Abstract
Temperature affects a cascade of ecological processes and functions of forests. With future higher global temperatures being inevitable it is critical to understand and predict how forest ecosystems and tree species will respond. This paper reviews experimental warming studies in boreal and temperate forests or tree species beyond the direct effects of higher temperature on plant ecophysiology by scaling up to forest level responses and considering the indirect effects of higher temperature. In direct response to higher temperature (1) leaves emerged earlier and senesced later, resulting in a longer growing season (2) the abundance of herbivorous insects increased and their performance was enhanced and (3) soil nitrogen mineralization and leaf litter decomposition were accelerated. Besides these generalizations across species, plant ecophysiological traits were highly species-specific. Moreover, we showed that the effect of temperature on photosynthesis is strongly dependent on the position of the leaf or plant within the forest (canopy or understory) and the time of the year. Indirect effects of higher temperature included among others higher carbon storage in trees due to increased soil nitrogen availability and changes in insect performance due to alterations in plant ecophysiological traits. Unfortunately only a few studies extrapolated results to forest ecosystem level and considered the indirect effects of higher temperature. Thus more intensive, long-term studies are needed to further confirm the emerging trends shown in this review. Experimental warming studies provide us with a useful tool to examine the cascade of ecological processes in forest ecosystems that will change with future higher temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is causing an increase in global mean temperature and a decrease in the diurnal temperature range (IPCC 2007), which may have wide-ranging implications for forest ecosystems (Saxe et al. 2001). Forests cover approximately 30 % of the land surface of the world and contribute to approximately 50 % of the net primary production in terrestrial ecosystems (Hyvönen et al. 2007). Therefore, forests can influence the climate via bidirectional transfer of energy, water, and carbon dioxide (CO2) with the atmosphere (Bonan 2008). Moreover, forests provide numerous ecosystem services, i.e., benefits from a multitude of resources and functions arising from the ecosystem, such as harboring biodiversity, regulating hydrologic cycles, and protecting soils from erosion. Temperature affects all biological processes in forests, including plant growth (Bronson et al. 2009; Farnsworth et al. 1995; Lahti et al. 2005), phenology (Morin et al. 2010; Nakamura et al. 2010), herbivory (Ayres and Lombardero 2000; Bale et al. 2002), and nitrogen (N) cycling (Peterjohn et al. 1994; Rustad et al. 2001). It is thus critical that we understand how tree species and forest ecosystems are likely to respond to future higher temperatures, and open-field warming experiments can be an effective approach in studying such response (Fig. 1). In experimental warming, different methods can be used to warm distinct ecosystem components, e.g., heating cables can be used to warm branches or soils, and infrared (IR) lamps or passive field chambers can be used to warm plants and soils.
Warming experiments are very useful for testing whether trends observed from long-term monitoring of natural populations will be maintained with a larger change in temperature, because temperatures can be experimentally controlled to reach levels that are outside the range of natural variability (Morin et al. 2010). Moreover, experimental warming enables us to directly test the effect of temperature elevation on ecosystem functions with fewer confounding factors, e.g., other variables that covary spatially and temporally with temperature. Results from warming experiments have also been used in models to predict changes in ecosystem functions, and thus to assess the vulnerability of ecosystems to climate change (Aronson and McNulty 2009; Peterjohn et al. 1993, 1994; Rastetter et al. 1991).
Given the usefulness of experimental warming studies in understanding the response of trees and forests to future climate change, it is important to determine the scope of these studies, and to assess the information and trends generated from them. The results of warming experiments have previously been analyzed in several review papers that have each focused on different aspects. For example, Shaver et al. (2000) provided a conceptual framework for implementing warming experiments and interpreting results derived from them, whereas Hyvönen et al. (2007) analyzed the impacts of increased temperature along with other environmental factors on carbon (C) sequestration in forests. Three other review papers presented meta-analyses that were conducted to determine the changes in important processes, such as plant growth, photosynthesis and respiration, and N mineralization, with temperature (Rustad et al. 2001; Way and Oren 2010; Wu et al. 2011). Although the findings and insights from these studies have been very useful for understanding the response of specific plants, we need further evaluation of the experimental results to scale them up in the context of forest ecosystem functions such as C and N cycles.
Our research has previously focused on the ecological processes of the C cycle and the budget of East Asian forest ecosystems, in which tree growth and the soil nutrient cycle play significant functional roles (special issue of the Journal of Plant Research 2010, vol 123). Through intensive studies on tree growth, leaf phenology, photosynthesis, and soil C and N cycles, it has become apparent that open-field warming experiments should be incorporated into research on ecosystem functions. In this paper, we review the key findings of warming studies conducted on tree species and forest ecosystems in temperate and boreal regions where large temperature changes are prominent during the year. We focus mainly on the processes responsible for forest ecosystem functions and ecological interactions, i.e., plant phenology and photosynthetic productivity, and their possible influence on herbivory and N cycling, as these have not previously been reviewed and are of relevance to the alterations in forest ecosystems under climate change. On the basis of this synthesis, we suggest future directions for experimental warming research on trees and forest ecosystems.
Changes in plant phenology in response to experimental warming
A change in plant phenology is one of the most sensitive and observable biological responses to climate change (Polgar and Primack 2011; Schwartz 2003), and is considered to be controlled by temperature and photoperiod at mid- to high latitudes, and by rainfall and evapotranspiration at low latitudes (Menzel et al. 2006; Spano et al. 1999). Shifts in the timing and length of the growing season have been reported in leaf phenology studies based on ground observations, remote sensing, and climatological analysis (Badeck et al. 2004; Linderholm 2006). During the last 50 years, leaf unfolding has, on average, been advanced by 1–3 days and leaf senescence has been delayed by 1–2 days per decade (Cleland et al. 2007; Menzel et al. 2006; Parmesan and Yohe 2003; Walther et al. 2002). Although a change in temperature is the dominant feature of climate change, the interaction between temperature and plant phenology via the ecophysiological functions of ecosystems is, surprisingly, not well understood (e.g., Way 2011). The effect of warming experiments on leaf phenology has been used to extrapolate the behavior of plants under future climate conditions, but this approach has recently been questioned by Wolkovich et al. (2012), who showed that warming experiments would underpredict the changes in leafing and flowering phenology for a wide range of species. To determine the effect of climate change on tree species and forest ecosystems, we must not only consider the direct effect of temperature on leaf phenology but also whether any changes in leaf phenology affect plant growth and allocation to reproduction, interactions with insects and pathogens, light availability to other plants via shading, and CO2 exchange between the atmosphere and ecosystems. In this section, we review the phenological responses of leaves of tree species and forest ecosystems to an increase in temperature through experimental warming.
Warming methods to investigate phenological changes in tree species and forest ecosystems
Different responses to warming may be observed depending on the warming method used (Bronson et al. 2009). However, to our knowledge, there has been no previous comparison of the different methods for the same species and/or the same degree of warming. When we compared studies using different methods for aboveground warming (Table 1), i.e., open-top chamber (OTC), whole-tree chamber, IR lamp, and branch heating cable, we found that none of these methods elicited a consistent trend in phenological changes. We acknowledge that the value of such a comparison is limited, as only single studies exist for some methods and different species have been used for each method. Each method, however, has its restrictions in what volume or area it can warm up; thus, the warming method needs to be carefully selected depending on the target of investigation. For example, Nakamura et al. (2010) noted that the heating cable should cover the terminal bud on branches in order to affect leaf phenology. In conclusion, all methods increased the temperature, and although not one comparison of methods was shown across one species or ecosystem it appeared that across all methods investigated effects on phenology depended more on the tree species or forest ecosystems in which the warming experiment was performed, and the degree of warming than the method used.
Phenological changes in response to the degree of warming in trees
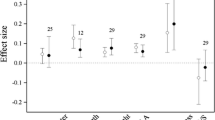
Field observations of leaf phenology are correlated with gradual increases in temperature over the course of decades, whereas experimental warming studies often set a target warming temperature, which may determine the magnitude of the plant phenological response. Linear extrapolation of the effect of temperature thus obtained beyond its historical range has been argued (Ibanez et al. 2010; Menzel et al. 2005; Richardson et al. 2010) and some long-term records show contrasting results for this relationship through time (Menzel et al. 2005; Sparks and Menzel 2002). However, there is a linear relationship between plant phenological response and temperature with increasing altitude (Vitasse et al. 2010). We conducted a meta-analysis and found that, within the range of experimental temperatures, there was no significant correlation between temperature increase and the days of advanced leaf unfolding or delayed leaf senescence (Fig. 2a, b). However, two studies, which each examined two levels of elevated temperature, found that spring phenology (budbreak and leaf expansion) occurred earlier under higher temperature conditions (Morin et al. 2010; Repo et al. 1996).
The number of days and rate of leaf phenological change in response to temperature (°C) increase by experimental warming; advancement in leaf unfolding (a), delay in leaf senescence (b), advancement rate of leaf unfolding (c), and delay rate of leaf senescence (d). Data are from Bronson et al. 2009; Guak et al. 1998; Gunderson et al. 2012; Kilpeläinen et al. 2006; Morin et al. 2010; Nakamura et al. 2010; Norby et al. 2003; Repo et al. 1996; Slaney et al. 2007; Xu et al. 2012; sensu Table 1
In the meta-analysis, we also estimated the rate of advancement or delay, i.e., the number of days of advancement in spring leaf phenology and delay in autumn leaf phenology divided by the increase in temperature (°C). We found that the advancement rate of leaf unfolding was negatively correlated with temperature increase (Fig. 2c), while the delay rate of leaf senescence was not correlated with temperature (Fig. 2d). Similarly, Morin et al. (2010) also found that the advancement rate of leaf unfolding decreased with increasing temperature when they compared two different temperature levels. This decrease in advancement rate may be caused by a lack of chilling requirements to break bud dormancy or a nonlinear effect of increasing temperature on cell growth (Morin et al. 2010).
Although the target temperatures in these studies reflect a climate change scenario (e.g., IPCC 2007) for the species and regions studied, care needs to be taken when designing such experiments, and ideally different degrees of warming should be included (Morin et al. 2010). When the selected target temperature is too high, it may reduce the phenological response (Table 1; Morin et al. 2010), leading to an underprediction of phenological changes compared with field observations, as shown by Wolkovich et al. (2012) for multiple species.
Most of the experimental warming studies on tree species investigated phenological change under warming and a few applied phenological models (Guak et al. 1998; Gunderson et al. 2012; Morin et al. 2010). Moreover, analyses using model or temperature-related parameters mostly focused on thermal sum, and other factors such as photoperiod and chilling requirements were not fully analyzed. For example, several studies reported budbreak did not advance under warming and suggested that other requirements, e.g., photoperiod and chilling, were not satisfied (Nakamura et al. 2010; Norby et al. 2003; Xu et al. 2012). In addition, belowground warming showed no change in leaf phenology (Bergh and Linder 1999; Bronson et al. 2009; Farnsworth et al. 1995), and thus in these temperate forest species soil temperature did not appear to control leaf phenology. In contrast, in non-temperate regions, where frozen soils determine spring phenology, soil warming could have an effect on leaf phenology (Totland and Alatalo 2002; Wookey et al. 1993). Unfortunately, none of these field studies examined which requirements were lacking, although studies carried out over multiple years may provide some insights into the role of photoperiod which is not expected to change between years.
To better understand the physiological mechanisms behind leaf phenology in spring, the effect of fluctuating temperature as well as the experimental combination of temperature with other environmental factors such as day length on the timing of leaf budbreak should be examined. From an ecological perspective, any damage caused by late frosts or a shortage of precipitation may be worth considering when predicting the effects of earlier leaf budbreak on photosynthetic C gain in the life of such leaves.
Changes in leaf and canopy photosynthesis under warming
Photosynthesis is the fundamental biochemical process for plant growth that determines vegetation structure and dynamics, and hence is one of the largest terrestrial C fluxes (Schlesinger 1997). A warmer temperature is expected to have significant influence across ecological hierarchy. Future warmer climate could affect photosynthesis and consequently also affect biomass allocation, growth, establishment, and survival of trees (Loik et al. 2004; Saxe et al. 2001). Plants increase their photosynthetic rate until the optimal temperature for maximum productivity is reached, but a change in growth temperature can shift the maximum productivity and optimal temperature (Berry and Björkman 1980). The thermal acclimation of photosynthesis is well documented (e.g., Hikosaka et al. 2006) but its response is species-specific and diverse (Berry and Björkman 1980) and the effects on plant growth are less well elaborated. A species-specific response can lead to changes in tree species composition under future climate change (Walther et al. 2002). In addition, phenotypic plasticity in traits responsible for photosynthetic C gain and growth should also be considered in predicting the effects of warming on plants (Nicotra et al. 2010). Therefore, it is important to experimentally determine how photosynthesis and plant growth may change under a warmer temperature.
Phenological changes in response to warming affect plant photosynthesis and growth by changing photosynthetic recovery in spring and the length of the growing season. Warmer spring temperatures can reduce the time needed for the dehardening of trees and help them to achieve earlier or even higher maximum photosynthetic rates, because the beginning of photosynthesis in spring is tightly linked to temperature (Polgar and Primack 2011; Strand and Lundmark 1995). Although few open-field experimental warming studies on trees have investigated the relationship between phenology and photosynthesis, advanced photosynthetic recovery in spring by soil warming treatment has been reported in boreal spruce stand (Bergh and Linder 1999) and temperate tree seedlings (Jo et al. 2011). Questions remain, however, regarding the quantitative consequences of leaf phenological changes and photosynthetic C gain at the single-leaf, whole-plant, and forest ecosystem scales. For example, warmer temperatures may extend the growing season and potentially increase the photosynthetic C gain, when it is not offset by increased leaf longevity, which is known to be negatively correlated with photosynthesis (Mediavilla and Escudero 2003; Reich et al. 1992).
The effect of experimental warming on photosynthesis and plant growth was determined on seedlings, saplings and juvenile trees especially in boreal, cool-temperate and temperate regions, while difficulty of accessing the crown of tall trees constraints warming experiments on forest canopy. Most of the studies were conducted on conifers (Bergh and Linder 1999; Bronson and Gower 2010; Danby and Hik 2007; Han et al. 2009; Wang et al. 1995; Yin et al. 2008; Zhao and Liu 2009), and in two studies, the response of oak and maple seedlings was determined (Arend et al. 2011; Gunderson et al. 2000). Responses of leaf photosynthesis or plant growth are species-specific and/or dependent on the ontogenetic stage of the trees. For example, seedlings/saplings responded positively to warmer conditions in Quercus species (+2 °C, Arend et al. 2011), Picea asperata and Abies faxoniana (+0.5–0.7 °C, Yin et al. 2008) and Picea glauca (+1.8 °C, Danby and Hik 2007), but negatively in Picea mariana (+5.0 °C, Bronson and Gower 2010) and Quercus rubra (+3 °C and +6 °C, Wertin et al. 2011). In adult trees Pinus sylvestris (+2.0 °C, Wang et al. 1995) responded negatively but Picea abies (+5.0 °C, Bergh and Linder 1999) responded positively. In Acer saccharum, Gunderson et al. (2000) pointed out that the photosynthetic response to warmer conditions differ between the ecotypes, and also between the temperature regime of the experiments, i.e., constantly high (in growth chamber) vs. fluctuating diurnally (open-field system).
From the available information, it is still difficult to discern a consistent relationship between photosynthesis, respiration and growth, and an increase in temperature (Arend et al. 2011; Bronson and Gower 2010; Danby and Hik 2007; Gunderson et al. 2000; Wang et al. 1995; Yin et al. 2008), because other factors such as N, water, and light availability also affect these processes. However, Way and Oren (2010) recently published a review and synthesis of the effect of temperature increases on leaf photosynthesis, respiration, and plant growth for various tree species of different biomes, and raised the possibility of making generalizations about plant responses to temperature increases if we consider broad ranges of species. For example, for an increase in temperature of 3–6 °C, they showed a positive biomass response ratio of 1.38 in temperate species. Also they showed that deciduous trees had higher growth rate than evergreen trees. This could be attributed to the higher photosynthetic capacity (measured at growth temperature, and represented by the maximum velocity of carboxylation, V cmax), higher biomass allocation to leaves and weak increase of respiratory C loss of deciduous trees under a higher growth temperature (Way and Oren 2010).
In addition to experimental studies on growth and photosynthetic responses to higher growth temperatures in seedlings/saplings, those on ecophysiological consequences between leaf-level photosynthesis, phenology, and biomass growth under variable environmental conditions are needed since plants must survive under a wider range of light, nutrient, and soil water conditions in the field. This issue would be critical for regeneration of forests. Under warmer temperatures leaves may show earlier expansion and delayed senescence which lead to longer leaf longevity (growing season length), but they may face severe high temperature stress under high light conditions either in open field or under large canopy gap since they decrease stomatal conductance and hence photosynthetic activity, especially in midday (Fig. 3b). On the other hand, in forest understory, higher temperature has weaker influence on photosynthetic activity (Fig. 3c). Indeed, Yin et al. (2008) showed higher growth in Abies faxoniana under low light conditions than under high light conditions in their warming experiment. In high light environment, soil water and nutrient availability should be critical since plants must cope with the higher transpirational demand and photoinhibitory damage (Muraoka et al. 2000; Valladares and Pearcy 1997) and the possible water stress is predicted by reduced water use efficiency in higher temperature (Fig. 3).
Instantaneous photosynthetic response of seedlings (a–c) and leaves in branches at canopy-top (d–f) to temperature conditions simulated by the authors using 3D model Y-plant (Pearcy et al. 2005). In the model three air temperature regimes were examined; daily minimum–maximum temperature of 15–25 °C (thick solid line), 20–30 °C (solid line), and 25–35 °C (broken line). For seedlings, high light (b) and understory light (c) conditions were examined, and for leaves in branches at canopy-top, two levels of atmospheric CO2 concentrations (Ca) were examined (385 ppm in panel e, and 470 ppm in panel f). Daily integrated values of daytime net photosynthesis (daily A) and water use efficiency (daily WUE) are also shown in the Table, with the reduction rate of daily A due to increasing temperature compared to that under current normal condition in parentheses
Understanding of the photosynthetic response to higher temperature is also crucial to predict the canopy-level photosynthesis of forest ecosystem. Again, warmer condition may expand the growing season of the canopy, but in summer, high light and high temperature conditions reduce stomatal conductance and hence photosynthetic activity at the canopy-top irrespective of the increasing atmospheric CO2 concentration (Fig. 3e, f). Indeed, there is evidence that combination of heat stress and severe drought reduced gross primary production (GPP) of forest ecosystems in western Europe in summer of 2003 (Reichstein et al. 2006). In Alaska, summer drought in 2004 decreased GPP of deciduous forests (Welp et al. 2007). In East Asia, prolonged dry season in tropical forests, and prolonged rainy season which led to less incident radiation in temperate forests in 2003, resulted in less GPP (Saigusa et al. 2008). Ito (2010) predicted by his model simulation that higher temperature may lengthen the growing season but increase of summer precipitation in central Japan would reduce GPP in the near future. Since climate change involves not only changes in temperature but also in the precipitation, sensitivity of forest canopy photosynthesis to the combined micrometeorological conditions should be studied by linking open-field experiments, long-term monitoring of CO2 flux and simulation models (cf. Muraoka et al. 2010). Open-field experiments on leaves and up-scaling analyses using simulation models are crucial to link plant phenological and ecophysiological changes with forest canopy functions under possible climate change. Furthermore, more warming studies in the forest canopy, where access is difficult, are needed. As a first attempt, heating cables (Nakamura et al. 2010) and an open-top canopy chamber (Muraoka, unpublished) are being applied to a cool-temperate, deciduous broadleaf tree (Quercus crispula) to determine leaf phenology, and photosynthetic and respiratory responses to temperature, and to estimate leaf-level effects on the forest canopy C budget. In these investigations, thermal acclimation of photosynthetic and respiration rates is also being examined.
Further potential issues relating to leaf, whole-plant and canopy photosynthesis and phenology
Based on the findings in the literature and our observations in the forest ecosystems, the photosynthetic response to warming can be illustrated as shown in Fig. 4 (see also Richardson et al. 2010). Here, we focus on both seedling and canopy-top photosynthesis, by considering the high and low light conditions, and also by considering photosynthetic ‘capacity’ and ‘activity’ separately. Also, photosynthetic and phenological responses should be separately considered for adult trees (canopy-top) and seedlings due to ontogenetic mechanisms (cf. Vitasse 2013). In both high and low light regimes, warming may lengthen the growing season by earlier leaf expansion and delayed senescence as mentioned above and shown in Table 1.
Hypothetical responses of photosynthetic productivity in deciduous trees and forest canopy to future warming environment throughout the seasons. The possible patterns were considered for high light environment for canopy-top leaves, juvenile and seedlings in open habitat (a–d) and low light environment for understory plants (e–h). Bold lines represent the present condition, and thin lines represent future conditions. See text for details
First, in high light regime, which is the case for leaves at canopy-top or seedling/juvenile plants in habitats with large gap in canopy, leaf-level photosynthetic capacity (represented by V cmax or light-saturated photosynthetic rate, A max) may increase or be similar to the current conditions (Fig. 4a, b). However, strong light and midday temperature along with high vapor pressure deficit (VPD), in summer and co-occurring drought may reduce the photosynthetic activity via stomatal closure (Fig. 4c). On the other hand, if the rainfall increases and radiation decreases in early summer as predicted for East Asia, photosynthetic C gain will be reduced because of shortage in light (Fig. 4d).
Second, in low light regime, which is the case for understory plants such as seedlings/saplings, photosynthetic capacity may increase weakly or show no-response under warming (Fig. 4e, f). Here photosynthetic activity of understory plants may depend on the density and phenology of forest canopy that determine the light availability of those plants. In the case warming does not change forest canopy density, warming may be beneficial to understory plants if they can expand their leaves earlier than the forest canopy (e.g., Augspurger and Bartlett 2003; Taylor and Pearcy 1976; Vitasse 2013) (Fig. 4g). However, if the canopy density was low with thin layer or with several significant gaps, photosynthetic activity of understory plants may be reduced in summer (Fig. 4h) since sunflecks would cause stomatal closure (Fig. 4f), while sunflecks are in general beneficial to understory plants (Pearcy et al. 1994).
Our findings shown here suggest the necessity of further research focusing on the photosynthetic ‘activity’ and not only photosynthetic ‘capacity’ of seedlings and forest canopy throughout the phenological phases, by considering multiple micrometeorological and ecological factors which affect leaf physiological reactions. Especially for seedlings/saplings, various light, nutrient, and soil water regimes should be also considered since they must survive in a given habitat and would be more susceptible to changing environments than mature trees. In these studies, in situ measurements and modeling for canopy photosynthesis, and measurements of leaf photosynthesis and biomass allocation of seedlings/saplings under artificial warming conditions should be beneficial for our further ecophysiological understanding of plant response or vulnerability to warming.
Changes in herbivory in response to experimental warming
Most herbivorous insects are very sensitive to changes in temperature, which can directly affect their activity, development, phenology, and survival (Bale et al. 2002; Karban and Strauss 2004). In addition to the direct effects of temperature elevation, insect responses also reflect indirect effects of temperature through plant ecophysiological changes (e.g., leaf phenology and photosynthesis) (Masters et al. 1998). The previous sections have shown that future warmer temperatures are expected to influence metabolic processes related to photosynthetic activity and to advance the timing of leaf emergence in trees. These interactions between climate change, plant ecophysiological processes, and forest disturbance as a result of herbivory may be critical in determining how climate change affects evapotranspiration, CO2 flux, and heat transfer in forest ecosystems, thereby creating feedbacks to climate (Ayres and Lombardero 2000; Dale et al. 2000; Kurz et al. 2008; Logan et al. 2003). For example, one alarming scenario is that global warming-induced insect outbreaks would increase the incidence of forest fires and exacerbate global warming by releasing C from forest ecosystems (Ayres and Lombardero 2000). However, the impact of insects owing to global warming on ecosystem functions of forests is not well documented (but see Kurz et al. 2008). In this section, we discuss how plant ecophysiological processes may alter herbivory under global warming and review the response of herbivorous insects to the experimental warming of tree species.
Indirect effects through changes in host plant chemistry
Variation in the quality of plant leaves can have a significant impact on the performance of herbivorous insects (e.g., Karban and Baldwin 1997). However, little information currently exists regarding elevated temperature-induced changes in host plant chemistry and their potential effects on insect performance, even from laboratory experiments (Bidart-Bouzat and Imeh-Nathaniel 2008; Zvereva and Kozlov 2006).
Temperature may affect the primary and secondary metabolism of plants by altering photosynthetic activity, which will have implications for the development of herbivores (Ayres and Lombardero 2000; Veteli et al. 2002; Williams et al. 2003). It is well known that secondary metabolites (e.g., phenolics and tannins) play an important role in plant defense against herbivory (Bidart-Bouzat and Imeh-Nathaniel 2008), but elevated temperatures may dilute these secondary metabolites due to increased C allocation to plant growth. Indeed, a meta-analysis of laboratory studies showed that increased temperatures had no effect on either N concentration or the C/N ratio or leaf mechanical characteristics, but led to decreased concentrations of carbohydrates and phenolics (Zvereva and Kozlov 2006).
Few warming experiments have investigated the effects of temperature elevation on plant chemistry and/or herbivory of tree species. However, in one such study, it was found that willow (Salix myrsinifolia) seedlings that were warmed using closed-top chambers exhibited increased stem biomass and a reduced concentration of several phenolic compounds in their leaves (Veteli et al. 2002), suggesting that the increased C allocation to growth may have diluted the concentration of these phenolics; this temperature elevation also increased the growth rate of beetle larvae (Phratora vitellinae). In a separate study on maple (Acer rubrum) seedling leaves, elevated temperatures within OTCs reduced N and leaf water but did not affect phenolics in mature leaves (Williams et al. 2003); these temperature-induced changes in host plant quality had no effect on the performance of gypsy moth larvae (Lymantria dispar). These species-specific responses of plant chemistry under warming may lead to distinct responses of plant–insect interactions in forests.
Indirect effects through phenological asynchrony between herbivorous insects and plants
Synchronization of egg hatching and budbreak is believed to be an important determinant of defoliation levels in forests (Harrington et al. 1999; Hunter 1992). When interacting species respond in different ways to increased temperatures, plant–insect interactions tend to be disrupted (Parmesan 2006). Under climate change scenarios, there is likely to be increased asynchrony between host plants and herbivorous insects (Bale et al. 2002), and a change in the timing of egg hatching relative to budbreak could lead to a reduction in herbivory. To date, only one warming experiment on tree species has examined the phenological stages of both host plants and herbivorous insects. In this study, elevated temperatures using cloches during the summer season markedly advanced the phenological stages of both the dwarf shrub (Dryas octopetala) and aphids (Acyrthosiphon svalbardicum) by approximately 5 days, and led to an 11-fold increase in the number of aphid overwintering eggs (Strathdee et al. 1993). It was also shown that temperature elevation had larger direct effects than indirect effects on the herbivore population, as experimental warming did not increase phenological asynchrony, which did not support the predictions made by climate change scenarios. There remains a paucity of experimental data on the responses of herbivorous insects to future higher temperatures in forest ecosystems, with only four studies having investigated this to date (Richardson et al. 2002; Strathdee et al. 1993; Veteli et al. 2002; Williams et al. 2003). Therefore, to better understand the mechanisms of how forest ecosystem functions will respond via herbivorous insect populations to future higher temperatures, we should continue to focus on the indirect effects of temperature through plant ecophysiological processes in open-field warming experiments.
Changes in N cycling and plant growth in response to experimental warming
Plant production in most temperate forests is limited by N, and thus it is important to determine how future increase in temperature will alter N cycling in forests (Aber et al. 1989; Vitousek and Horwarth 1991). In the majority of the warming experiments reviewed, N mineralization increased under a warming treatment (Butler et al. 2012; Lükewille and Wright 1997; Melillo et al. 2011; Peterjohn et al. 1994). In Harvard forest, a mixed deciduous forest located in Massachusetts, USA, a soil warming treatment using heating cables increased the net N mineralization rates and ammonium concentrations (Peterjohn et al. 1994). Similarly, in a catchment of a Norwegian boreal forest, soil warming using heating cables increased N release by mineralization (Lükewille and Wright 1997). In contrast, no change in soil inorganic N was observed in an experiment conducted on the Tibetan Plateau, where spruce seedlings were warmed with IR lamps (Liu et al. 2011). In addition, decrease in mineral N during winter under soil warming treatment in a cool temperate forest was also reported (Ueda et al. 2013).
Increased rates of litter decomposition in forests under experimental warming are also consistent with the higher rates of N mineralization observed. For example, soil warming using heating cables induced an increase in mass loss of maple and spruce litter (Rustad and Fernandez 1998). In a northern hardwood forest, the percentage mass of beech litter in soil warmed by 7.5 °C was significantly lower than that in the control and soil warmed by 2.5 °C, and there was a greater decrease in N concentration of the beech litter as the heating level increased (McHale et al. 1998).
Increased N mineralization in forests at higher temperatures will result in more N being available for plants, which can support increased plant growth and alter plant allometry (Melillo et al. 2011). In the Harvard forest experiment, where higher N mineralization rates were observed after six months of soil warming (Peterjohn et al. 1994), additional seven years of warming treatment led to increased C storage in woody tissues (Melillo et al. 2011). Although the growing season was lengthened under the warming treatment, most of the plant C gain was attributed to higher N availability, because N is the major limiting factor for plant growth in this forest ecosystem. In addition, fine root biomass decreased under the warming treatment, probably also due to more N made available for plants because fewer C resources needed to be allocated to belowground parts to acquire N (Melillo et al. 2011).
Increased N availability in response to warming also has the potential to alter forest composition, since tree species have distinct strategies for acquiring N (Butler et al. 2012). Seven years of warming in Harvard forest induced a large increase in net N mineralization, with the greatest increase being observed in the foliar N content and relative growth rate of red maple (A. rubrum). Although the foliar N content of red oak (Q. rubra) and white ash (Fraxinus americana) also increased under the warming treatment, this increase was lower than that for red maple. These species-specific responses to increased N mineralization under warming may lead to changes in canopy dominance in the long term, which would gradually change forest functions such as photosynthesis. Thus, to better understand the interaction between plants and biogeochemical cycles in forest ecosystems, further investigations via open-field warming approach are needed.
Concluding remarks
In this paper, we reviewed current knowledge on the influence of increased temperature on plant phenology, leaf photosynthesis, leaf traits and herbivory, and N cycling and plant growth, mainly by analyzing open-field warming studies on tree species and forest ecosystems. Direct effects of higher temperature included (1) earlier emergence and later senescence of leaves which resulted in a longer growing season (2) increased abundance and enhanced performance of herbivorous insects and (3) accelerated soil N mineralization and leaf litter decomposition. As we mentioned above further experimental cases are still needed to achieve general understanding on the possible response of plants and forests to rising temperature.
For further understanding and prediction of plant ecophysiological and soil biogeochemical responses, several issues should be addressed in current and future research on the assessment of climate change impacts on terrestrial ecosystems (Rustad 2008; Saxe et al. 2001; Shaver et al. 2000). These issues include (1) the application of experimental knowledge to obtain a general understanding and prediction of ecological processes, (2) cross-scale or cross-hierarchy analyses and/or integration of the experimental results over space and time, and (3) identifying interactions between the physiological and biogeochemical processes, the responses of which occur across different time-scales for a given ecosystem (Fig. 5). For example, in the evaluation of photosynthetic and growth responses in plants, we should consider the in situ conditions of the habitats and the spatial position in the forests, depending on whether we are addressing questions on forest regeneration processes or forest canopy photosynthesis in C cycling. Experimental results from seedling experiments can be applied in answering the former question but not for the latter. This indicates that further research at forest-level must consider the ontogenetic difference of tree responses to environmental changes (Vitasse 2013). In the cases for herbivory and soil C and N cycles, high biodiversity and their ecological interaction throughout the seasons should be considered. As shown in Fig. 5 questions on mechanistic and quantitative consequences of plant ecophysiological and biogeochemical responses, together with biological interactions, should be tackled.
Representation of the reviewed influence of higher temperatures on the ecological components of forest ecosystems. Solid lines indicate positive (+) or negative (−) effects of temperature on the processes. The dashed line indicates a possible influence that was not reviewed in detail in this paper. Numerous studies have examined the direct effects of higher temperatures on individual processes, but their indirect effects on ecological and/or biogeochemical processes and their interactions in a given forest ecosystem must also be investigated to determine the dynamic influences of local and global warming
The ecophysiological processes involved in the above dynamics may not only respond to mean temperatures, but also to the range of temperatures, which are likely to provide the environmental cues for physiological and developmental activities. Consideration of such temperature regimes in the design of warming experiments will be critical for determining the “tipping point” of ecosystem functions and associated physiological processes, plant growth, species persistence and ecological interactions. To detect any signature changes in ecosystem functions and to provide mechanistic understanding on the changes due to recent and future climate change, combined studies of long-term observation and warming experiments should also be conducted.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM (1989) Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–386
Arend M, Kuster T, Günthardt-Goerg MS, Dobbertin M (2011) Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol 31:287–297
Aronson EL, McNulty SG (2009) Appropriate experimental ecosystem warming methods by ecosystem, objective, and practicality. Agric For Meteorol 149:1791–1799
Augspurger CK, Bartlett EA (2003) Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol 23:517–523
Ayres MP, Lombardero MJ (2000) Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci Total Environ 262:263–286
Badeck F-W, Bondreau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Responses of spring phenology to climate change. New Phytol 162:295–309
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8:1–16
Bergh J, Linder S (1999) Effects of soil warming during spring on photosynthetic recovery in boreal Norway spruce stands. Glob Change Biol 5:245–253
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Bidart-Bouzat MG, Imeh-Nathaniel A (2008) Global change effects on plant chemical defenses against insect herbivores. J Integr Plant Biol 50:1339–1354
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–1449
Bronson DR, Gower ST (2010) Ecosystem warming does not affect photosynthesis or aboveground autotrophic respiration for boreal black spruce. Tree Physiol 30:441–449
Bronson DR, Gower ST, Tanner M, van Herk I (2009) Effect of ecosystem warming on boreal black spruce bud burst and shoot growth. Glob Change Biol 15:1534–1543
Butler SM, Melillo JM, Johnson JE, Mohan J, Steudle PA, Lux H, Burrows E, Smith RM, Vario CL, Scott L, Hill TD, Aponte N, Bowles F (2012) Soil warming alters nitrogen cycling in a New England forest: implications for ecosystem function and structure. Oecologia 168:819–828
Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shifting plant phenology in response to global change. Trends Ecol Evol 22:357–365
Dale VH, Joyce LA, McNulty S, Neilson RP (2000) The interplay between climate change, forests, and disturbances. Sci Total Environ 262:201–204
Danby RK, Hik DS (2007) Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Glob Change Biol 13:437–451
Farnsworth EJ, Nunez-Farfan J, Careaga SA, Bazzaz FA (1995) Phenology and growth of three temperate forest life forms in response to artificial soil warming. J Ecol 83:967–977
Guak S, Olsyzk DM, Fuchigami LH, Tingey DT (1998) Effects of elevated CO2 and temperature on cold hardiness and spring bud burst and growth in Douglas-fir (Pseudotsuga menziesii). Tree Physiol 18:671–679
Gunderson CA, Norby RJ, Wullschleger SD (2000) Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiol 20:87–96
Gunderson CA, Edwards NT, Walker AV, O’Hara KH, Campion CM, Hanson PJ (2012) Forest phenology and a warmer climate—growing season extension in relation to climatic provenance. Glob Change Biol 18:2008–2025
Han C, Liu Q, Yang Y (2009) Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regul 58:153–162
Harrington R, Woiwod I, Sparks T (1999) Climate change and trophic interactions. Trends Ecol Evol 14:146–150
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Hunter MD (1992) A variable insect plant interaction—the relationship between tree budburst phenology and population levels of insect herbivores among trees. Ecol Entomol 17:91–95
Hyvönen R, Ågren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, Kellomäki S, Lindroth A, Loustau D, Lundmark T, Norby RJ, Oren R, Pilegaard K, Ryan MG, Sigurdsson BD, Strömgren M, Van Oijen M, Wallin G (2007) The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173:463–480
Ibanez I, Primack RB, Miller-Rushing AJ, Ellwood E, Higuchi H, Lee SD, Kobori H, Silander JA (2010) Forecasting phenology under global warming. Philos Trans R Soc Lond B 365:3247–3260
IPCC (Intergovernmental Panel on Climate Change) (2007) The physical sciences basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, London
Ito A (2010) Changing ecophysiological processes and carbon budget in East Asian ecosystems under near-future changes in climate: implications for long-term monitoring from a process-based model. J Plant Res 123:577–588
Jo W, Son Y, Chung H, Noh NJ, Yoon TK, Han S, Lee SJ, Lee SK, Yi K, Jin L (2011) Effect of artificial warming on chlorophyll contents and net photosynthetic rate of Quercus variabilis seedlings in an open-field experiment. J Korean For Soc 100:733–737 (in Korean with English abstract)
Karban R, Baldwin IT (1997) Induced responses to herbivory. The University of Chicago Press, Chicago
Karban R, Strauss SY (2004) Physiological tolerance, climate change, and a northward range shift in the spittlebug, Philaenus spumarius. Ecol Entomol 29:251–254
Kilpeläinen A, Peltola H, Rouvinen I, Kellomaki S (2006) Dynamics of daily height growth in Scots pine trees at elevated temperature and CO2. Trees-Struct Funct 20:16–27
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990
Lahti M, Apalo P, Finér L, Ryyppö A, Lehto T, Mannerkoski H (2005) Effects of soil temperature on shoot and root growth and nutrient uptake of 5-year-old Norway spruce seedlings. Tree Physiol 25:115–122
Linderholm HW (2006) Growing season changes in the last century. Agric For Meteorol 137:1–14
Liu Q, Yin H, Chen J, Zhao C, Cheng X, Wei Y, Lin B (2011) Belowground responses of Picea asperata seedlings to warming and nitrogen fertilization in the eastern Tibetan Plateau. Ecol Res 26:637–648
Logan JA, Régnière J, Powell JA (2003) Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ 1:130–137
Loik ME, Still CJ, Huxman TE, Harte J (2004) In situ photosynthetic freezing tolerance for plants exposed to a global warming manipulation in the Rocky Mountains, Colorado, USA. New Phytol 162:331–341
Lükewille A, Wright RF (1997) Experimentally increased soil temperature causes release of nitrogen at a boreal forest catchment in southern Norway. Glob Change Biol 3:13–21
Masters GJ, Brown VK, Clarke IP, Whittaker JB, Hollier JA (1998) Direct and indirect effects of climate change on insect herbivores: Auchenorrhyncha (Homoptera). Ecol Entomol 23:45–52
McHale PJ, Mitchell MJ, Bowles FP (1998) Soil warming in a northern hardwood forest: trace gas fluxes and leaf litter decomposition. Can J For Res 28:1365–1372
Mediavilla S, Escudero A (2003) Photosynthetic capacity, integrated over the lifetime of a leaf, is predicted to be independent of leaf longevity in some tree species. New Phytol 159:203–211
Melillo JM, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L, Vario C, Hill T, Burton A, Zhou Y, Tang J (2011) Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA 108:9508–9512
Menzel A, Estrella N, Testka A (2005) Temperature response rates from long-term phenological records. Clim Res 30:21–28
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-kübler K, Bissolli P, Braslavská O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl A, Defila C, Donnelly A, Filella Y, Jatczak K, Mage F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remišová V, Scheifinger H, Striz M, Susnik A, Van vliet AJH, Wielgolaski F-E, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976
Morin X, Roy J, Sonié L, Chuine I (2010) Changes in leaf phenology of three European oak species in response to experimental climate change. New Phytol 186:900–910
Muraoka H, Tang Y, Terashima I, Koizumi H, Washitani I (2000) Contributions of diffusional limitation, photoinhibition and photorespiration to the midday depression of photosynthesis in Arisaema heterophyllum in the natural high light. Plant, Cell Environ 23:235–250
Muraoka H, Saigusa N, Nasahara KN, Noda H, Yoshino J, Saitoh TM, Nagai S, Murayama S, Koizumi H (2010) Effects of seasonal and interannual variation in leaf photosynthesis and canopy leaf area index on gross primary production in a cool-temperate deciduous broadleaf forest in Takayama, Japan. J Plant Res 123:563–576
Nakamura M, Muller O, Tayanagi S, Nakaji T, Hiura T (2010) Experimental branch warming alters tall tree leaf phenology and acorn production. Agric For Meteorol 150:1026–1029
Nicotra AB, Atkin OK, Bonser SP et al (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692
Norby RJ, Hartz-Rubin JS, Verbrugge MJ (2003) Phenological responses in maple to experimental atmospheric warming and CO2 enrichment. Glob Change Biol 9:1792–1801
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Pearcy RW, Chazdon RL, Gross LJ, Mott KA (1994) Photosynthetic utilization of sunflecks: a temporally patchy resource on a time scale of seconds to minutes. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants. Academic Press, San Diego, pp 175–204
Pearcy RW, Muraoka H, Valladares F (2005) Crown architecture in sun and shade environments: assessing function and tradeoffs with a 3-D simulation model. New Phytol 166:791–800
Peterjohn WT, Melillo JM, Bowles FP, Steudler PA (1993) Soil warming and trace gas fluxes: experimental design and preliminary flux results. Oecologia 93:18–24
Peterjohn WT, Melillo JM, Steudler PA, Newkirk KM, Bowles FP, Aber JD (1994) Responses of trace gas fluxes and N availability to experimentally elevated soil temperatures. Ecol Appl 4:617–625
Polgar CA, Primack RB (2011) Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytol 191:926–941
Rastetter EB, Ryan MG, Shaver GR, Melillo JM, Nadelhoffer KJ, Hobbie JE, Aber JD (1991) A general biogeochemical model describing the responses of the C and N cycles in terrestrial ecosystems to changes in CO2, climate, and N deposition. Tree Physiol 9:101–126
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reichstein M, Ciais P, Papale D, Valentini R, Running S, Viovy N, Cramer W, Granier A, Ogée J, Allard V, Aubinet M, Bernhofer C, Buchmann N, Carrara A, Grünwald T, Heimann M, Heinesch B, Knohl A, Kutsch W, Loustau D, Manca G, Matteucci G, Miglietta F, Ourcival JM, Pilegaard K, Pumpanen J, Rambal S, Schaphoff S, Seufert G, Soussana JF, Sanz MJ, Vesala T, Zhao M (2006) Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: a joint flux tower, remote sensing and modelling analysis. Glob Change Biol 12:1–18
Repo T, Hänninen H, Kellomaki S (1996) The effects of long-term elevation of air temperature and CO2 on the frost hardiness of Scots pine. Plant Cell Environ 19:209–216
Richardson SJ, Press MC, Parsons AN, Hartley SE (2002) How do nutrients and warming impact on plant communities and their insect herbivores? A 9-year study from a sub-Arctic heath. J Ecol 90:544–556
Richardson AD, Black TA, Ciais P, Delbart N, Friedl MA, Gobron N, Hollinger DY, Kutsch WL, Longdoz B, Luyssaert S, Migliavacca M, Montagnani L, Munger JW, Moors E, Piao SL, Rebmann C, Reichstein M, Saigusa N, Tomelleri E, Vargas R, Varlagin A (2010) Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos Trans R Soc Lond B 365:3227–3246
Rustad LE (2008) The response of terrestrial ecosystems to global climate change: towards an integrated approach. Sci Total Environ 404:222–235
Rustad LE, Fernandez IJ (1998) Soil warming: consequences for foliar litter decay in a spruce-fir forest in Maine, USA. Soil Sci Soc Am J 62:1072–1080
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J, GCTE-NEWS (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Saigusa N, Yamamoto S, Hirata R, Ohtani Y, Ide R, Asanuma J, Gamo M, Hirano T, Kondo H, Kosugi Y, Li SG, Nakai Y, Takagi K, Tani M, Wang H (2008) Temporal and spatial variations in the seasonal patterns of CO2 flux in boreal, temperate, and tropical forests in East Asia. Agric For Meteorol 148:700–713
Saxe H, Cannell MGR, Johnsen Ø, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytol 149:369–400
Schlesinger (1997) Biogeochemistry: an analysis of global change. Academic Press, San Diego
Schwartz MD (2003) Phenology: an integrative environmental science. Springer, Dordrecht
Shaver GR, Canadell J, Chapin FS, Gurevitch J, Harte J, Henry G, Ineson P, Jonasson S, Melillo J, Pitelka L, Rustad L (2000) Global warming and terrestrial ecosystems: a conceptual framework for analysis. Bioscience 50:871–882
Slaney M, Wallin G, Medhurst J, Linder S (2007) Impact of elevated carbon dioxide concentration and temperature on bud burst and shoot growth of boreal Norway spruce. Tree Physiol 27:301–312
Spano D, Cesaraccio C, Duce P, Snyder RL (1999) Phenological stages of natural species and their use as climate indicators. Int J Biometeorol 42:124–133
Sparks TH, Menzel A (2002) Observed changes in seasons: an overview. Int J Climatol 22:1715–1725
Strand M, Lundmark T (1995) Recovery of photosynthesis in 1-year-old needles of unfertilized and fertilized Norway spruce (Picea abies (L.) Karst.) during spring. Tree Physiol 15:151–158
Strathdee AT, Bale JS, Block WC, Coulson SJ, Hodkinson ID, Webb NR (1993) Effects of temperature elevation on a field population of Acyrthosiphon svalbardicum (Hemiptera: Aphididae) on Spitsbergen. Oecologia 96:457–465
Taylor RJ, Pearcy RW (1976) Seasonal patterns of the CO2 exchange characteristics of understory plants from a deciduous forest. Can J Bot 54:1094–1103
Totland O, Alatalo JM (2002) Effects of temperature and date of snowmelt on growth, reproduction, and flowering phenology in the arctic/alpine herb, Ranunculus glacialis. Oecologia 133:168–175
Ueda MU, Muller O, Nakamura M, Nakaji T, Hiura T (2013) Soil warming decreases inorganic and dissolved organic nitrogen pools by preventing the soil from freezing in a cool temperate forest. Soil Biol Biochem 61:105–108
Valladares F, Pearcy RW (1997) Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ 20:25–36
Veteli TO, Kuokkanen K, Julkunen-Tiitto R, Roininen H, Tahvanainen J (2002) Effects of elevated CO2 and temperature on plant growth and herbivore defensive chemistry. Glob Change Biol 8:1240–1252
Vitasse Y (2013) Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytol 198:149–155
Vitasse Y, Bresson CC, Kremer A, Michalet R, Delzon S (2010) Quantifying phenological plasticity to temperature in two temperate tree species. Funct Ecol 24:1211–1218
Vitousek PM, Horwarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J, Hoegh-Guldber O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wang K, Kellomäki S, Laitinen K (1995) Effects of needle age, long-term temperature and CO2 treatments on the photosynthesis of Scots pine. Tree Physiol 15:211–218
Way DA (2011) Tree phenology responses to warming: spring forward, fall back? Tree Physiol 31:469–471
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Welp LR, Randerson JT, Liu HP (2007) The sensitivity of carbon fluxes to spring warming and summer drought depends on plant functional type in boreal forest ecosystems. Agric For Meteorol 147:172–185
Wertin TM, McGuire MA, Teskey RO (2011) Higher growth temperatures decreased net carbon assimilation and biomass accumulation of northern red oak seedlings near the southern limit of the species range. Tree Physiol 31:1277–1288
Williams RS, Lincoln DE, Norby RJ (2003) Development of gypsy moth larvae feeding on red maple saplings at elevated CO2 and temperature. Oecologia 137:114–122
Wolkovich EM, Cook BI, Allen JM, Crimmins TM, Betancourt JL, Travers SE, Pau S, Regetz J, Davies TJ, Kraft NJB, Ault TR, Bolmgren K, Mazer SJ, McCabe GJ, McGill BJ, Parmesan C, Salamin N, Schwartz MD, Cleland EE (2012) Warming experiments underpredict plant phenological responses to climate change. Nature 485:494–497
Wookey PA, Parsons AN, Welker JM, Potter JA, Callaghan TV, Lee JA, Press MC (1993) Comparative responses of phenology and reproductive development to simulated environmental change in sub-arctic and high arctic plants. Oikos 67:490–502
Wu Z, Dijkstra P, Koch GW, Penuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Change Biol 17:927–942
Xu Z, Hu T, Zhang Y (2012) Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China. Eur J For Res 131:811–819
Yin HJ, Liu Q, Lai T (2008) Warming effects on growth and physiology in the seedlings of the two conifers Picea asperata and Abies faxoniana under two contrasting light conditions. Ecol Res 23:459–469
Zhao CZ, Liu Q (2009) Growth and physiological responses of Picea asperata seedlings to elevated temperature and to nitrogen fertilization. Acta Physiol Plant 31:163–173
Zvereva EL, Kozlov MV (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Glob Change Biol 12:27–41
Acknowledgments
This work was supported by the “National Research Foundation of Korea (2010-0014620)”, “Korea Forest Service (S111112L030100)”, “Korea University (2013)”, “JSPS Funding Program for Next Generation World-Leading Researchers (NEXT Program)”, “JSPS-NRF-NSFC A3 Foresight Program”, and the Environment Research and Technology Development Fund (S-9-3) and Global Environment Research Fund (D-0909) of the Ministry of the Environment, Japan. HM thanks Taku M. Saitoh (Gifu University) for information of predicted changes in temperature and precipitation in central Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was contributed at the invitation of the Editorial Committee.
Rights and permissions
About this article
Cite this article
Chung, H., Muraoka, H., Nakamura, M. et al. Experimental warming studies on tree species and forest ecosystems: a literature review. J Plant Res 126, 447–460 (2013). https://doi.org/10.1007/s10265-013-0565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-013-0565-3