Abstract
Using a process-based model, I assessed how ecophysiological processes would respond to near-future global changes predicted by coupled atmosphere–ocean climate models. An ecosystem model, Vegetation Integrative SImulator for Trace gases (VISIT), was applied to four sites in East Asia (different types of forest in Takayama, Tomakomai, and Fujiyoshida, Japan, and an Alpine grassland in Qinghai, China) where observational flux data are available for model calibration. The climate models predicted +1–3°C warming and slight change in annual precipitation by 2050 as a result of an increase in atmospheric CO2. Gross primary production (GPP) was estimated to increase substantially at each site because of improved efficiency in the use of water and radiation. Although increased respiration partly offset the GPP increase, the simulation showed that these ecosystems would act as net carbon sinks independent of disturbance-induced uptake for recovery. However, the carbon budget response relied strongly on nitrogen availability, such that photosynthetic down-regulation resulting from leaf nitrogen dilution largely decreased GPP. In relation to long-term monitoring, these results indicate that the impacts of global warming may be more evident in gross fluxes (e.g., photosynthesis and respiration) than in the net CO2 budget, because changes in these fluxes offset each other.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ongoing global environmental changes, such as accumulation of atmospheric CO2 and climate change, are expected to affect the structure and function of terrestrial ecosystems [Intergovernmental Panel on Climate Change (IPCC) 2007]. Understanding the plant and soil response to such changes is necessary, because ecosystems provide invaluable services to human society and can ameliorate or accelerate anthropogenic climate change (Foley et al. 2003). Predicting near-future (e.g., by the middle of this century) ecosystem response is especially important for planning measures of adaptation and conservation.
Many previous studies have explored ecological, physiological, and biogeochemical mechanisms of ecosystem responses to future global changes, particularly in terms of the carbon budget. Photosynthetic response to elevated atmospheric CO2 concentration (i.e., the CO2 fertilization effect) has been one of the most intensively studied processes. At the single leaf level, mechanistic models (e.g., Farquhar et al. 1980; Field et al. 1995) have described the short-term response of photosynthesis and stomatal conductance. Laboratory and free-air CO2 enrichment (FACE) experiments have revealed that the magnitudes of the CO2 fertilization effect differ among plant functional types, and that the initial response can decrease through acclimation or down-regulation (Hikosaka et al. 2005; Norby et al. 2005; Ainsworth and Rogers 2007). In particular, growth limitation by nutrients such as nitrogen is expected to become more evident under a higher CO2 environment. Plant and microbial respiration will also be affected by global environmental changes in response to increased temperature and chemical changes in substrates (Amthor 1991; Ryan 1991; Hamilton et al. 2001). Climate warming will affect phenological processes such as leaf display and shedding of deciduous forests and grasslands, leading to longer growing periods for temperate and boreal ecosystems (e.g., Badeck et al. 2004; but see Piao et al. 2008). These ecophysiological responses may propagate to larger scales such as plant demography, the food web (including animals), the ecosystem carbon budget, and biodiversity. Indeed, many experimental and monitoring studies have implied that climate change can affect the terrestrial ecosystem (e.g., Woodward et al. 1991; Long et al. 2004; Parmesan 2006). However, the response of physiological and ecological processes to global environmental change is still not well understood, especially in terms of the long-term response to variations in multiple factors. To integrate the responses of ecophysiological processes into the ecosystem level, several mechanistic ecosystem models have been developed to simulate the ecosystem response to elevated CO2 concentrations and other aspects of climate change (e.g., Oikawa 1986; Sitch et al. 2003; Luo et al. 2008). Such models effectively allow us to make long-term projections and to separate the complicated impacts of multiple factors by means of sensitivity analysis, although the results must be interpreted with care because of insufficient model constraints and mechanistic understanding, as well as a lack of validation with observational data.
This study assesses the impacts of near-future climate changes on the carbon budget of East Asian terrestrial ecosystems, using a process-based model that captures ecosystem structure and ecophysiological processes. Because the Asian region has characteristic ecosystems under a monsoon climate (Kira 1991) and makes considerable contributions to the global carbon cycle and climate system, many studies have addressed the contemporary carbon budget of this region (e.g., Kato and Tang 2008; Saigusa et al. 2008; Piao et al. 2009; Ichii et al. 2009). However, few studies have explored the response of plant and soil processes to future environmental changes in this region. In this study, I apply a model to estimate ecophysiological processes and the carbon budget of East Asian terrestrial ecosystems using model-projected scenarios of the near-future (through 2050) rising level of atmospheric CO2 and climate change. This type of projection may be useful for future ecosystem impact assessment, management for mitigation, and adaptation to climate change. Based on the simulation results, I discuss collaboration between observational monitoring and modeling.
Method
Site description
Four sites with representative East Asian biomes were studies (Fig. 1). Three of the sites are in Japan: Takayama (TKY), with a cool-temperate deciduous broad-leaved forest; Tomakomai (TMK), with a deciduous coniferous forest; and Fujiyoshida (FJY), with a warm-temperate mixed evergreen conifer forest. The fourth site is an alpine grassland in Qinghai-Haibei (QHB), China. A summary of site characteristics is presented in Table 1. These sites cover the spectrum of climate conditions and biome diversity in the East Asian region, so that geographical heterogeneity in ecophysiological responses under different conditions can be retrieved. The four sites have observational towers for micrometeorological flux measurements by the eddy-covariance method (e.g., Hirata et al. 2008), and ecosystem models have been calibrated to CO2 flux data at each site: TKY by Saigusa et al. (2005), TMK by Hirata et al. (2007), FJY by Ohtani et al. (2005), and QHB by Kato et al. (2006). Also, plant ecophysiological parameters such as maximum photosynthesis rate are available from field studies: TKY by Muraoka and Koizumi (2005) and TMK by Kitao et al. (2005). The sites have undergone different disturbance regimes. The forests at TKY and FJY were logged in the 1960s and 1910s, respectively, the TMK larch forest is an artificial forest established in the 1950s, and the alpine grassland at the QHB site has been utilized as a meadow for livestock such as yak and sheep.
Model description
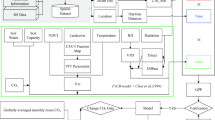
A process-based model of the terrestrial ecosystem, Vegetation Integrative SImulator for Trace gases (VISIT), was adopted in this study. As demonstrated in Ito (2008), comparisons with the previously mentioned observations by the eddy-covariance method indicate that VISIT captured CO2 flux appropriately: at TKY for 1999–2006 (r = 0.67, RMSE = 1.09 g C m−2 day−1), at TMK for 2001–2003 (r = 0.74, RMSE = 1.31 g C m−2 day−1), at FJY for 2000–2004 (r = 0.56, RMSE = 1.05 g C m−2 day−1), and at QHB for 2001–2004 (r = 0.70, RMSE = 0.80 g C m−2 day−1). A brief description of the model is given below; see Ito and Oikawa (2002) and Ito et al. (2007) for a more detailed description, including equations and parameters.
The model was developed on the basis of a simple model (Sim-CYCLE: Ito and Oikawa 2002), and it simulates carbon and nitrogen cycles in an ecophysiological manner. The ecosystem carbon stock is separated into four sectors: overstory woody plants, understory herbaceous plants, dead biomass (litter), and mineral soil (humus). These carbon stock components are divided into sub-compartments that have different physiological functions (e.g., leaf, stem, and root) and turnover times (e.g., active to passive humus). Net ecosystem production (NEP) is calculated as the difference between gross primary production (GPP) and ecosystem respiration (ER) at a daily time-step (in g C m−2 year−1 or Mg C ha−1 year−1).
In VISIT, GPP is a function of the leaf area index (LAI), incident photosynthetically active radiation (PAR), air temperature and humidity, soil water content, and ambient CO2 concentration. A two-component canopy model by de Pury and Farquhar (1997) was adopted, in which absorption of beam and diffuse radiation and conversion to carbon assimilation are calculated separately. In the canopy scheme, leaf photosynthetic capacity (maximum carboxylation rate) is assumed to be proportional to leaf nitrogen concentration, and the vertical gradient of leaf nitrogen within the canopy is considered using exponential approximation. LAI is predicted by leaf carbon amount and specific leaf area, which is a biome-specific parameter. GPP responds to an increase in temperature through three processes: (1) a decrease in photosynthetic quantum yield, (2) an increase in light-saturated photosynthetic rate unless it is excessively hot owing to increase of photorespiration, and (3) a decrease in stomatal gas conductance through elevated vapor pressure deficit of ambient air. VISIT includes a semi-empirical model of stomatal conductance by Leuning (1995). Additionally, for deciduous forests and grasslands, elevated temperatures result in earlier leaf display and later leaf shedding, which leads to longer growing periods. GPP also responds to elevated concentrations of atmospheric CO2 through four processes: (1) an increase in photosynthetic quantum yield, (2) an increase in light-saturated photosynthetic rate, (3) an increase in optimal photosynthetic temperature, and (4) a decrease in stomatal gas conductance. ER is composed of plant autotrophic respiration and soil microbial heterotrophic respiration. The difference between GPP and autotrophic respiration is termed net primary production (NPP), and it indicates the amount of useful carbon resource. Autotrophic respiration rate responds to temperature, directly for maintenance respiration and indirectly for growth respiration via the response to carbon acquisition by GPP. The heterotrophic respiration rate increases exponentially with rising temperature, as parameterized by Lloyd and Taylor (1994). Furthermore, elevated temperatures can indirectly affect photosynthesis and decomposition rates via soil water content, which is affected by the evapotranspiration rate.
Allocation of assimilated carbon to plant organs is determined on the basis of theoretically optimum LAI (Monsi and Saeki 2005) and the allometric relationship between aboveground and belowground standing biomass. Elevated CO2 concentration instantaneously increases carbon allocation to leaf production and then expands LAI, unless other environmental factors have a stronger effect in the opposite direction. Photosynthetic down-regulation under elevated CO2 concentration is not explicitly included in the reference model. Mortality was assumed to be specific to plant organs and independent of environmental conditions. Leaf phenology of deciduous forests and grasslands is determined using cumulative temperature (i.e., growing degree-days) above a critical temperature. In this study, leaf display occurs when cumulative temperature above 1°C exceeds +110°C to 280°C days. Leaf shedding occurs when cumulative temperature below 12°C exceeds −20°C to 50°C days. I assume that no acclimation occur in the leaf phenology regime as a result of long-term climate change.
Simulation analysis
A model simulation requires driving variables such as atmospheric CO2 concentration and climate conditions at daily time-steps, including downward shortwave radiation, cloudiness, air temperature, air specific humidity, precipitation, soil temperature, and wind velocity. Daily PAR and its beam/diffuse fraction are estimated from downward shortwave radiation and cloudiness by using empirical equations (Ito and Oikawa 2006). The model simulation is carried out in three phases: spinning-up, historical run, and prediction. In the spinning-up phase, an equilibrium carbon budget state is obtained through repeated calculations (2,000 years) under current atmospheric conditions: i.e., ambient CO2 concentration was fixed to 306 ppmv (parts per million in volume)—the level in 1947. Next, the historical simulation is conducted for the period from 1 January 1948 to 31 December 2007 using time-series climate data from the United States National Centers for Environmental Prediction and the United States National Center for Atmospheric Research (NCEP/NCAR; Kistler et al. 2001). In parallel, ambient CO2 concentration was raised to 384 ppmv by 2007. Then, the prediction simulation is conducted for the period from 1 January 2008 to 31 December 2050 using climate projection scenarios estimated by atmosphere–ocean coupled global climate models. Taking account of uncertainty in the climate predictions, this study used daily climate projections from three climate models: (1) a high-resolution version of MIROC by the Center for Climate System Research, University of Tokyo and the National Institute for Environmental Studies, Japan (Emori et al. 2005); (2) CCCma by the Canadian Center for Climate Modeling and Analysis (Boer et al. 2004); and (3) ECHAM by the Max-Planck Institute for Meteorology, Germany (Mikolajewicz et al. 2007). These climate models were driven by the anthropogenic greenhouse gas emission scenarios presented by the IPCC. Climate projections based on the standard scenario, that is, A1B by the IPCC Special Report on Emission Scenarios [SRES; Intergovernmental Panel on Climate Change (IPCC) 2000], was used to indicate the uncertainty among climate models. Additionally, for the CCCma model, climate projections based on different IPCC emission scenarios were available: the SRES A2 scenario, which provides a higher increase in atmospheric CO2; and the SRES B2 scenario, which provides a lower one. Under these scenarios, the concentration of atmospheric CO2 would increase to 475–525 ppmv by 2050. Within this short prediction period (i.e., 43 years), ecophysiological processes are expected to dominate the ecosystem response; in other words, demographic changes such as species compositional change may not be a major factor, except for human land-use and rapid invasion of herbaceous species.
Figure 2 shows the trajectories of the annual temperature and precipitation changes estimated by the climate models at the four study sites, obtained by linear interpolation from the four nearest climate model grid points. To avoid discontinuity between historical NCEP/NCAR climate data and predicted climate values, deviation components of the future climate (Fig. 2) were overlaid on the average value of the historical climate. Each model predicts that annual mean temperature would increase by +1 to +3°C by 2050, but no clear trend was found for precipitation. Differences among the climate models are evident at TKY, FJY, and QHB: the MIROC climate model estimated higher rates of temperature increase than other models after 2030. On the other hand, differences among emission scenarios were less evident for the CCCma projections. Thus, the simulations using these climate scenarios allow examination of the range of uncertainty in model-based projections. To separate the effect of elevated CO2 from climate change, an additional prediction simulation was conducted assuming a stationary atmospheric CO2 concentration (380 ppmv) for the period from 2008 to 2050.
Annual mean temperature and precipitation projections at the four study sites, estimated by three climate models (MIROC high-resolution, ECHAM, and CCCma) for the 1980–2050 period using the IPCC SRES A1B emission scenario. For the CCCma climate model, projections using the SRES A2 and B1 scenarios are also shown
The focus was on the response of ecophysiological processes in relation to the ecosystem carbon budget, including the NPP/GPP ratio (i.e., carbon use efficiency), leaf area expansion, leaf phenology and growing period, water-use efficiency (WUE, g C kg−1 H2O), and radiation-use efficiency (RUE, mol C mol−1 photon). WUE and RUE are defined as follows:
where GPP (g C m−2 year−1 or mol C m−2 year−1) is the photosynthetic CO2 assimilation rate, TR (kg H2O m−2 year−1) is the transpiration rate estimated in the water-budget scheme of VISIT, and APAR (mol photon m−2 year−1) is the absorbed PAR. An additional simulation was conducted to specify the effect of nitrogen limitation (e.g., Kitao et al. 2005; Ollinger et al. 2008), which can cause down-regulation of plant growth response. Namely, the total amount of canopy nitrogen was fixed to the current level (average for 1998–2007), simulating severe nitrogen limitation. In this case, leaf area expansion caused by the CO2 fertilization effect will result in a decrease in leaf nitrogen concentration and in photosynthetic capacity (i.e., dilution). For clarity, the nitrogen limitation effect on respiration was not included. The range between the control (no nitrogen limitation) and the severe nitrogen limitation simulations is expected to indicate the possible magnitude of actual ecosystem response.
Results and discussion
Contemporary state
Model simulations using historical climate data captured the contemporary ecophysiological processes and carbon budget at each study site. Gross primary production at the TKY, TMK, FJY, and QHB sites during the 1998–2007 period was estimated to be 13.3, 15.8, 16.8, and 11.0 Mg C ha−1 year−1, respectively. Plant autotrophic respiration consumed 40% (QHB) to 45% (TKY, TMK, FJY) of GPP. Because of the past disturbance at the forest sites and climate change, these ecosystems were estimated as a net carbon sink by 3.1 ± 0.5 (TKY), 2.8 ± 0.6 (TMK), 2.1 ± 0.8 (FJY), and 1.7 ± 0.8 (QHB) Mg C ha−1 year−1 (average ± standard deviation of interannual variability). The deciduous forests (TKY and TMK) showed clear seasonal changes in LAI and CO2 fluxes.
Response to future climate changes: carbon budget
Along with the near-future climate changes (Fig. 2) and a rise in atmospheric CO2 levels, the carbon budget of East Asian ecosystems was estimated to change gradually (Fig. 3). At the cool-temperate deciduous forest (TKY), annual GPP increased by 6–9 Mg C ha−1 year−1 dependent on climate scenarios, from the present (1998–2007) level to the 2040s. As a result of a longer growing period and a higher peak LAI, APAR increased from 5,089 mol photon m−2 year−1 in the present to 6,728 mol photon m−2 year−1 in the 2040s. Annual NEP also increased but to a lesser extent (1.2–2.3 Mg C ha−1 year−1), because increased ecosystem respiration largely consumed the increased carbon assimilation. At the deciduous conifer forest (TMK), annual GPP increased by 4.7–11.3 Mg C ha−1 year−1, with a wide variability among the climate projection scenarios, especially after 2030. NEP increased by only 0.16 Mg C ha−1 year−1 in the case of the MIROC climate projection, whereas a large NEP increase of 3.0 Mg C ha−1 year−1 was estimated using the CCCma-A1B climate projection. The warm-temperate evergreen conifer forest (FJY) had the highest current GPP, and the CO2 assimilation rate was estimated to increase by 8.5–13.7 Mg C ha−1 year−1. However, NEP did not increase substantially (0.2–1.7 Mg C ha−1 year−1), primarily as a result of increased levels of respiratory emissions under the warmer climate. It seems that the carbon budget of the evergreen forest was most greatly affected by the increased respiration rates. At the alpine meadow (QHB) site, where temperature and water conditions are the most severely limiting (Table 1), annual GPP was estimated to increase by 9.3–16.3 Mg C ha−1 year−1; the largest response was simulated using the MIROC climate projection, which gave higher temperature increases (Fig. 2). As a consequence, annual NEP increased by 1.3–2.9 Mg C ha−1 year−1. Differences in the estimated GPP and NEP values among the emission scenarios (SRES A1B, A2, and B1 for the CCCma climate model) were not evident, even though these scenarios differ in atmospheric CO2 concentration by 50 ppmv at the end of simulation period. The study sites are all in a humid monsoonal climate, so no clear response to precipitation change was found.
CO2 fertilization effect
To clarify the CO2 fertilization effect, contemporary climate conditions were applied to the future projections. For example, GPP at the TKY site increased by 3.9 Mg C ha−1 year−1 from the 1990s to the 2040s as a result of the CO2 fertilization effect, or 43–67% of total change, including climate impact. Canopy photosynthesis by shaded leaves was more responsive to future environmental changes, because LAI expansion increased CO2 assimilation by shaded leaves. The CO2 fertilization effect accounted for only 29% of the total GPP increase at the QHB site under the MIROC-A1B climate scenario, and climatic warming exerted a stronger influence. At the other sites, about half of the GPP increase was attributable to the CO2 fertilization effect. At the TKY site, GPP by sunny and shaded leaves increased by 1.5 (30%) and 3.0 (41%) Mg C ha−1 year−1, respectively. Alternatively, as a result of increased shading by overstory trees, photosynthesis of understory herbaceous plants decreased from 1.7 to 1.2 Mg C ha−1 year−1. Hence, the large increase in GPP was attributable to both the CO2 fertilization effect and climate impacts on the leaf-level response of photosynthetic gas exchange and canopy-level response of LAI expansion.
Phenological changes under global change
Projected near-future climate changes affected the seasonal pattern of ecosystem processes. Figure 4 shows average annual seasonal changes in GPP and ER at the TKY site for the 1998–2007 and 2041–2050 periods as a typical example. Under the warmer climate, spring leaf display occurred 25 days earlier and autumn leaf shedding occurred 20 days later, leading to a longer growing period. Peak photosynthetic rate also increased with elevated CO2 concentrations; the peak LAI of canopy trees increased from 4.6 to 8.5. A slight decline in GPP from day of year (DOY) 150–200 was caused by a decline in solar radiation during the rainy season. ER for 2041–2050 increased throughout the year compared to 1990–1999, because of the temperature rise and biomass accumulation. The increased ER in mid-summer to autumn (DOY 210–280) resulted in a suppression of net carbon uptake in this period.
Changes in resource-use efficiencies
As a result of elevated CO2 and climate changes, plant resource-use efficiencies were improved. Figures 5 and 6 compare GPP, TR, ARAP, WUE, and RUE at the four sites between the 1990s and the 2040s. WUE increased by +2.5 to +7 g C kg−1 H2O, because water loss by TR did not increase at the same rate as GPP (Fig. 5b: by only 5–10%). The WUE improvement was especially clear at the QHB site, primarily as a result of considerable GPP increase and a lower TR (Fig. 5b: 150–185 mm year−1). Note that, in VISIT, stomatal conductance decreases as atmospheric CO2 concentration increases (Leuning 1995). RUE increased by about +0.005 mol C mol−1 photon; at each site, APAR increased by 1,103–3,513 mol photon m−2 year−1 (Fig. 5c). As exemplified in Fig. 7, the increase of GPP was attributable to increased APAR due to LAI expansion and improved RUE due to instantaneous CO2 fertilization effect. These results imply that East Asian ecosystems would utilize resources more efficiently in terms of production under the predicted near-future climate change. On the other hand, the NPP/GPP ratio, an index of plant carbon use efficiency, of the study sites was not affected by environmental change during the experimental period.
Potential effect of down-regulation
An additional simulation, in which the total amount of canopy nitrogen was fixed at the current level, showed the importance of nitrogen limitation for future projections of ecophysiology and the carbon budget. As a result of nitrogen dilution caused by LAI expansion, leaf-level photosynthetic capacity was reduced such that total GPP did not increase despite elevated levels of atmospheric CO2 and climate warming (Fig. 8). This result seems consistent with the experimental outcome of Hirose et al. (1997), which showed that optimal LAI for maximizing carbon gain might not increase under elevated CO2 levels when nitrogen availability for plants is limited. Because the control simulation assumed a conservative leaf nitrogen concentration (70–80 mmol m−2 at the canopy-top), it showed little down-regulation or acclimation resulting from nitrogen limitation. Similar qualitative results were obtained from simulations at the other three sites. This result implies a problem in long-term prediction based on short-term experimental findings; that is, ecosystem response can be largely overestimated. Therefore, we should acknowledge that the two simulations represent extreme cases, i.e., complete adaptation and strong down-regulation, and the results indicate the possible range of ecophysiological responses. In reality, many leaf properties such as respiration, thickness, and longevity, and allocation can also change under elevated CO2 concentration (e.g., Long et al. 2004); such an extensive analysis remains to be addressed in a future study.
Simulated annual GPP at the Tomakomai (TMK) site, with and without photosynthetic down-regulation resulting from leaf nitrogen dilution. In the case of diluted canopy N, the total nitrogen in the canopy leaves was fixed, and the expansion in leaf area induced by the CO2 fertilization effect results in nitrogen dilution
Concluding remarks
The model simulations conducted in this study imply that substantial impacts may occur in the carbon budget of East Asian terrestrial ecosystems as a result of near-future increases in atmospheric CO2 levels and climate change. Such considerable changes in the ecosystem carbon budget should be taken into account for planning ecosystem management for mitigation of climatic change, because forest carbon sequestration is included in the national greenhouse gas inventory (e.g., the Kyoto Protocol). For example, forest carbon stock or cumulative NEP was estimated to change at decadal time scale, e.g., between the periods 2008–2012 (the first commitment period of the Kyoto Protocol) and 2018–2022. At a young cool-temperate forest of the TKY site, cumulative NEP increased substantially from 17–20 Mg C ha−1 for 2008–2012 to 20–24 Mg C ha−1 for 2018–2022. In contrast, at a semi-mature warm-temperate forest of FJY, cumulative NEP did not always increase: 10–18 Mg C ha−1 for 2008–2012 to 11–17 Mg C ha−1 for 2018–2022. There remain, however, uncertainties in our understanding of the long-term response and in the results of the model simulations. For example, it is still difficult to estimate how much the down-regulation would occur in various ecophysiological processes such as respiration and allocation, which have complicated interactions within ecosystems. Other environmental changes such as an increasing amount of anthropogenic nitrogen deposition in East Asia may have local and ancillary impacts on plant and soil processes.
It is expected that these simulation results using observation-calibrated model and multiple scenarios carry robust implications for long-term monitoring of terrestrial ecosystems. Indeed, although several model studies investigated ecophysiological responses to elevated CO2 and climate change (e.g., McMurtrie and Wang 1993), few have provided future perspectives on long-term ecosystem monitoring. The perspectives presented in this study may strengthen motivation to continue monitoring at flux measurement sites. First, GPP time-series (Fig. 3) show a clearer trend (i.e., slope of temporal change) than NEP time-series and are thus more useful to detect the impacts of elevated levels of atmospheric CO2 and climate warming. It seems feasible to detect significant changes (i.e., exceeding the range of statistical observational error) in GPP or other gross fluxes such as ER in the near future. Second, the experimental sites showed differences in future climate conditions and carbon budget responsiveness. Systematic and extensive monitoring networks are required to capture the regional tendency of climatic impacts on terrestrial ecosystems. Third, seasonal change in fluxes and parameters seem to clearly represent changes in the ecosystems (Fig. 4), indicating the importance of monitoring plant phenology as done in several studies (e.g., Doi and Takahashi 2008). Monitoring of phenology would be a low-cost, effective way of detecting ecosystem responses to global climate change. Fourth, down-regulation or acclimation caused by nitrogen limitation appears to substantially alter the ecosystem response, leading to lower carbon uptake in this region. Because most ecosystem models adopt a photosynthetic scheme similar to that used in VISIT, they can largely overestimate long-term ecosystem response (e.g., Thornton et al. 2007). Therefore, further experimental and monitoring studies are required to better understand these processes. Fifth, the simulated ecophysiological changes suggest that the ecosystem responses are related with various fields of research and ecosystem management. For example, the increase of WUE implies that human-available water from a specific amount of precipitation could increase through runoff drainage in the future. Sixth, the simulations imply that LAI would gradually increase at each site in the future. Because many studies have attempted air- and space-borne measurement of LAI (e.g., Asner et al. 2003; Nasahara et al. 2008), remote sensing of LAI may effectively enable the use of broad-scale monitoring of the impacts of global change on terrestrial ecosystems.
Further experimental and monitoring studies should be conducted to elucidate the mechanisms determining the ecosystem carbon budget. These findings are essential for evaluating ecosystem functions and global change impacts anticipated in the future with process-based models.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Amthor JS (1991) Respiration in a future, higher CO2 world. Plant Cell Environ 14:13–20
Asner GP, Scurlock JMO, Hicke JA (2003) Global synthesis of leaf area index observations: implications for ecological and remote sensing studies. Glob Ecol Biogeogr 12:191–205
Badeck F-W, Bondeau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Responses of spring phenology to climate change. New Phytol 162:295–309
Boer GJ, Yu B, Kim S-J, Flato GM (2004) Is there observational support for an El Niño-like pattern of future global warming? Geophys Res Lett 31:L06201. doi:10.1029/2003GL018722
de Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20:537–557
Doi H, Takahashi M (2008) Latitudinal patterns in the phenological responses of leaf colouring and leaf fall to climate change in Japan. Glob Ecol Biogeogr 17:556–561
Emori S, Hasegawa A, Suzuki T, Dairaku K (2005) Validation, parameterization dependence, and future projection of daily precipitation simulated with a high-resolution atmospheric GCM. Geophys Res Lett 32:L06708. doi:10.1029/2004GL022306
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Field CB, Jackson RB, Mooney HA (1995) Stomatal responses to increases CO2: implications from the plant to global scale. Plant Cell Environ 18:1214–1225
Foley JA, Costa MH, Delire C, Ramankutty N, Snyder P (2003) Green surprise? How terrestrial ecosystems could affect earth’s climate. Front Ecol Environ 1:38–44
Hamilton JG, Thomas RB, Delucia EH (2001) Direct indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant Cell Environ 24:975–982
Hikosaka K, Onoda Y, Kinugasa T, Nagashima H, Anten NPR, Hirose T (2005) Plant responses to elevated CO2 concentration at different scales: leaf, whole plant, canopy, and population. Ecol Res 20:243–253
Hirata R, Hirano T, Saigusa N, Fujinuma Y, Inukai K, Kitamori Y, Takahashi Y, Yamamoto S (2007) Seasonal and interannual variations in carbon dioxide exchange of a temperate larch forest. Agric For Meteorol 147:110–124
Hirata R, Saigusa N, Yamamoto S, Ohtani Y, Ide R, Asanuma J, Gamo M, Hirano T, Kondo H, Kosugi Y, Li S-G, Nakai Y, Takagi K, Tani M, Wang H (2008) Spatial distribution of carbon balance in forest ecosystem across East Asia. Agric For Meteorol 148:761–775
Hirose T, Ackerly DD, Traw MB, Damseier D, Bazzaz FA (1997) CO2 elevation, canopy photosynthesis, and optimal leaf area index. Ecology 78:2339–2350
Ichii K, Suzuki T, Kato T, Ito A, Hajima T, Ueyama M, Sasai T, Hirata R, Saigusa N, Ohtani Y, Takagi K (2009) Multi-model analysis of terrestrial carbon cycles in Japan: reducing uncertainties in model outputs among different terrestrial biosphere models using flux observations. Biogeosci Discuss 6:8455–8502
Intergovernmental Panel on Climate Change (IPCC) (2000) Special report on emissions scenarios. Cambridge University Press, Cambridge
Intergovernmental Panel on Climate Change (IPCC) (2007) Climate change 2007: the physical science basis. Cambridge University Press, Cambridge
Ito A (2008) The regional carbon budget of East Asia simulated with a terrestrial ecosystem model and validated using AsiaFlux data. Agric For Meteorol 148:738–747
Ito A, Oikawa T (2002) A simulation model of the carbon cycle in land ecosystems (Sim-CYCLE): a description based on dry-matter production theory and plot-scale validation. Ecol Model 151:147–179
Ito A, Oikawa T (2006) Absorption of photosynthetically active radiation, dry matter production, and light-use efficiency of terrestrial vegetation: a global model simulation. In: Kawahata H, Awaya Y (eds) Global Climate change and response of carbon cycle in the equatorial Pacific and Indian oceans and adjacent landmasses, vol 73. Elsevier, Amsterdam, pp 335–359
Ito A, Inatomi M, Mo W, Lee M, Koizumi H, Saigusa N, Murayama S, Yamamoto S (2007) Examination of model-estimated ecosystem respiration by use of flux measurement data from a cool-temperate deciduous broad-leaved forest in central Japan. Tellus 59B:616–624
Kato T, Tang Y (2008) Spatial variability and major controlling factors of CO2 sink strength in Asian terrestrial ecosystems; evidence from eddy covariance data. Glob Chang Biol 14:2333–2348
Kato T, Tang Y, Gu S, Hirota M, Du M, Li Y, Zhao X (2006) Temperature and biomass influences on interannual changes in CO2 exchange in an alpine meadow on the Qinghai-Tibetan Plateau. Glob Chang Biol 12:1285–1298
Kira T (1991) Forest ecosystems of east and southeast Asia in a global perspective. Ecol Res 6:185–200
Kistler R, Kalnay E, Collins W, Saha S, White G, Woollen J, Chelliah M, Ebisuzaki W, Kanamitsu M, Kousky V, van den Dool H, Jenne R, Fiorino M (2001) The NCEP-NCAR 50-year reanalysis: monthly means CD-ROM and documentation. Bull Am Meteorol Soc 82:247–267
Kitao M, Koike T, Tobita H, Maruyama Y (2005) Elevated CO2 and limited nitrogen nutrition can restrict excitation energy dissipation in photosystem II of Japanese white birch (Betula platyphylla var. japoninca) leaves. Physiol Plant 125:64–73
Leuning R (1995) A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ 18:339–355
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Luo Y, Gerten D, Le Maire G, Parton WJ, Weng E, Zhou X, Keough C, Beier C, Ciais P, Cramer W, Dukes JS, Emmett B, Hanson PJ, Knapp A, Linder S, Nepstad D, Rustad L (2008) Modeled interactive effects of precipitation, temperature, and [CO2] on ecosystem carbon and water dynamics in different climatic zones. Glob Chang Biol 14:1986–1999
McMurtrie RE, Wang Y-P (1993) Mathematical models of the photosynthetic response of tree stands to rising CO2 concentrations and temperatures. Plant Cell Environ 16:1–13
Mikolajewicz U, Gröger M, Maier-Reimer E, Schurgers G, Vizcaíno M, Winguth AME (2007) Long-term effects of anthropogenic CO2 emissions simulated with a complex earth system model. Clim Dyn 28:599–633
Monsi M, Saeki T (2005) On the factor light in plant communities and its importance for matter production. Ann Bot 95:549–567
Muraoka H, Koizumi H (2005) Photosynthetic and structural characteristics of canopy and shrub trees in a cool-temperate deciduous broad-leaved forest: implications to the ecosystem carbon gain. Agric For Meteorol 134:39–59
Nasahara KN, Muraoka H, Nagai S, Mikami H (2008) Vertical integration of leaf area index in a Japanese deciduous broad-leaved forest. Agric For Meteorol 148:1136–1146
Norby RJ, Delucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJ, Ceulemans RD, De Angelis P, Finzi AC, Karnosky DF, Kubiske ME, Lukac M, Pregitzer KS, Scarascia-Mugnozza GE, Schlesinger WH, Oren R (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA 102:18052–18056
Ohtani Y, Saigusa N, Yamamoto S, Mizoguchi Y, Watanabe T, Yasuda Y, Murayama S (2005) Characteristics of CO2 fluxes in cool-temperate coniferous and deciduous broadleaf forests in Japan. Phyton 45:73–80
Oikawa T (1986) Simulation of forest carbon dynamics based on dry-matter production model: 3. Effects of increasing CO2 upon a tropical rainforest ecosystem. Bot Mag 99:419–430
Ollinger SV, Richardson AD, Martin ME, Hollinger DY, Frolking SE, Reich PB, Plourde LC, Katul GG, Munger JW, Oren R, Smith M-L, KT PawU, Bolstad PV, Cook BD, Day MC, Martin TA, Monson RK, Schmid HP (2008) Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. Proc Natl Acad Sci USA 105:19336–19341
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Piao S, Ciais P, Friedlingstein P, Peylin P, Reichstein M, Luyssaert S, Margolis H, Fang J, Barr A, Chen A, Grelle A, Hollinger DY, Laurila T, Lindroth A, Richardson AD, Vesala T (2008) Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451:49–52
Piao S, Fang J, Ciais P, Peylin P, Huang Y, Sitch S, Wang T (2009) The carbon balance of terrestrial ecosystems in China. Nature 458:1009–1013
Ryan MG (1991) Effects of climate change on plant respiration. Ecol Appl 1:157–167
Saigusa N, Yamamoto S, Murayama S, Kondo H (2005) Inter-annual variability of carbon budget components in an AsiaFlux forest site estimated by long-term flux measurements. Agric For Meteorol 134:4–16
Saigusa N, Yamamoto S, Hirata R, Ohtani Y, Ide R, Asanuma J, Gamo M, Hirano T, Kondo H, Kosugi Y, Li S-G, Nakai Y, Takagi K, Tani M, Wang H (2008) Temporal and spatial variations in the seasonal patterns of CO2 flux in boreal, temperate, and tropical forests in East Asia. Agric For Meteorol 148:700–713
Sitch S, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer W, Kaplan JO, Levis S, Lucht W, Sykes MT, Thonicke K, Venevsky S (2003) Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob Chang Biol 9:161–185
Thornton PE, Lamarque J-F, Rosenbloom NA, Mahowald NM (2007) Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Global Biogeochem Cycles 21:GB4018. doi:10.1029/2006GB002868
Woodward FI, Thompson GB, McKee IF (1991) The effects of elevated concentrations of carbon dioxide on individual plants, populations, communities and ecosystems. Ann Bot 67:23–38
Acknowledgments
This study was partly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 19310017) and from the Japan Ministry of Education, Culture, Sports, Science and Technology (no. 21114010). This is a contribution to the A3 Foresight Program “Quantifying and predicting terrestrial carbon sinks in East Asia: toward a network of climate change research”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, A. Changing ecophysiological processes and carbon budget in East Asian ecosystems under near-future changes in climate: implications for long-term monitoring from a process-based model. J Plant Res 123, 577–588 (2010). https://doi.org/10.1007/s10265-009-0305-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0305-x