Abstract
Interactions among the functionally specialized organs of higher plants ensure that the plant body develops and functions properly in response to changing environmental conditions. When an incision or grafting procedure interrupts the original organ or tissue connection, cell division is induced and tissue reunion occurs to restore physiological connections. Such activities have long been observed in grafting techniques, which are advantageous not only for agriculture and horticulture but also for basic research. To understand how this healing process is controlled and how this process is initiated and regulated at the molecular level, physiological and molecular analyses of tissue reunion have been performed using incised hypocotyls of cucumber (Cucumis sativus) and tomato (Solanum lycopersicum) and incised flowering stems of Arabidopsis thaliana. Our results suggest that leaf gibberellin and microelements from the roots are required for tissue reunion in the cortex of the cucumber and tomato incised hypocotyls. In addition, the wound-inducible hormones ethylene and jasmonic acid contribute to the regulation of the tissue reunion process in the upper and lower parts, respectively, of incised Arabidopsis stems. Ethylene and jasmonic acid modulate the expression of ANAC071 and RAP2.6L, respectively, and auxin signaling via ARF6/8 is essential for the expression of these transcription factors. In this report, we discuss recent findings regarding molecular and physiological mechanisms of the graft union and the tissue reunion process in wounded tissues of plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatially and temporally controlled intercellular attachment is essential for the organization of multicellular organisms. In animal cell division, the cells divide by constriction, and just after cell division the daughter cells separate, although the cells may remain adhered depending on their characteristics (Barr and Gruneberg 2007). In plants, however, each cell is surrounded by a cell wall that defines its shape and fixes its position within tissues (Smith 2001). In most plants, the cells divide via formation of the cell plate, and daughter cells remain attached just after cytokinesis. In higher plants, however, the cell plate is formed during the last step of cell division to produce two daughter cells that remain attached to each other. Epigenetic re-adhesion of separated cells usually occurs in animal systems, but it rarely occurs in higher plants, such as in carpel fusion during gynoecium development (van der Schoot et al. 1995; Siegel and Verbeke 1989; Walker 1975), tissue union during grafting (Flaishman et al. 2008; Kollmann and Glockmann 1985; Richardson et al. 1996; Wang and Kollmann 1996), and cell repair in incised tissues. In Arabidopsis thaliana mutants, the genes encoding fiddlehead (Lolle et al. 1992, 1997; Lolle and Cheung 1993; Lolle and Pruitt 1999) and wax-1 (Jenks et al. 1996) induce the ectopic fusion or adhesion of aerial tissues.
Plants have the ability to undertake defense responses against environmental stresses and biotic attacks as well as by wounding. Wounding threatens plants survival, especially if the injury occurs to the stem, because it alone connects each shoot to the plant (Satoh 2006). Stobbe et al. (2002) reported that the wound-induced calli of lime trees (Tilia sp.) showed subsequent development of a wound tissue comprised of xylem, cambium, and phloem in particular cases. At the cellular level, plants generally respond to wounding by activating defense systems required for healing wounded tissues and protecting them from subsequent pathogen invasion. The physiological responses include the repair and reinforcement of the cell wall and the activation of wound-signaling pathways that induce the production of both local and systemic defense-related proteins, including basic-type pathogenesis-related (PR) proteins and wound-related hormones such as ethylene and jasmonic acid (JA) (Bostock and Stermer 1989; Leon et al. 2001; Sasaki et al. 2002). When wounding or grafting interrupts the original vascular connection, the formation of new vascular tissues can be induced. Although the molecular mechanisms controlling vascular differentiation are not fully understood, the involvement of phytohormones, such as auxin and cytokinin, in xylem and phloem differentiation has been suggested (Mattsson et al. 1999; Sachs 2000; Roberts 1988; Ye 2002).
In contrast to vascular regeneration and defense responses, the molecular basis of tissue repair, including tissue reunion in the ground tissues such as the cortex and pith, remains largely unknown (Reid and Ross 2011). The reunion process in those tissues also includes cell division and cell differentiation to recover the lost tissue (Asahina et al. 2002, 2006, 2011). Although plant cell is known to exert totipotency upon stresses and has been much studied in tissue culture, the underlying molecular mechanisms are beginning to be understood. Several studies suggest that certain transcription factors (TFs) are involved in regulating the expression of many genes that govern the regeneration process (Ikeuchi et al. 2013; Sena et al. 2009; Zimmerman 1993); it is unclear, though, how this healing process is initiated and regulated, including the underlying molecular mechanisms.
In this review, we describe recent findings regarding the molecular and physiological studies of the graft union, and the control mechanisms of tissue reunion in incised tissues, with a specific focus on the involvement of phytohormones.
Molecular and physiological studies of the graft union
The root and aboveground organs of higher plants are connected by the xylem and phloem of the vascular bundles, and vascular tissues provide the long-distance transport system for water, nutrients and various organic materials within the plant (Jesko 1989; Sakuta and Satoh 2000, Satoh et al. 1998; Satoh 2006). When wounding or grafting interrupts the original vascular connection, the formation of new vascular tissues should be induced. Such activities have long been observed in grafting techniques, which are advantageous for agriculture and horticulture (Davisa et al. 2008). Tsukaya et al. (1993) developed the grafting technique used on Arabidopsis flowering stems, and grafting experiments using Arabidopsis have been performed to study different aspects of plant biology. Flaishman et al. (2008) used homografts and heterografts of Arabidopsis inflorescence stems to study the compatibility and ontogeny of graft union formation. Micrografting, a seedling grafting technique, was first reported in Arabidopsis by Turnbull et al. (2002) and has been applied to basic research on systemic physiological events, such as flower induction (Corbesier et al. 2007; Notaguchi et al. 2008, 2009) and hormone actions (Matsumoto-Kitano et al. 2008). Yin et al. (2012) developed a simple and effective micrografting method that improved on the previous protocols (Bainbridge et al. 2006; Turnbull et al. 2002).
Previous studies on grafting and the repairing of wounded tissues have demonstrated the involvement of phytohormones, such as auxin and cytokinin in the repairing process of vascular tissues (Sachs 2000). As related findings, several groups have reported the role of several NAC (NAM, ATAF1, 2 and CUC2) TFs when the vascular elements are regenerated (Kubo et al. 2005; Mitsuda et al. 2005; Yamaguchi et al. 2008). Recently, Yin et al. (2012) performed histochemical and microarray analyses to show gene expression profiles during the grafting of Arabidopsis seedlings. Their work addressed the molecular events underlying graft union development and provided evidence that auxin and wound-inducible hormones, such as ethylene and JA, are involved in the development of the graft union in Arabidopsis seedlings, although the gene expression patterns might be different from those of the partially incised stems which will be mentioned in the later sections, depending on the continuity of polarity and auxin polar transport through the non-incised part of the stem.
Differences of tissue participation for the reunion in different plant species
Tissue reunion of cucumber and tomato hypocotyls
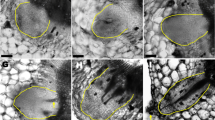
To investigate the tissue reunion process in the cortex of cut tissues, cucumber and tomato hypocotyls have been transversely cut to half their diameters, and morphological and histochemical analyses were then performed (Asahina et al. 2002). Cell division in the cortex commences 3 days after cutting, and the cortex is nearly fully reunited within 7 days (Fig. 1a). Cell division during tissue reunion is strongly inhibited when the cotyledons were removed from the cucumber seedlings. In Japan, the grafting of cucumber hypocotyls onto squash rootstocks is often performed to prevent damage from soil borne diseases during cultivation (Satoh 1996). Although the exact role of the cotyledons in the formation of the graft union is not understood, cotyledons of the scion and rootstock are preferentially left on the hypocotyl to improve the grafting efficiency. These facts are consistent with our result that tissue reunion in incised cucumber hypocotyls does not occur after the removal of the cotyledons.
The tissue reunion process in the incised cucumber hypocotyl and Arabidopsis flowering stem. a Incised hypocotyl of cucumber. After 7 days of germination, the hypocotyls were incised through half of their diameter transversely 3 cm from the base using a razor blade and the plant was then grown for an additional 7 days. b Incised flowering stem of Arabidopsis. After 7–10 days of bolting, flowering stem between 1st or 2nd cauline leaf and rosette leaf was incised through half of their diameter with a micro-surgical knife and the plant was then grown for an additional 7 days. Photographs of section were taken from 1 to 7 days after incision. Arrowheads indicate the incision site. pi, pith; co, cortex; vb, vascular bundle. Scale bars 100 µm. Microscopy images in (a) are reprinted and modified from Asahina et al. (2002) and in (b) are from Asahina et al. (2011). Detailed experimental procedures are described in previous studies (Asahina et al. 2002, 2011)
This inhibition is reversed by applying gibberellin (GA) to the apical tip of the plants without cotyledons, but not restored by indole-3-acetic acid (Asahina et al. 2002). Supporting this observation, cell division in the cortex is inhibited by the treatment of the cotyledons with uniconazole-P, a GA biosynthesis inhibitor, and this inhibition is also reversed by the simultaneous application of GA. The requirement of GA for tissue reunion in cut hypocotyls is also evident in gib-1, the GA-deficient mutant of tomato (Asahina et al. 2002). In contrast to the essential role of the cotyledons, normal tissue reunion in cut hypocotyls is still observed when shoot apexes are removed (Asahina et al. 2002). Moreover, normal tissue-reunion occurred in the cortex of the hypocotyl when 10−4 M 2,3,5-triiodobenzoic acid (TIBA), an inhibitor of polar auxin transport, was applied to the surface of the shoot above the cut on the hypocotyl. These results indicate that shoot apex-producing auxin is less important in tissue reunion process of hypocotyls in cucumber and tomato seedlings.
To study the effects of cotyledons on the GA content in hypocotyls directly, cotyledons have been removed from seedlings, and endogenous GA levels in the hypocotyls have then been determined (Asahina et al. 2007). Compared with intact seedlings, those without cotyledons contain reduced levels of bioactive GA4 (Gibberellin A4) and its precursors in the cucumber and tomato hypocotyls. These results suggest that GA is required for cell division during tissue reunion in the cortex of wounded hypocotyls from cucumber and tomato seedlings and that cotyledons are necessary for maintaining normal GA levels in hypocotyls (Fig. 2).
Schematic model for tissue reunion in cucumber and tomato incised hypocotyl. Gibberellin (GA) is required for cell division during tissue reunion in the cortex of incised hypocotyls of cucumber and tomato seedlings, and cotyledons are necessary for maintaining normal GA levels in hypocotyl. The microelements boron (B), zinc (Zn) and manganese (Mn) ions from the roots are required for intrusive cell elongation during tissue reunion in the cortex of cucumber hypocotyls
In the hypocotyls of root-eliminated cucumber seedlings, cell division occurs in the cortex during the tissue reunion process, but intrusive cell elongation and the interdigitation of cortex cells at the cut surface to form a tight tissue connection does not occur, even after 7 days. Experimental results, including the application of particular compounds contained in xylem sap (Satoh 2006), suggest that boron, zinc and manganese ions, as well as their uptake from soil to xylem sap, are required for the intrusive cell elongation that occurs during tissue reunion in the cortex of cucumber hypocotyls (Fig. 2; Asahina et al. 2006).
Tissue reunion of Arabidopsis incised stem
The technique of grafting has been widely used in agriculture, horticulture and the basic studies of plant biology. Apart from the grafting of hypocotyls in Cucurbita plants, grafting is usually performed on the stems of fruit trees and Solanaceae plants. In Arabidopsis, both flowering stem grafting and hypocotyl grafting have been described (Tsukaya et al. 1993; Turnbull et al. 2002). Because Arabidopsis seedlings are so small that manipulating their hypocotyls is difficult, we have examined the tissue reunion process in incised flowering stems. When the flowering stem of Arabidopsis is incised through half of its diameter, cell division occurs in the pith 3 days after incision (Fig. 1b). However, the process is strongly inhibited by the decapitation of the inflorescence and when the polar transport of auxin is inhibited, either by an inhibitor or in the pin1 mutant, which affects PIN-FORMED 1, a transmembrane protein involved in auxin transport (Asahina et al. 2011). The incised stems of the GA-deficient Arabidopsis mutant ga3ox1/ga3ox2 recover with no difficulty, indicating that GA is not involved in wound healing of stems in Arabidopsis (Asahina et al. 2011).
Different hormone requirements for tissue reunion in wounded hypocotyls and stems
Differences in the hormone requirements for the tissue reunion process between Arabidopsis incised flowering stems and cucumber and tomato incised hypocotyls may be due to differences in the tissues and the developmental stages (Table 1). After the incision of cucumber and tomato hypocotyls, cell division and cell elongation occur mainly in the cortex to restore the stem connection, which requires GA for cortex cell division during tissue reunion. The hypocotyl, an embryonic organ, consists of epidermal, cortical, endodermal and vascular tissues, but this is not true of the pith tissue. Its growth is thought to depend on the cotyledons, which supply nutrients and hormones at the early post-germination stage (Reid and Ross 2011). In contrast, the Arabidopsis flowering stem is a reproductive organ that develops after the juvenile stage and contains epidermal, cortical, endodermal and vascular tissues, as well as the pith, which occupies most of the central part of the stem.
Genetic networks in the tissue reunion process
A microarray analysis of Arabidopsis incised stems revealed differential gene expression levels between cut and uncut stems with intact or decapitated inflorescences and during the tissue reunion process; these findings are summarized in Fig. 3. Up-regulated genes included those that are related to cell division and phytohormones, as well as genes that encode TFs, which were predominantly expressed 1–3 days after cutting. The expression levels of INDOLE-3-ACETIC ACID INDUCIBLE 5 (IAA5), which encodes an Aux/IAA auxin response protein, 1-AMINO-CYCLOPROPANE-1-CARBOXYLATE SYNTHASE 2 (ACS2) and LIPOXYGENASE 2 (LOX2), which are involved in ethylene and JA production, respectively, peak at 1 day post-incision.
Schematic model of the phytohormone regulation of ANAC071 and RAP2.6L expression during tissue reunion in Arabidopsis incised stem (modified from Asahina et al. 2011). The tissue reunion process is regulated differently in the upper and lower parts of the incision. PIN1 protein is involved in polar transport of auxin in flowering stem. In the upper part, ANAC071 is up-regulated by IAA (a) and ethylene (b), a wound-inducible hormone. In the lower part, RAP2.6L, which was down-regulated by IAA, is activated because of auxin depletion due to blocked polar auxin transport. JA, a key regulator of plant responses to environmental stresses and biotic challenges, also induces RAP2.6L expression (c). Auxin signaling via ARF6/8 is essential for the expression of ANAC071 and RAP2.6L in the upper and lower parts of incised stems, respectively, and the production of JA, via induction of DAD1 (d) induces RAP2.6L expression in the tissue reunion process. The gene expression of ACS2 and LOX2/3, which are involved in ethylene and JA production, respectively, peak at 1 day post-incision
NAC DOMAIN CONTAINING PROTEIN 71 (ANAC071), which encodes a TF, is predominantly expressed at 1–3 days post-incision, and its expression is promoted by auxin. Auxin-responsive genes contain auxin response elements (AuxREs) in their promoter regions that specifically bind ARFs (Hagen and Guilfoyle 2002; Liscum and Reed 2002). In the promoter of ANAC071, AuxREs are present at −2654 bp from translational start site. In addition, in ANAC071-promoter::GUS transgenic plants, GUS activity was high in the distal region of the incision as well as after indole-3-acetic acid (IAA) application in decapitated plants (Pitaksaringkarn et al. 2014b), suggesting that the auxin may control the expression of ANAC071 in the tissue reunion process. The arf6arf8 mutant shows an inhibition of cell division in the pith tissue a week after incision, whereas no inhibition is observed in each single mutant and in the mutants for the other ARFs (Pitaksaringkarn et al. 2014b). Our qPCR analysis indicated that the expression of ANAC071 decreases in both incised and non-incised stems of the arf6arf8 mutant (Pitaksaringkarn et al. 2014b). Thus increased auxin levels in the upper part of the incision might induce expression of ANAC071 with mediation by ARF6 and ARF8.
The expression of RELATED TO AP2 6L (RAP2.6L), a member of the ethylene response factor (ERF) subfamily B-4 of the ERF/AP2 TF family, also peaks at 1 day post-incision and is up-regulated by JA but is suppressed by auxin. The expression level of RAP2.6L, another early induced gene by incision, is higher in non-incised stems but is lower in incised stems of the arf6arf8 double mutants than in those of wild-type plants (Pitaksaringkarn et al. 2014b).
Tabata et al. (2010) reported that, in the AUXIN RESPONSE FACTOR (ARF) double mutant arf6arf8, the amount of JA is reduced in flower buds, and, of several JA biosynthetic genes studied, only the expression of DEFECTIVE ANTHER DEHISCENCE 1 (DAD1) is significantly decreased compared with wild type. Our qPCR analyses showed that some of the JA biosynthesis genes are up-regulated and that JA levels are increased during the tissue reunion process (unpublished data). In addition, DAD1 expression levels were dramatically decreased in the arf6 arf8 mutant as compared with the wild-type (Pitaksaringkarn et al. 2014a, b). From these results, we hypothesized that auxin signaling via ARF6/8 is essential for the expression of ANAC071 in the upper parts of incised stems (Fig. 3a) and that the production of JA, via the induction of DAD1 (Fig. 3d), induces RAP2.6L expression in the lower parts of the incised stems during the tissue reunion process (Pitaksaringkarn et al. 2014a, b).
Ethylene also promotes ANAC071 expression but suppresses RAP2.6L expression. Plants expressing dominant negative form of RAP2.6L (RAP2.6L-SRDX) or ANAC071 (ANAC071-SRDX) have defects in tissue reunion, and the tissue reunion site in the integral membrane protein ETHYLENE INSENSITIVE 2 (EIN2) mutant, ein2, has an abnormal morphology. Five days after cutting the ein2 stem, cell division was observed only in the cortex neighboring the incision and not in the pith, unlike in the wild-type stem. Complete reunion was not observed up to 7 days after cutting the ein2 stem (Asahina et al. 2011). Although the control mechanisms for the downstream targets of the ANAC071 and RAP2.6L remain unknown, these results indicate that ANAC071 and RAP2.6L are essential for tissue reunion in Arabidopsis incised flowering stems but are conversely affected by polar-transported auxin, with fine-tuning by the wound-inducible hormones JA and ethylene (Fig. 3; Asahina et al. 2011).
XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE 20 (XTH20) is a selected gene from a microarray analysis that is induced by auxin and is highly expressed in the upper part of cut inflorescence stems, in a manner similar to ANAC071 (Asahina et al. 2011; Pitaksaringkarn et al. 2014a). Our current analysis indicated that ANAC071 binds directly to the promoters of XTH20 and XTH19 (which is phylogenetically closest to XTH20) to induce their expression, and the xth19xth20 double mutant shows inhibition of pith cell proliferation (Pitaksaringkarn et al. 2014a). Although further research is needed to elucidate how XTHs control cell proliferation and/or cytokinesis during tissue reunion, this work revealed that XTH19 and XTH20 are directly controlled by ANAC071, which regulates the tissue reunion process in Arabidopsis incised inflorescence stems.
Wounding promotes callus formation in various parts of Arabidopsis seedlings in tissue culture (Iwase et al. 2011; Ikeuchi et al. 2013). Iwase et al. (2011) reported that WOUND INDUCED DEDIFFERENTIATION 1 (WIND1), another AP2/ERF TF gene, is up-regulated in wounded Arabidopsis hypocotyls and that WIND1 promotes cell dedifferentiation, suggesting that WIND1 proteins, along with other functionally redundant factors, regulates callus formation upon wounding. Iwase et al. (2013) also reported that the WIND1-mediated signaling cascade for cell dedifferentiation during callus formation is conserved in several species of Brassicaceae and Solanaceae and may be conserved more widely. We found that WIND1 is not up-regulated during tissue reunion in Arabidopsis incised stems at 1, 3, and 5 days after incision (Asahina et al. 2011) and this gene is not responsive to auxin (Iwase et al. 2011). We also found that WIND1 was not significantly up-regulated at 1–24 h after incision and the incised stems of WIND1-SRDX showed normal reunion (unpublished results). This suggests that the different responses or signaling cascades found between the tissue reunion of partially incised inflorescence stems and the wound-induced callus formation of explants may be due to the presence or absence of auxin polar transport along the vertical axis.
Conclusion
The stem is an essential organ that supports plant body structure and subsequent organ development and delivers water, nutrients and chemical information to the roots and shoots of plants (Kehr and Buhtz 2008; Satoh 2006). Therefore, as soon as injuries occur to the stem, defense responses and repair processes are activated to protect the plant from further damage and to recover normal physiology (Reid and Ross 2011). Because auxin is produced in the apical meristem and is transported in stems with polarity, its distribution triggers corresponding developmental responses (Friml 2003; Robert and Friml 2009; Sachs 1991) and several physiological responses, including formation of adventitious roots and the breaking of apical dominancy, which are associated with the type of wound and its depth. The wound depth may not disturb a functional route of auxin transportation or may lead to complete interruption of its transportation.
Various compounds and physical factors are involved in the wound signal transduction pathway, and cross-talk between some signaling pathways results in a different pattern of responses to mechanical damage (Che et al. 2006). A detailed analysis of temporal and spatial gene expression levels in cells undergoing tissue reunion will be required for a full understanding of the molecular events during tissue reunion (Fig. 4). How wound-signal transduction is involved in the tissue reunion process, especially in the gene regulatory network, and whether tissue reunion is controlled by similar TFs and plant hormones in different plant species remain to be examined in the future.
Abbreviations
- GA:
-
Gibberellin A
- JA:
-
Jasmonic acid
- qPCR:
-
Quantitative reverse transcription PCR
References
Asahina M, Iwai H, Kikuchi A, Yamaguchi S, Kamiya Y, Kamada H, Satoh S (2002) Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiol 129:201–210
Asahina M, Gocho Y, Kamada H, Satoh S (2006) Involvement of inorganic elements in tissue reunion in the hypocotyl cortex of Cucumis sativus. J Plant Res 119:337–342
Asahina M, Yamauchi Y, Hanada A, Kamiya Y, Kamada H, Satoh S, Yamaguchi S (2007) Effects of the removal of cotyledons on endogenous gibberellin levels in hypocotyls of young cucumber and tomato seedlings. Plant Biotechnol 24:99–106
Asahina M, Azuma K, Pitaksaringkarn W, Yamazaki T, Mitsuda N, Ohme-Takagi M, Yamaguchi S, Kamiya Y, Okada K, Nishimura T, Koshiba T, Yokota T, Kamada H, Satoh S (2011) Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc Natl Acad Sci USA 108:16128–16132
Bainbridge K, Bennett T, Turnbull C, Leyser O (2006) Grafting. Methods Mol Biol 323:39–44
Barr FA, Gruneberg U (2007) Cytokinesis: placing and making the final cut. Cell 131:847–860
Bostock RM, Stermer BA (1989) Perspectives on wound healing in resistance to pathogens. Annu Rev Phytopathol 27:343–371
Che P, Lall S, Nettleton D, Howell SH (2006) Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol 141:620–637
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Davisa AR, Perkins-Veazie P, Sakata Y, López-Galarza S, Maroto JV, Lee SG, Huh YC, Sun ZY, Miguel A, King SR, Cohen R, Lee JM (2008) Cucurbit grafting. Crit Rev Plant Sci 27:50–74
Flaishman M, Loginovsky K, Golobowich S, Lev-Yadun S (2008) Arabidopsis thaliana as a model system for graft union development in homografts and heterografts. J Plant Growth Regul 27:231–239
Friml J (2003) Auxin transport—shaping the plant. Curr Opin Plant Biol 6:7–12
Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49:373–385
Ikeuchi M, Sugimoto K, Iwase A (2013) Plant Callus: mechanisms of Induction and Repression. Plant Cell 2:3159–3173
Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, Ohme-Takagi M (2011) The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol 21:508–514
Iwase A, Mitsuda N, Ikeuchi M, Ohnuma M, Koizuka C, Kawamoto K, Imamura J, Ezura H, Sugimoto K (2013) Arabidopsis WIND1 induces callus formation in rapeseed, tomato, and tobacco. Plant Signal Behav 8(12):e27432
Jenks MA, Rashotte AM, Tuttle HA, Feldmann KA (1996) Mutants in Arabidopsis thaliana altered in epicuticular wax and leaf morphology. Plant Physiol 110:377–385
Jesko J (1989) Physiology of the plant root system. In: Kolek J, Kozinka V (eds) Developments in plant and soil sciences, vol 46. Kluwer academic publishers, London, pp 1–30
Kehr J, Buhtz A (2008) Long distance transport and movement of RNA through the phloem. J Exp Bot 59:85–92
Kollmann R, Glockmann C (1985) Studies on graft unions. I. Plasmodesmata between cells of plants belonging to different unrelated taxa. Protoplasma 124:224–235
Kubo M, Udagawa M, Nishikubo N, Horiguch G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19:1855–1860
Leon J, Rojo E, Sanchez-Serrano JJ (2001) Wound signaling in plants. J Exp Bot 52:1–9
Liscum E, Reed JW (2002) Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol 49:387–400
Lolle SJ, Cheung AY (1993) Promiscuous germination and growth of wild-type pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant, fiddlehead. Dev Biol 155:250–258
Lolle SJ, Pruitt RE (1999) Epidermal cell interactions: a case for local talk. Trends Plant Sci 4:14–20
Lolle SJ, Cheung AY, Sussex IM (1992) Fiddlehead: an Arabidopsis mutant constitutively expressing an organ fusion program that involves interactions between epidermal cells. Dev Biol 152:383–392
Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter WD, Pruitt RE (1997) Developmental regulation of cell interactions in the Arabidopsis fiddlehead 1 mutant: a role for the epidermal cell wall and cuticle. Dev Biol 189:311–321
Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105:20027–20031
Mattsson J, Sung ZR, Berleth T (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126:2979–2991
Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17:2993–3006
Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T (2008) Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol 49:1645–1658
Notaguchi M, Daimon Y, Abe M, Araki T (2009) Adaptation of a seedling micro-grafting technique to the study of long-distance signaling in flowering of Arabidopsis thaliana. J Plant Res 122:201–214
Pitaksaringkarn W, Matsuoka K, Asahina M, Miura K, Sage-Ono K, Ono M, Yokoyama R, Nishitani K, Ishii T, Iwai H, Satoh S (2014a) XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J 80:604–614
Pitaksaringkarn W, Ishiguro S, Asahina M, Satoh S (2014b) ARF6 and ARF8 contribute to tissue reunion in incised Arabidopsis inflorescence stems. Plant Biotechnol 31:49–53
Reid JB, Ross JJ (2011) Regulation of tissue repair in plants. Proc Natl Acad Sci USA 108:17241–17242
Richardson FVM, Saoir SMA, Harvey BMR (1996) A study of the graft union in in vitro micrografted apple explants. Plant Growth Regul 20:17–23
Robert HS, Friml J (2009) Auxin and other signals on the move in plants. Nat Chem Biol 5:325–332
Roberts LW (1988) Hormonal aspects of vascular differentiation. In: Roberts LW, Gahan PB, Aloni R (eds) Vascular differentiation and plant growth regulators. Springer, Berlin, pp 22–37
Sachs T (1991) Cell polarity and tissue patterning in plants. Development 113:83–93
Sachs T (2000) Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41:649–656
Sakuta C, Satoh S (2000) Vascular tissue-specific gene expression of xylem sap glycine-rich proteins in root and their localization in the walls of metaxylem vessels in cucumber. Plant Cell Physiol 41:627–638
Sasaki K, Hiraga S, Ito H, Seo S, Matsui H, Ohashi Y (2002) A wound-inducible Tobacco peroxidase gene expresses preferentially in the vascular system. Plant Cell Physiol 43:108–117
Satoh S (1996) Inhibition of flowering of cucumber grafted on rooted squash stock. Physiol Plant 97:440–444
Satoh S (2006) Organic substances in xylem sap delivered to above-ground organs by the roots. J Plant Res 119:179–187
Satoh S, Kuroha T, Wakahoi T, Inouye Y (1998) Inhibition of the formation of adventitious roots on cucumber hypocotyls by the fractions and methoxybenzylglutamine from xylem sap of squash root. J Plant Res 111:541–546
Sena G, Wang X, Liu HY, Hofhuis Birnbaum KD (2009) Organ regeneration does not require a functional stem cell niche in plants. Nature 45:1150–1153
Siegel BA, Verbeke JA (1989) Diffusible factors essential for epidermal cell redifferentiation in Catharanthus roseus. Science 244:580–582
Smith LG (2001) Plant cell division: building wall in the right places. Nat Rev Mol Cell Biol 2:33–39
Stobbe H, Schmitt U, Eckstein D, Dujesiefken D (2002) Developmental stages and fine structure of surface callus formed after debarking of living lime trees (Tilia sp.). Ann Bot (Lond) 89:773–782
Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, Ueno Y, Yamamoto KT, Machida Y, Nakamura K, Ishiguro S (2010) Arabidopsis auxin response factor 6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol 51:164–175
Tsukaya N, Naito S, Rédei G, Komeda Y (1993) A new class of mutations in Arabidopsis thaliana, acaulis1, affecting the development of both inflorescences and leaves. Development 118:751–764
Turnbull CGN, Booker JP, Leyser HMO (2002) Micrografting techniques for testing long-distance signaling in Arabidopsis. Plant J 32:255–262
van der Schoot C, Dietrich MA, Storms M, Verbeke JA, Lucas WJ (1995) Establishment of a cell-to-cell communication pathway between separate carpels during gynoecium development. Planta 195:450–455
Walker DB (1975) Postgenital carpel fusion in Catharanthus roseus (Apocynaceae). I. Light and scanning electron microscopic study of gynoecial ontogeny. Am J Bot 62:457–467
Wang Y, Kollmann R (1996) Vascular differentiation in the graft union of in-vitro grafts with different compatibility. Structural and functional aspects. J Plant Physiol 147:521–533
Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55:652–664
Ye ZH (2002) Vascular tissue differentiation and pattern formation in plants. Annu Rev Plant Biol 53:183–202
Yin H, Yan B, Sun J, Jia P, Zhang Z, Yan X, Chai J, Ren Z, Zheng G, Liu H (2012) Graft-union development: a delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J Exp Bot 63:4219–4232
Zimmerman JL (1993) Somatic Embryogenesis: a Model for Early Development in Higher Plants. Plant Cell 5:1411–1423
Acknowledgments
We thank Prof. H. Yamane, Prof. T. Yokota and Dr. K. Matsuoka (Teikyo University) for valuable suggestions and technical assistance. This work was supported in part by JSPS-KAKENHI (Grant-in-Aid for Young Scientists B; 26840098 to M.A. and a Grant-in-Aid for Scientific Research on Innovative Areas; 24114006 to S.S.), the MEXT-supported Program for the Strategic Research Foundation at Private Universities (S1311014 to M.A.) and Special grant for promoting education and research at Faculty of Science and Engineering of Teikyo University (to M.A.).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Asahina, M., Satoh, S. Molecular and physiological mechanisms regulating tissue reunion in incised plant tissues. J Plant Res 128, 381–388 (2015). https://doi.org/10.1007/s10265-015-0705-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-015-0705-z