Abstract
Key message
Jasmonic acid and RAP2.6L are induced upon wounding but are not involved in cell proliferation during healing in Arabidopsis hypocotyls.
Abstract
Plants produce jasmonic acid in response to wounding, but its role in healing, if any, has not been determined. Previously, the jasmonic acid–induced transcription factor, RAP2.6L, related to APETALA 2.6-like, was identified as a spatially expressed factor involved in tissue reunion in partially incised flowering stems of Arabidopsis. In the present study, we investigated the function of JA and RAP2.6L on wound healing using an Arabidopsis hypocotyl-grafting system, in which separated tissues are reattached by vascular tissue cell proliferation. The jasmonic acid–responsive genes AOS and JAZ10 were transiently expressed immediately after grafting. We confirmed that the endogenous content of jasmonic acid-Ile, which is the bioactive form of jasmonic acid, increased in hypocotyls 1 h after grafting. Morphological analysis of the grafted tissue revealed that vascular tissue cell proliferation occurred in a similar manner in wild-type Arabidopsis, the jasmonic acid–deficient mutant aos, the jasmonic acid–insensitive mutant coi1, and in Arabidopsis that had been exogenously treated with jasmonic acid. RAP2.6L expression was also induced during graft healing. Because RAP2.6L expression occurred during graft healing in aos and coi1, its expression must be regulated via a jasmonic acid–independent pathway. The rap2.6L mutant and dominant repressor transformants for RAP2.6L showed normal cell proliferation during graft healing. Taken together, our results suggest that JA and RAP2.6L, induced by grafting, are not necessary for cell proliferation process in healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have the ability to heal after being mechanically damaged. Their injured tissues are repaired by a stepwise process of cellular dedifferentiation, proliferation, and re-differentiation. Specifically, when functioning properly, cell proliferation contributes to the mechanical strength of the healed wound, and these cells are the source of newly differentiated cells. Certain phytohormones seem to be involved in the regulation of cell proliferation during tissue healing. Gibberellin was found to be needed to induce cell proliferation in incised tomato and cucumber hypocotyls (Asahina et al. 2002), and auxin appears to control cell proliferation in incised Arabidopsis stems (Asahina et al. 2011). These two phytohormones act at the wound site from long distances as they are produced distally in apical buds or leaves. Conversely, the phytohormone jasmonic acid (JA), which is involved in wound signaling and various biotic and abiotic stress signaling pathways, is produced at the wound site. JA induces expression of numerous genes after mechanical wounding (Creelman et al. 1992; Tian et al. 2003; Glauser et al. 2008; Chung et al. 2008). JA induce the defense compounds to increase the resistance of plants to feeding by insects and act to suppress growth for saving an energy cost for defense response (Koo 2017). Wound healing is also important for the survival and reproduction of plants. Thus, the responses of plants to wounding include tissue healing, immunity to insect feeding, and suppression of growth. The involvement of JA during wound healing has not been fully elucidated because characterizing and quantifying wound healing in plants is difficult. We previously established a method for the morphological analysis of wound repair after grafting in the hypocotyl of Arabidopsis and found that a successful graft was formed by cell proliferation of vascular tissue induced by auxin (Matsuoka et al. 2016).
The biosynthetic pathway of JA-Ile, which is the active form of JA, involves the following enzymatically controlled steps (Schaller and Stintzi 2009; Wasternack and Hause 2013). Fatty acids are first converted sequentially into 12-oxo-phytodienoic acid (OPDA) in the chloroplast by the lipases lipoxygenase, allene oxide synthase (AOS), and allene oxide cyclase. Then, JA is synthesized from OPDA by various enzymes, including OPDA reductase 3. Finally, jasmonate resistant 1 catalyzes the formation of JA-Ile, which is a ligand of the coronatine insensitive1 (COI1) receptor (Yan et al. 2009). Expression of these JA-biosynthesis enzymes is rapidly induced upon wounding and by JA as feedback (Bell and Mullet 1993; Bate et al. 1998; Stenzel et al. 2003; Suza and Staswick 2008). Because wounding and an increase in JA also rapidly induce the JA transcriptional repressor, jasmonate ZIM-domain (JAZ) (Chung et al. 2008), JA is thought to be produced transiently and rapidly after wounding owing to both positive and negative feedback loops.
The expression of the genes encoding RAP2.6 and RAP2.6L, which are transcription activators containing similar AP2 DNA-binding domains, are induced by JA (Wang et al. 2008; Krishnaswamy et al. 2011). RAP2.6L has been characterized as a spatially expressed transcription factor involved in healing of incised stem tissues in Arabidopsis in conjunction with the transcription factor ANAC071 (Asahina et al. 2011). RAP2.6L was found to be expressed in the lower regions of injured stems after depletion of indole acetic acid (IAA). Exogenously applied JA also induced RAP2.6L expression. In plants, callus cells are considered to be undifferentiated and are often observed at the wound site. Shoot regeneration occurs in calli at wound sites under ex vitro conditions in potatoes and tomatoes (Lauer 1963; Johkan et al. 2008). When RAP2.6L was nearly absent, regeneration of Arabidopsis shoots was prevented even though the culture medium contained auxin and cytokinin (Che et al. 2006), which suggests that RAP2.6L is involved in cellular reprogramming and wound healing.
For the study reported herein, we demonstrated that JA biosynthesis and expression of RAP2.6L were induced during grafting in Arabidopsis hypocotyls. However, JA and RAP2.6L activities were not required for hypocotyl tissue healing during grafting.
Materials and methods
Plant materials and growth conditions
Seeds of Arabidopsis thaliana ecotype Colombia-0 (Col-0) were surface-sterilized with sodium hypochlorite containing 1% (w/v) active chloride for 6 min, vernalized at 4 °C overnight, and then germinated and grown on 1.5% (w/v) agar medium spread over rectangular plastic plates containing half-strength Murashige and Skoog medium (denoted culture medium hereafter; Murashige and Skoog 1962) and 0.25% (w/v) sucrose in 1.5% (w/v) solidified agar under white light (60 µmol m−2 s−1) at 22 °C. The plastic plates were placed vertically in a growth chamber. The 35S::RAP2.6L-SRDX #1/#34 lines were constructed as described (Asahina et al. 2011). Seeds of the rap2.6 (SAIL_1225_G09) line were purchased from the Nottingham Arabidopsis Stock Centre (Nottingham, UK), and seeds of the aos (dde2-2), coi1 (SALK_035548), and rap2.6L (SALK_032496) lines were purchased from the Arabidopsis Biological Resource Center (Columbus, USA). The presence of the T-DNA insertion was confirmed by PCR using the primer pair described in Table S1. The frame-shift mutation in aos (dde2-2) was confirmed by BstU I cleavage of the PCR product (von Malek et al. 2002). For treatment of 6 day-old roots with exogenous MeJA, the plants were transferred into culture medium that contained 10 µM MeJA and 0.1% (v/v) dimethylsulfoxide (DMSO).
Construction of vectors and transformants
To generate promoter-β-glucuronidase (GUS) reporters, the promoter regions of JAZ10, RAP2.6, and RAP2.6L were individually PCR-amplified with genomic DNA as the template (primers are shown in Table S1). The PCR-amplified promoter fragments pJAZ10 and pRAP2.6L were individually cloned into pENTR/D-TOPO vectors (ThermoFisher Scientific) and then individually transferred into pKGWFS7 vectors (Karimi et al. 2002) by the kit Gateway LR Clonase II Plus enzyme mix (ThermoFisher Scientific). The amplified fragment for pRAP2.6 was cloned into pCR2.1-Topo (ThermoFisher Scientific). The resulting construct, pCR2.1-Topo-pRAP2.6, was then digested with Hind III and Xba I, and pRAP2.6 was then ligated into Hind III/Xba I–digested pBI121.
Vector constructs were introduced into Agrobacterium and then into Arabidopsis by the floral dip method (Clough and Bent 1998). Arabidopsis transformants were selected according to their resistance to kanamycin (50 µg/ml culture medium). Homozygous T3 or T4 lines were used for the study.
Grafting of Arabidopsis hypocotyls
Self-grafting was conducted according to the procedure of Matsuoka et al. (2016). From 6 day-old plants, grown as described above, hypocotyls were cut out at the middle, and then grafted to the remaining stumps using silicone tubings (0.3 mm i.d., 0.4 mm o.d.). Successfully grafted hypocotyls did not form adventitious roots on the hypocotyl surface. When grafting was not carried out, the cut surface of hypocotyls was allowed to contact agar culture medium. In order to examine the effect of exogenous MeJA on grafted plants, seedlings were transplanted to a culture medium containing 1 µM MeJA and 0.1% (v/v) DMSO prior to grafting. If necessary, cotyledons of the grafted plants were treated with 100 µM triiodobenzoic acid (TIBA) in 0.1% (v/v) DMSO as previously described (Matsuoka et al. 2016).
Confocal microscopy
Hypocotyl samples were fixed in ice-cold Farmer’s solution (3:1 (v/v) ethanol:acetic acid) overnight and then serially rehydrated starting from a 70% (v/v) ethanol(aq) solution. The rehydrated samples were incubated in 0.2 M NaOH, 1% (w/v) sodium dodecyl sulfate at 37 °C overnight and then in 0.01% (w/v) α-amylase in phosphate-buffered saline at 37 °C overnight. For staining by the pseudo-Schiff propidium iodide method, samples were first incubated in 1% (w/v) periodic acid for 40 min and then in 100 mM sodium metabisulfite, 100 mg/ml propidium iodide, 0.15 M HCl at room temperature for 90 min (Truernit et al. 2008). Histological sections were observed through a confocal laser-scanning microscope (TCS SP8, Leica) after incubation overnight in a chloral hydrate/glycerol/water solution (4:1:2 w/v/v). The excitation wavelength was 488 nm, and the emission wavelength region was from 550 to 720 nm. Z-stack images were acquired at 1 µm intervals for a total depth of at least 100 µm. Each experiment was carried out with at least 20 plants. Vascular tissue dimensions at the graft union were measured as previously described (Matsuoka et al. 2016).
Histochemistry of GUS activity
Plant samples were soaked in ice-cold 90% (v/v) acetone for 5 min. After washing with distilled water, samples were vacuum infiltrated with 100 mM sodium phosphate, pH 7.0, 10 mM EDTA, 0.1% (v/v) Triton X-100, 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 2 mM 5-bromo-4-chloro-3-indolyl-β-glucuronide, 20% (v/v) methanol at room temperature for 5 min and then the infiltrated samples were incubated at 37 °C for 1–3 h. Samples were then soaked in 70% (v/v) ethanol(aq) and then mounted in a chloral hydrate/glycerol/water solution (4:1:2 w/v/v). Photographs of the GUS-stained plant samples were acquired under a stereomicroscope (M165FC, Leica).
qRT-PCR
Harvested hypocotyls from ~ 30 plants were preserved in RNAlater solution (Qiagen). After removing that solution from the microcentrifuge tube, samples were ground with a micropestle in liquid nitrogen. Total RNA was extracted in a QIAshredder (Qiagen) using RNeasy Micro kit reagents (Qiagen). Total RNA (100 ng) was reverse transcribed using components from a PrimeScript RT Reagent kit with gDNA Eraser (Takara). qRT-PCR (in 20 µl total reaction volume; 10 µl Fast SYBR Green Master Mix (ThermoFisher Scientific), 1 µl cDNA and 0.2 µM of each primer; Table S1) was performed in a 7500 Fast Real-Time PCR system (ThermoFisher Scientific). Relative expression was normalized to the expression of ACT2 and calculated from three independent replicates.
Quantification of endogenous phytohormone
Intact and grafted hypocotyls (100 each) were harvested directly into liquid nitrogen, and the frozen samples were suspended in 1 ml of 80% (v/v) methanol(aq) containing [2H2]JA, [13C6]JA-Ile, and [13C6]IAA as internal standards. Samples were homogenized for extraction of endogenous phytohormone, and the supernatants were individually loaded onto a Bond Elut C18 cartridge (Agilent Technologies). The solutions were concentrated to ~ 40 µl under a stream of nitrogen. Phytohormone analysis was carried out as previously described (Enomoto et al. 2017). Aliquots (2 µl) of each extract were subjected to liquid chromatography-electrospray ionization-tandem mass spectrometry. A quadrupole tandem mass spectrometer (Agilent 6460 Triple Quadrupole mass spectrometer) with an electrospray ion source and an Agilent 1200 separation module was used in conjunction with a ZORBAX Eclipse XDB-C18 Rapid Resolution HD (2.1 mm × 50 mm, 1.8 µm particle size; Agilent Technologies).
Results
Expression time course of JA-related genes during grafting
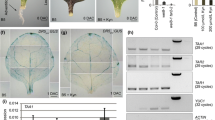
To examine the roles of JA during grafting, we investigated the expression of AOS and JAZ10 using qRT-PCR. Expression of AOS and JAZ10 was drastically induced in response to grafting, peaking at 1 h after grafting (HAG) and then gradually decreasing to nearly the level found for intact hypocotyls by 24 HAG, but still significantly higher than intact hypocotyls (Fig. 1a, b). Induction of these genes by 1 HAG occurred in the scions and stocks of the grafted plants and in non-grafted hypocotyls, suggesting that expression was a response to wounding (Fig. 1e, f). From days 1–7 after grafting (DAG), the expression AOS and JAZ10 remained significantly elevated compared with intact hypocotyls (Fig. 2a, b). For the histochemical assays of GUS staining induced by AOS and JAZ10 promoters, staining was mainly observed in the vascular tissue of the hypocotyls in pAOS::GUS and pJAZ10::GUS plants (Fig. 2e, f).
Gene expression during the initial 24 h after grafting. Expression of AOS (a, e), JAZ10 (b, f), RAP2.6 (c, g), and RAP2.6L (d, h) analyzed by qRT-PCR and reported relative to ACT2 expression. Values are the mean ± standard deviation. Three independent experiments were performed. a–d Time-courses for 0 h (before grafting) and at 1, 3, 6, and 24 HAG. Asterisks indicate statistically significant differences compared with expression in intact hypocotyls (*P < 0.05, **P < 0.01; Dunnett’s test). Different letters indicate statistically significant differences (P < 0.05; Tukey’s test). e–h Scions and stocks from hypocotyls were each examined for GUS activity at 1 and 24 HAG. Scions and stocks that were sampled immediately after cutting in the middle of the hypocotyl are labeled “Intact.” Non-grafted describes hypocotyls that were cut but not grafted
Gene expression during days 1 and 7 after grafting. Expression of AOS (a), JAZ10 (b), RAP2.6 (c), and RAP2.6L (d) analyzed by qRT-PCR and reported relative to ACT2 expression. Values are the mean ± standard deviation. Three independent experiments were performed. Time-courses for 0 day (before grafting) and at 1, 3, 5, and 7 DAG. Asterisks indicate statistically significant differences compared with expression in intact hypocotyls (*P < 0.05, **P < 0.01; Dunnett’s test). e–h Histochemistry of GUS staining under the control of the promoter-containing constructs pAOS::GUS, pJAZ10::GUS, pRAP2.6::GUS, and pRAP2.6L::GUS. Cleared hypocotyls are shown for 0 h (before grafting) and at 1, 3, 5 and 7 DAG. Red arrowheads indicate the position of each graft. Scale bars, 100 µm
Expression of RAP2.6 also drastically increased between 1 and 3 HAG (Fig. 1c) and remained significantly elevated between 1 and 7 DAG (Fig. 2c), which was similar to that observed for AOS and JAZ10. RAP2.6 was also upregulated at 1 HAG in the scions and stocks in grafted and non-grafted hypocotyls (Fig. 1g). GUS staining owing to pRAP2.6::GUS expression was uniformly distributed in the grafted hypocotyls at 1 DAG, but GUS activity was not detected in the grafted region at 5 DAG (Fig. 2g). Conversely, expression of RAP2.6L occurred at 1 HAG, increased to its highest level by 3 HAG, and remained highly expressed until 5 DAG (Figs. 1d, 2d). Expression of RAP2.6L was greater in the stocks than in scions until 24 HAG (Fig. 1h), and the greater expression in the stocks was detected in the vascular tissue of plants expressing pRAP2.6L::GUS at 1 DAG (Fig. 2h). GUS staining was observed in the vascular tissue of scions and stocks after 3 DAG. RAP2.6 and RAP2.6L showed completely different expression patterns during grafting.
Determination of endogenous JA content during grafting
JA-responsive genes, AOS and JAZ10 were rapidly induced by 1 HAG and remained at significantly higher levels until 7 DAG. The endogenous levels of JA and JA-Ile in the grafted hypocotyls were determined using liquid chromatography-tandem mass spectrometry. JA was not detected in intact hypocotyls but was found in grafted hypocotyls by 1 HAG (Table 1). The amount of JA-Ile per grafted hypocotyl increased by ~ 180% of that found per intact hypocotyl at 1 HAG, although by 3 DAG no significant difference in the JA-Ile concentration was found for intact and grafted hypocotyls. Conversely, endogenous IAA significantly increased by 3 DAG.
Morphology of the grafted sites in JA-related mutants
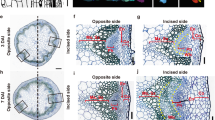
Because the production of JA and expression of RAP2.6L were induced during hypocotyl grafting, we characterized the morphology of the graft site at 7 DAG in WT and the JA-related mutants aos, coi1, rap2.6, rap2.6L, rap2.6 rap2.6L, RAP2.6L-SRDX #1, and RAP2.6L-SRDX #34. Using confocal laser-scanning microscopy, longitudinal sections were obtained from the hypocotyls of the plants that had been stained by the modified pseudo-Schiff propidium iodide method. In WT, horizontal growth of vascular tissue was seen as a result of cell proliferation at the graft site, and the vascular tissue filled the gap between the scion and stock (Fig. 3a; yellow arrowheads). In addition, vertical growth of vascular tissue was also seen at 7 DAG (Fig. S1A). We previously reported that cell proliferation at the graft site of the anac071 anac096 double mutant was suppressed at 7 DAG (Fig. S2; Matsuoka et al. 2016). Vertical cell proliferation was observed in aos, a mutant deficient in JA biosynthesis, and in coi1, a mutant insensitive to the action of JA (Fig. 3b, c and S1b, c). The width and length of the new vascular tissue in the WT graft were not significantly different from those in the aos and coi1 grafts (Fig. 3i, j). Cell proliferation of vascular tissue was also observed in the graft site of the rap2.6 and rap2.6L mutants, the rap2.6 rap2.6L double mutant, and the RAP2.6L-SRDX transformants that had suppressed the function of RAP2.6L (Fig. 3d–j, S1d–h and S3).
Morphology of the healed tissue at the graft site in the mutants and transformants. Longitudinal sections of vascular tissues from the propidium iodide–stained hypocotyls of WT (a), aos (b), coi1 (c), rap2.6 (d), rap2.6L (e), rap2.6 rap2.6L (f), RAP2.6L-SRDX #1 (h), and RAP2.6L-SRDX #34 (i) at 7 DAG observed by confocal laser-scanning microscopy. Yellow arrowheads indicate each cut surface. Scale bars, 100 µm. i, j Boxplots of the widths (i) and lengths (j) of the healed hypocotyl vascular tissues in the mutants and transformants at 7 DAG. Diamond marks mean outlier. Red pluses denote statistically significant differences compared with intact hypocotyls (++, P < 0.01; Steel–Dwass test). n.s. not significant (P > 0.05)
Gene expression in JA-related mutants during grafting
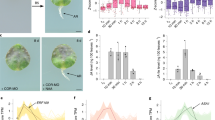
We analyzed expression of JA-responsive genes in the grafted hypocotyls of the JA-related mutants to examine the effects of JA on gene regulation during grafting. RAP2.6 was significantly suppressed in mutant coi1 compared with WT at 3 DAG (Fig. 4a). RAP2.6 expression in aos was slightly, but not significantly, decreased in comparison with expression in WT at the same time. In contrast, no difference in RAP2.6L expression was seen for WT and its JA-related mutants (Fig. 4b). These results were supported by GUS staining, which was equally intense for pRAP2.6L::GUS in WT and coi1 background (Fig. 4d). To confirm that expression of the JA-responsive genes could be induced by JA, the roots of the promoter::GUS lines were treated with MeJA. Induction of JAZ10 and RAP2.6 promoter activities in the roots was obviously enhanced by treatment with MeJA in comparison with the absence of MeJA (control); however, the RAP2.6L promoter activity was not affected by the presence of MeJA (Fig. S4). These results suggested that JA does not regulate RAP2.6L expression. Graft-induced expression of RAP2.6L further increased upon treatment of the auxin transport inhibitor, TIBA (Fig. S5).
Gene expression in the JA-related mutants. a–c Expression of RAP2.6, RAP2.6L, and CYCB1;1 in WT, aos, and coi1 hypocotyls measured by qRT-PCR and reported relative to ACT2 expression at 3 DAG. Values are the mean ± standard deviation. Three independent experiments were performed. Asterisks indicate statistically significant differences compared with WT (*P < 0.05; Dunnett’s test); n.s. not significant (P > 0.05). d Histochemistry of GUS staining at 3 DAG in hypocotyl vascular tissues from pRAP2.6L::GUS in WT (left) and coi1 (right) background. Red arrowheads indicate the graft site. Scale bars, 100 µm
The WT and JA-related mutants were not significantly different with respect to expression of CYCB1;1, a cell division marker (Fig. 4c), suggesting that JA did not affect graft-related cell proliferation. The relative expression of ANAC071 and ANAC096, which are important for graft-related cell proliferation, was similar for WT and the JA-related mutants (Fig. S6).
Effects of exogenous JA on grafting
To investigate the effects of exogenous JA on grafting, plants were grafted on culture medium containing 1 µM MeJA; this increased GUS activity in the graft vascular tissue of pJAZ10::GUS in the WT background with at 3 DAG (Fig. 5a). In the aos background, GUS activity of pJAZ10::GUS was observed only upon MeJA application. These results indicated that the graft response to JA was enhanced when MeJA was present in the culture medium. In WT, shoot growth was inhibited by MeJA, but cells in vascular tissue in the grafts proliferated upon treatment with MeJA at 7 DAG (Fig. 5b and S7). No significant differences in the width and length of the vascular tissue were apparent upon treatment with MeJA (Fig. 5c, d), suggesting that cell proliferation was not affected by exogenous MeJA application during grafting.
Effect of exogenous MeJA on grafting. a pJAZ10::GUS plants were self-grafted in culture medium with (+) or without (−) 1 µM MeJA with 0.1% (v/v) DMSO. GUS staining of vascular tissue at the graft site zone at 3 DAG is shown. Top: WT background; bottom: aos background. Red arrowheads identify the graft position. Scale bars, 100 µm. b Top: shoot morphology at 7 DAG. Scale bars, 1 mm. Middle: longitudinal section of the graft at 7 DAG. Scale bars, 100 µm. Bottom: cross-section of the graft at 7 DAG. Scale bars, 50 µm. Yellow arrowheads indicate each cut surface. Cross-sections were obtained from Z-stack images through the cut surface of the scion. Dotted white lines indicate the outer borders of the vascular tissue. c, d Boxplots showing the vascular tissue widths (c) and lengths (d) at 7 DAG. n.s, not significant (P > 0.05; Steel–Dwass test)
Discussion
JA is produced by grafting but does not contribute to the graft-healing process
The production of JA and expression of genes that respond to JA occur rapidly at non-wounded sites that are directly connected via vascular tissue to the wounded site (Stratmann 2003; Glauser et al. 2009). To understand the systemic JA-signaling mechanism(s), grafting experiments have been performed using WT and JA-related mutants of tomato and Arabidopsis (Li et al. 2002, 2005; Thatcher et al. 2009; Gasperini et al. 2015a). However, the effect(s) of JA on healing of the graft wound has not been studied in depth. Microarray analyses for a late time after grafting provided results for a few of the genes involved in JA biosynthesis in Arabidopsis and grapevine (Yin et al. 2012; Cookson et al. 2013). JA-responsive genes, including AOS and JAZ10, were induced within 1 h after wounding, and their expression levels would have reflected endogenous JA levels (Stenzel et al. 2003; Chung et al. 2008). During grafting of the hypocotyl in our study, expression of AOS and JAZ10 was rapidly induced at 1 HAG and remained upregulated from 1 to 7 DAG (Figs. 1a, b, 2a, b). The endogenous JA-Ile level increased at 1 HAG and returned to its normal level at 3 DAG (Table 1), suggesting that JA biosynthesis occurred only immediately after grafting. AOS and JAZ10 were expressed to the same extent even when the hypocotyl was cut but then not grafted (Fig. 1e, f). Therefore, the JA response is thought not to be affected by the conditions of the cut tissues (touching or not) during grafting, i.e., the wound response will occur during grafting.
Studies that used Arabidopsis hypocotyls to examine grafting events found that healing is controlled by proliferation and differentiation of vascular tissue cells and that auxin induces the process (Yin et al. 2012; Melnyk et al. 2015; Matsuoka et al. 2016). We observed proliferation of vascular tissue cells in the graft-repair site in WT and in mutants aos and coi1 (Fig. 3a–c, i, j). In addition, CYCB1;1 was expressed to approximately the same level in WT, aos, and coi1 by 3 DAG (Fig. 4c). We recently reported that ANAC071 and ANAC096 are essential for the proliferation of vascular tissue cells during grafting (Matsuoka et al. 2016). ANAC071 and ANAC096 were induced at the graft site in WT and in the JA-related mutants (Figs. S6a, b). These results are supported by studies that demonstrated grafting in JA-related mutants of Arabidopsis and tomato (Li et al. 2002, 2005; Thatcher et al. 2009; Gasperini et al. 2015a). Therefore, JA, which is produced concomitantly with grafting, appears not to be essential for cell proliferation at the graft site. This result was supported by Ikeuchi et al. (2017), who reported that JA related mutants showed callus formation at wound site of hypocotyl. The amounts of endogenous JA-Ile in intact hypocotyls were not different from those in hypocotyls at 3 DAG (Table 1). Repeated wounding increases the endogenous JA content and inhibits shoot growth (Zhang and Turner 2008), and root growth is reduced by repeated shoot wounding in a JA-dependent manner (Gasperini et al. 2015b). That is, repeated wounding would be in fact necessary for continuous production of JA to inhibit cell division. Our results indicated that JA production induced by grafting would not be sufficient to affect graft healing (Fig. 3a–c; Table 1). In addition, we also found that treatment with exogenous JA did not inhibit cell proliferation in the graft of WT (Fig. 5a–c), we found that treatment with exogenous JA did not inhibit cell proliferation in the graft of WT (Fig. 5a–c). Therefore, neither endogenous nor exogenous JA induces cell proliferation during grafting. JA is known to inhibit cell division, and treatment with JA prevents the G1/S cell-cycle transition in tobacco Bright Yellow 2 cells (Swiatek et al. 2002). Leaf growth is inhibited by application of exogenous JA, which has been shown to repress cell-cycle progression and DNA replication (Noir et al. 2013). At the grafted site, a new vascular cambium was found to form to allow for dedifferentiation of vessel elements and increase the mechanical strength of the healed site (Rhee and Somerville 1995; Vijayan et al. 1998; Estrada-Luna et al. 2002). Treatment with exogenous JA does not inhibit secondary growth, which gives rise to cell division in the cambium (Tian et al. 2003; Sehr et al. 2010). Grafting and secondary growth may, therefore, have some common mechanisms that are related to cell division in the cambium.
To our knowledge, no studies have been reported concerning JA-regulated wound healing—especially any that have focused on tissue healing. Conversely, many studies have found that an increase in JA correlates with an accumulation of metabolites and differences in the expression of many proteins after wounding (Angelini et al. 2007; Gfeller et al. 2011; Lulai et al. 2011; Yuan-Yuan et al. 2013). JA is also known to induce a defense against attacks by pathogens as part of the wounding response (McConn et al. 1997; Vijayan et al. 1998; Yan et al. 2013). Inoculation with Phaeomoniella chlamydospora suppressed the cell proliferation of vascular tissue during wound healing in grapevine trunk (Pierron et al. 2016), suggesting that defense response is important for normal wound healing. In our present study, because grafting and plant growth were aseptically performed, the effect(s) of JA on graft healing may not have been observable.
RAP2.6L is induced through a JA-independent pathway and does not contribute to graft healing
After grafting hypocotyls, RAP2.6L expression was induced in the stock, which corresponds to the lower region of an incised stem (Figs. 1h, 2h). Treatment with TIBA strongly enhanced RAP2.6L expression at the graft site (Fig. S5), a result consistent with the observation that RAP2.6L level increased with concomitant deprivation of auxin (Asahina et al. 2011). However, RAP2.6L expression was induced in WT and mutants aos and coi1 by grafting (Fig. 4b, d and S5). Treatment with exogenous JA did not induce RAP2.6L expression in roots (Fig. S4). In the time-course experiments, RAP2.6L expression remained high between 1 and 5 DAG in WT (Figs. 1d, 2d), although the expression of other JA-responsive genes had diminished by 3 HAG (Fig. 1a–c). These data suggest that RAP2.6L expression is induced via a JA-independent pathway during grafting. This observation may be related to a previous report that found that application of JA to intact flowering stems did not strongly promote RAP2.6L expression (Asahina et al. 2011).
Although we hypothesized that RAP2.6L deficiency would inhibit cell proliferation at the graft site, cell proliferation was normal at the graft in the rap2.6L mutant (Fig. 3b, i, j) and at the graft site of the rap2.6 rap2.6L double mutant and at these of the RAP2.6L-SRDX transformants, which dominantly represses the target genes (Fig. 3f–j). These results indicate that RAP2.6L is not an activator of proliferation of vascular tissue cells during grafting. The difference in the results for the incised stem and hypocotyl grafting may be a consequence of the different tissues in the organs because pith tissue is only found in the stem, which would cause the healing mechanism to differ somewhat according to the organ. The other possibilities are that the difference is attributed to the method of wounding (half cut or cut thoroughly), or RAP2.6L indirectly promotes cell division through a stress response in the incised flowering stem. Iwase et al. reported that RAP2.6L acts downstream of WOUND INDUCED DEDIFFERENTIATION1, which is necessary for wound-induced cellular reprogramming and is involved in shoot regeneration, but rap2.6 l mutant did not affect to cell proliferation of callus formation at wound hypocotyl (Iwase et al. 2016; Ikeuchi et al. 2017). In addition, adventitious buds have been observed to emerge from the graft sites in tomatoes (Winkler 1907; Bausher 2011). These observations demonstrate that shoot regeneration is possible during grafting. Therefore, RAP2.6L might promote shoot regeneration instead of cell proliferation during grafting, and this may be an additional function of RAP2.6L that includes allowing plants to tolerate being waterlogged and resist pathogens (Che et al. 2006; Sun et al. 2010; Liu et al. 2012).
In conclusion, our results show that JA production and RAP2.6L expression induced by wounding do not contribute to wound healing—as defined by cell proliferation that occurs during grafting.
Abbreviations
- AOS:
-
Allene oxide synthase
- COI1:
-
Coronatine insensitive 1
- CYCB:
-
Cyclin B
- DMSO:
-
Dimethyl sulfoxide
- GUS:
-
β-glucuronidase
- DAG:
-
Days after grafting
- HAG:
-
Hours after grafting
- IAA:
-
Indole-3-acetic acid
- JA:
-
Jasmonic acid
- JAZ:
-
Jasmonate ZIM-domain
- MeJA:
-
Methyl jasmonate
- OPDA:
-
12-oxo-phytodienoic acid
- qRT-PCR:
-
Quantitative reverse transcription PCR
- RAP2.6:
-
Related to APETALA2.6
- RAP2.6L:
-
Related to APETALA2.6-like
- SRDX:
-
Superman repression domain X
- TIBA:
-
Triiodobenzoic acid
- WT:
-
Wild type
References
Angelini R, Tisi A, Rea G, Chen MM, Botta M, Federico R, Cona A (2007) Involvement of polyamine oxidase in wound healing. Plant Physiol 146:162–177. https://doi.org/10.1104/pp.107.108902
Asahina M, Iwai H, Kikuchi A, Yamaguchi S, Kamiya Y, Kamada H, Satoh S (2002) Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiol 129:201–210. https://doi.org/10.1104/pp.010886
Asahina M, Azuma K, Pitaksaringkarn W, Yamazaki T, Mitsuda N, Ohme-Takagi M, Yamaguchi S, Kamiya Y, Okada K, Nishimura T, Koshiba T, Yokota T, Kamada H, Satoh S (2011) Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc Natl Acad Sci USA 108:16128–16132. https://doi.org/10.1073/pnas.1110443108
Bate NJ, Sivasankar S, Moxon C, Riley JMC, Thompson JE, Rothstein SJ, Department (1998) Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol 117:1393–1400. https://doi.org/10.1104/pp.117.4.1393
Bausher MG (2011) Grafting technique to eliminate rootstock suckering of grafted tomatoes. Hortscience 46:596–598
Bell E, Mullet JE (1993) Characterization of an Arapdopsis lipoxygenase gene responsive to methyl jasmonate and woundig. Plant Physiol 103:1133–1137
Che P, Lall S, Nettleton D, Howell SH (2006) Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Development 141:620–637. https://doi.org/10.1104/pp.106.081240.620
Chung HS, Koo AJK, Gao X, Jayanty S, Thines B, Jones AD, Howe G (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146:952–964. https://doi.org/10.1104/pp.107.115691
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cookson SJ, Clemente Moreno MJ, Hevin C, Nyamba Mendome LZ, Delrot S, Trossat-Magnin C, Ollat N (2013) Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J Exp Bot 64:2997–3008. https://doi.org/10.1093/jxb/ert144
Creelman R, Tierney ML, Mullet JE (1992) Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA 89:4938–4941. https://doi.org/10.1073/pnas.89.11.4938
Enomoto H, Sensu T, Sato K, Sato F, Paxton T, Yumoto E, Miyamoto K, Asahina M, Yokota T, Yamane H (2017) Visualisation of abscisic acid and 12-oxo-phytodienoic acid in immature Phaseolus vulgaris L. seeds using desorption electrospray ionisation-imaging mass spectrometry. Sci Rep 7:42977. https://doi.org/10.1038/srep42977
Estrada-Luna AA, López-Peralta C, Cárdenas-Soriano E (2002) In vitro micrografting and the histology of graft union formation of selected species of prickly pear cactus (Opuntia spp.). Sci Hortic 92:317–327. https://doi.org/10.1016/S0304-4238(01)00296-5
Gasperini D, Chauvin A, Acosta IF, Kurenda A, Stolz S, Chetelat A, Wolfender J-L, Farmer EE (2015a) Axial and radial oxylipin transport. Plant Physiol 169:2254–2254. https://doi.org/10.1104/pp.15.01104
Gasperini D, Chételat A, Acosta IF, Goossens J, Pauwels L, Goossens A, Dreos R, Alfonso E, Farmer EE (2015b) Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genet 11:e1005300. https://doi.org/10.1371/journal.pgen.1005300
Gfeller A, Baerenfaller K, Loscos J, Chételat A, Baginsky S, Farmer EE (2011) Jasmonate controls polypeptide patterning in undamaged tissue in wounded Arabidopsis leaves. Plant Physiol 156:1797–1807. https://doi.org/10.1104/pp.111.181008
Glauser G, Grata E, Dubugnon L, Rudaz S, Farmer EE, Wolfender JL (2008) Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J Biol Chem 283:16400–16407. https://doi.org/10.1074/jbc.M801760200
Glauser G, Dubugnon L, Mousavi SAR, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284:34506–34513. https://doi.org/10.1074/jbc.M109.061432
Ikeuchi M, Iwase A, Rymen B, Lambolez A, Kojima M, Takebayashi Y, Heyman J, Watanabe S, Seo M, De Veylder L, Sakakibara H, Sugimoto K (2017) Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol 175:1158–1174. https://doi.org/10.1104/pp.17.01035
Iwase A, Harashima H, Ikeuchi M, Rymen B, Ohnuma M, Komaki S, Morohashi K, Kurata T, Nakata M, Ohme-Takagi M, Grotewold E, Sugimoto K (2016) WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 29:54–69. https://doi.org/10.1105/tpc.16.00623
Johkan M, Mori G, Mitsukuri K, Mishiba K, Morikawa T, Oda M (2008) In vivo shoot regeneration promoted by shading the cut surface of the stem in tomato plants. Hortscience 43:220–222
Karimi M, Inze D, Van Lijsebettens M, Hilson P (2002) Gateway vectors for transformation of cereals. Trends Plant Sci 7:4–7. https://doi.org/10.1016/S1360-1385(02)02251-3
Koo AJ (2017) Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress response. Phytochem Rev 13:1–30. https://doi.org/10.1007/s11101-017-9510-8
Krishnaswamy S, Verma S, Rahman MH, Kav NNV (2011) Functional characterization of four APETALA2-family genes (RAP2.6, RAP2.6L, DREB19 and DREB26) in Arabidopsis. Plant Mol Biol 75:107–127. https://doi.org/10.1007/s11103-010-9711-7
Kubigsteltig I, Laudert D, Weiler EW (1999) Structure and regulation of the Arabidopsis thaliana allene oxide synthase gene. Planta 208:463–471
Lauer FI (1963) Influence of high and low levels of N and K on adventitious bud formation in the potato. 40:302–307
Li L, Li C, Lee GI, Howe G (2002) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA 99:6416–6421. https://doi.org/10.1073/pnas.072072599
Li C, Schilmiller AL, Liu G, Lee GI, Jayanty S, Sageman C, Julia V, James JG, Kaori Y, Yuichi K, Gregg AH, Kobayashi Y, Howe GA (2005) Role of β-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell Online 17:971–986. https://doi.org/10.1105/tpc.104.029108
Liu P, Sun F, Gao R, Dong H (2012) RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol Biol 79:609–622. https://doi.org/10.1007/s11103-012-9936-8
Lulai E, Huckle L, Neubauer J, Suttle J (2011) Coordinate expression of AOS genes and JA accumulation: JA is not required for initiation of closing layer in wound healing tubers. J Plant Physiol 168:976–982. https://doi.org/10.1016/j.jplph.2010.12.001
Matsuoka K, Sugawara E, Aoki R, Takuma K, Terao-Morita M, Satoh S, Asahina M (2016) Differential cellular control by cotyledon-derived phytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant Cell Physiol 57:2620–2631. https://doi.org/10.1093/pcp/pcw177
McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94:5473–5477
Melnyk CW, Schuster C, Leyser O, Meyerowitz EM (2015) A developmental framework for graft formation and vascular reconnection in Arabidopsis thaliana. Curr Biol 25:1306–1318. https://doi.org/10.1016/j.cub.2015.03.032
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Noir S, Bömer M, Takahashi N, Ishida T, Tjir-Li T, Balbi V, Shanahan H, Sugimoto K, Devoto A (2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161:1930–1951. https://doi.org/10.1104/pp.113.214908
Pierron RJG, Pouzoulet J, Couderc C, Judic E, Compant S, Jacques A (2016) Variations in early response of grapevine wood depending on wound and inoculation combinations with Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Front Plant Sci 7:1–14. https://doi.org/10.3389/fpls.2016.00268
Rhee SY, Somerville CR (1995) Flat-surface grafting in Arabidopsis thaliana. Plant Mol Biol Rep 13:118–123. https://doi.org/10.1111/j.1399-3054.2009.01220.x
Schaller A, Stintzi A (2009) Enzymes in jasmonate biosynthesis—structure, function, regulation. Phytochemistry 70:1532–1538. https://doi.org/10.1016/j.phytochem.2009.07.032
Sehr EM, Agusti J, Lehner R, Farmer EE, Schwarz M, Greb T (2010) Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J 63:811–822. https://doi.org/10.1111/j.1365-313X.2010.04283.x
Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, Ziegler J, Feussner I, Wasternack C (2003) Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol Biol 51:895–911. https://doi.org/10.1023/A:1023049319723
Stratmann J (2003) Long distance run in the wound response—jasmonic acid is pulling ahead. Trends Plant Sci 8:247–250. https://doi.org/10.1016/S1360-1385(03)00106-7
Sun F, Liu P, Xu J, Dong H (2010) Mutation in RAP2.6L, a transactivator of the ERF transcription factor family, enhances Arabidopsis resistance to Pseudomonas syringae. Physiol Mol Plant Pathol 74:295–302. https://doi.org/10.1016/j.pmpp.2010.04.004
Suza WP, Staswick PE (2008) The role of JAR1 in jasmonoyl-L-isoleucine production during Arabidopsis wound response. Planta 227:1221–1232. https://doi.org/10.1007/s00425-008-0694-4
Swiatek A, Lenjou M, Van Bockstaele D, Inzé D, Van Onckelen H (2002) Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol 128:201–211. https://doi.org/10.1104/pp.010592.1
Thatcher LF, Manners JM, Kazan K (2009) Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J 58:927–939. https://doi.org/10.1111/j.1365-313X.2009.03831.x
Tian W-M, Shi M-J, Yu F-Y, Wu J-L, Hao B-Z, Cui K-M (2003) Localized effects of mechanical wounding and exogenous jasmonic acid on the induction of secondary laticifer differentiation in relation to the distribution of jasmonic acid in Hevea brasiliensis. Acta Bot Sin 45:1366–1372
Truernit E, Bauby H, Dubreucq B, Grandjean O, Runions J, Barthélémy J, Palauqui J-C (2008) High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 20:1494–1503. https://doi.org/10.1105/tpc.107.056069
Vijayan P, Shockey J, Lévesque C, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95:7209–7214. https://doi.org/10.1073/pnas.95.12.7209
von Malek B, van der Graaff E, Schneitz K, Keller B (2002) The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216:187–192. https://doi.org/10.1007/s00425-002-0906-2
Wang Z, Cao G, Wang X, Miao J, Liu X, Chen Z, Qu LJ, Gu H (2008) Identification and characterization of COI1-dependent transcription factor genes involved in JA-mediated response to wounding in Arabidopsis plants. Plant Cell Rep 27:125–135. https://doi.org/10.1007/s00299-007-0410-z
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058. https://doi.org/10.1093/aob/mct067
Winkler H (1907) Über Propfbastarde und pflanzliche Chimären. Ber Deutsch Bot Gesell 25:568–576
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236. https://doi.org/10.1105/tpc.109.065730
Yan L, Zhai Q, Wei J, Li S, Wang B, Huang T, Du M, Sun J, Kang L, Li CB, Li C (2013) Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genet 9:e1003964. https://doi.org/10.1371/journal.pgen.1003964
Yin H, Yan B, Sun J, Jia P, Zhang Z, Yan X, Chai J, Ren Z, Zheng G, Liu H (2012) Graft-union development: a delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J Exp Bot 63:4219–4232. https://doi.org/10.1093/jxb/ers109
Yuan-Yuan D, Chuang-Shu S, Gui-Lin C (2013) Effect of methyl jasmonate on wound healing and antioxidant enzyme activities of stem in Cynomorium songaricum Rupr. Plant Physiol J 49:787–792
Zhang Y, Turner JG (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS ONE 3:e3699. https://doi.org/10.1371/journal.pone.0003699
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science (Grant-in-Aid for Young Scientists B No. 16K18572 to M.A.), Promotion and Mutual aid corporation for Private School of Japan (to M.A.) and the Ministry of Education, Culture, Sports, Science, and Technology Program for the Strategic Research Foundation at Private Universities (Grant No. S1311014 to T.Y., H.Y., and M.A.). We thank Dr. Ines Kubigsteltig (Ruhr University Bochum, Bochum, Germany; Kubigsteltig et al. 1999) for providing the pAOS::GUS vector, and Drs. Nobutaka Mitsuda (National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan) and Masaru Ohme-Takagi (Saitama University, Saitama, Japan) for the kind gift of the RAP2.6L-SRDX seeds.
Author information
Authors and Affiliations
Contributions
KM contributed to all experiments and manuscript preparation. KM and MA designed the overall study. RY performed the microscopic experiments. EY and TY performed the phytohormone assays. MA, SS, and HY helped develop the manuscript text.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Research involving human and animal rights
This study did not involve human and animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuoka, K., Yanagi, R., Yumoto, E. et al. RAP2.6L and jasmonic acid–responsive genes are expressed upon Arabidopsis hypocotyl grafting but are not needed for cell proliferation related to healing. Plant Mol Biol 96, 531–542 (2018). https://doi.org/10.1007/s11103-018-0702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0702-4