H. Embryology of Phyllonoma (Phyllonomaceae, Aquifoliales): characteristics and character evolution. J Plant Res 128, 633–642 (2015). https://doi.org/10.1007/s10265-015-0730-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-015-0730-y