Abstract
Paphiopedilum and Cypripedium are closely related in phylogeny, but have contrasting leaf traits and habitats. To understand the divergence in leaf traits of Paphiopedilum and Cypripedium and their adaptive significance, we analyzed the leaf anatomical structures, leaf dry mass per area (LMA), leaf lifespan (LL), leaf nitrogen concentration (Nmass), leaf phosphorus concentration (Pmass), mass-based light-saturated photosynthetic rate (Amass), water use efficiency (WUE), photosynthetic nitrogen use efficiency (PNUE) and leaf construction cost (CC) for six species. Compared with Cypripedium, Paphiopedilum was characterized by drought tolerance derived from its leaf anatomical structures, including fleshy leaves, thick surface cuticles, huge adaxial epidermis cells, lower total stoma area, and sunken stomata. The special leaf structures of Paphiopedilum were accompanied by longer LL; higher LMA, WUE, and CC; and lower Nmass, Pmass, Amass, and PNUE compared with Cypripedium. Leaf traits in Paphiopedilum helped it adapt to arid and nutrient-poor karst habitats. However, the leaf traits of Cypripedium reflect adaptations to an environment characterized by rich soil, abundant soil water, and significant seasonal fluctuations in temperature and precipitation. The present results contribute to our understanding of the divergent adaptation of leaf traits in slipper orchids, which is beneficial for the conservation of endangered orchids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The diandrous orchid subfamily Cypripedioideae consists of five genera: Selenipedium, Phragmipedium, Mexipedium, Paphiopedilum, and Cypripedium (Cox et al. 1997). Among them, Selenipedium, Phragmipedium, and Mexipedium are found in tropical America, while China is the geographical distribution center for Paphiopedilum and Cypripedium (Cribb 1997, 1998). Though Paphiopedilum and Cypripedium are closely related in phylogeny (Cox et al. 1997), they have contrasting leaf traits and geographical distribution.
Paphiopedilum, containing approximately 66 species in the world, occurs mainly in tropical and subtropical forests from Asia to the islands of the Pacific. More than 18 species can be found in southwest China, usually on karst limestone hills below an altitude of 2,000 m (Cribb 1998; Chen et al. 2005). In karst areas, limestone and other soluble rocks dissolve easily, resulting in the rugged topography of highly permeable and cavernous rocks. Consequently, the mantle soil layer in karst areas is shallow, with a scarcity of soil and surface streams (LeGrand 1973). In many areas where Paphiopedilum grows, rainfall and consequent humidity is high. However, rainfall is also usually seasonal and water is not easily retained, so plants in such areas often have to survive considerable dry periods (Cribb 1998).
Cypripedium, consisting of 50 species, is widely distributed in temperate and subtropical zones of America, Europe, and Asia. About 32 species are found in China, with most growing in the shade of forest at altitudes above 1,800 m in southwest China (Cribb 1997; Chen et al. 2005). In contrast to Paphiopedilum, the soil layer in areas where Cypripedium grows is usually thicker than 60 cm, contains more nutrients, and can store abundant water during the growing season.
In terms of leaf traits, Cypripedium is distinguished by its broad plicate leaves attached to a distinct or abbreviated stem, whereas Paphiopedilum is characterized by its ligulate, conduplicate leaves produced in a basal distichous rosette (Karasawa and Saito 1982; Atwood 1984). Leaves of Paphiopedilum are evergreen and fleshy, and have distinct epidermal cuticles, whereas leaves of Cypripedium are deciduous, thin, and have no obvious cuticle (Karasawa and Saito 1982; Atwood 1984).
In the evolution of plants, leaves are more sensitive and plastic to the environmental change than other organs. Leaf traits are key factors in terms of reflecting the influence of the environment on the plant and adaptation of the plant to the environment (Dunbar-Co et al. 2009). Leaf anatomical structures are the foundations of leaf physiological functions. Consequentially, the change of leaf anatomical structures greatly affects plant growth and metabolism (Vendramini et al. 2002; Pandey et al. 2009). For example, plants with xeromorphic features usually grow in an environment where leaf photosynthesis is limited by water availability (Haworth and McElwain 2008). Features such as stomatal furrows, sunken stomata, and epidermal cuticles are commonly viewed as adaptations to aridity, as they result in enhanced boundary layer resistance, thereby limiting transpiration (Haworth and McElwain 2008; Mill and Stark Schilling 2009).
In leaf economics, leaf dry mass per area (LMA) is a pivotal trait that characterizes both the investment (mass) and potential for return (photosynthetic area) (Wright and Westoby 2002; Poorter et al. 2009). The leaf lifespan (LL)–LMA relationship among species reflects a trade-off between investment and return: species with low LMA have a greater potential for rapid growth, whereas species with long LL have a longer duration of revenue stream from the investment (Wright and Westoby 2002). The mass-based light-saturated photosynthetic rate (A mass) and maintenance cost decrease with increasing LL, whereas leaf construction cost (CC) shows an increasing trend (Reich et al. 1999). LMA and LT (leaf thickness) are significantly correlated with plant resource-use strategy (Cunningham et al. 1999). For example, along gradients in nutrient and water availability in southeast Australia, LT and LMA increase with decreasing resource availability (Cunningham et al. 1999). In general, plants in nutrient-poor or arid habitats share common leaf traits, such as lower leaf nitrogen concentration (N mass), phosphorus concentration (P mass), A mass, and dark respiration rate (Rd mass); higher LMA, water use efficiency (WUE), and CC; and longer LL and payback time (Poorter and Bongers 2006; Reich et al. 2007; Vernescu and Ryser 2009).
As renowned horticultural plants bearing large, peculiar, and beautiful flowers, Paphiopedilum and Cypripedium have attracted much attention from botanists and horticulturists (Cribb 1997, 1998). However, all species in the two genera are listed in Appendix 1 of the Convention on International Trade in Endangered Species of Wild Fauna and Flora. Some species are even at risk of extinction due to commercial over-collection and habitat loss or destruction (Li et al. 2008; Yuan et al. 2010). Knowledge of their adaptive strategies is essential for their conservation and continued use in the ornamental trade. However, little is known about the leaf traits in Cypripedium and Paphiopedilum, or their adaptive strategies.
In the present study, we investigated the leaf anatomical structures and related leaf physiological functions of three Paphiopedilum species and three Cypripedium species to understand the divergent adaptation of leaf traits in Paphiopedilum and Cypripedium. We hypothesize that the divergence of leaf anatomical structures in Paphiopedilum and Cypripedium reflect adaptation to their habitats, and that Paphiopedilum should exhibit more leaf xeromorphic features than Cypripedium because of its adaptation to periodic water deficiency in limestone soil.
Materials and methods
Plant materials
Six species of Paphiopedilum and Cypripedium (Paphiopedilum bellatulum (Rchb. f.) Stein, Paphiopedilum armeniacum S.C. Chen et F.Y. Liu, Paphiopedilum dianthum T. Tang et F.T. Wang, Cypripedium flavum P.F. Hunt et Summerh, Cypripedium yunnanense French., and Cypripedium lichiangense S.C. Chen et Cribb) were collected from their natural habitats. The ecological characteristics and biological traits of the considered species are shown in Table 1 (Cribb 1997, 1998). As the environmental requirements of the two genera are different, they were cultivated at two sites. Three Paphiopedilum species were cultivated in a greenhouse at Kunming (alt. 1,990 m; 102°41′E, 25°01′N). The growing conditions of Paphiopedilum were 30–40% of full sunlight and an air temperature of 20–25°C in the day and approximately 15°C at night. Three Cypripedium species were cultivated in a greenhouse at Zhongdian (alt. 3,260 m; 99°50′E, 27°48′N). The seedlings were given 50–70% of full sunlight and an air temperature of 15–20°C in the day and approximately 10°C at night. The seedlings were watered as needed. After cultivation for 2–3 years, the seedlings were used for measurements in the present study.
All six species were sampled for leaf histological observations, measurements of LMA, stable carbon isotope ratio (δ13C), N mass, and P mass. Previous studies indicate that leaf physiological functions in the same genera of Paphiopedilum and Cypripedium showed many interspecific similarities (Donovan and Arditti 1984; Johnson 1992; Zhang et al. 2006). Thus, only P. armeniacum and C. flavum were selected to investigate the physiological functions because of the limited experimental materials.
Histological observations
The middle parts of mature leaves were fixed in FAA (formalin/glacial acetic acid/ethanol/distilled water, 10:5:50:35, v/v) for at least 24 h. They were then dehydrated in an ethanol series and embedded in paraffin for sectioning. Transverse sections, made on a Leica RM2126RT rotary microtome (Leica Inc., Bensheim, Germany) were mounted on glass slides. The samples were examined and photographed under a light microscope (OlympusU-CMAD3, Olympus Inc., Tokyo, Japan). Cuticle, epidermis, mesophyll, and leaf thickness were measured at the midpoint of each transverse section with imaging software (Adobe Photoshop 7.0, Adobe Systems Inc., CA, USA).
The adaxial and abaxial epidermis of middle mature leaf parts were peeled from fresh leaves and photographed under a light microscope. Digital images were manually analyzed with Adobe Photoshop 7.0. The stomatal density (d), and stomatal apparatus length (l) and width (w) were measured. The stomatal apparatus area (A s) was calculated as follows: A s = 1/4 × π × l × w (Shelley and David 2001). Total stoma area (A t) was calculated as follows: A t = A s × d × 100%.
The existence of leaf guard cell chloroplasts was examined with a fluorescence microscope (Zeiss Axioplan 2, Carl Zeiss Inc., Jena, Germany) on the freshly stripped epidermal peels. We could clearly see the intrinsic fluorescence of the guard cell chloroplasts within 30 min of stripping under green light (500–530 nm).
Leaf fragments were mounted on aluminium stubs and coated with gold to an approximate thickness of 10 nm. They were then examined under a scanning electron microscope (KYKY Amray 1000B, KYKY Inc., Beijing, China) at 30 kV and were photographed.
For leaf histological observations, four leaves from four different individuals were examined for each species, and more than ten images per leaf were analyzed.
Measurements of leaf traits
The leaf photosynthetic abilities of P. armeniacum at a leaf age of 1–2 years and of C. flavum at a leaf age of 60 days were measured with a Li-6400 portable open gas exchange system (Li-Cor Inc., Lincoln, USA). At these leaf age stages, the leaf photosynthetic capabilities of the two species are the highest of their whole LLs (Guan 2010). To ensure the results could be compared, the two genera were cultivated and analyzed under their optimal growth conditions. Therefore, the temperatures and light intensities used in photosynthetic measurements were different between P. armeniacum and C. flavum. Leaf photosynthetic responses to light were measured at 11 light intensities (0–1,000 μmol m−2 s−1 for P. armeniacum and 0–2,000 μmol m−2 s−1 for C. flavum) under a controlled CO2 concentration (370 μmol mol−1 for P. armeniacum and C. flavum) and temperature (25°C for P. armeniacum and 20°C for C. flavum). Because the absence of guard cell chloroplast in Paphiopedilum makes the response of stomatal opening insensitive to red light (Zeiger et al. 2002), we used a lower ratio of red–blue light in Paphiopedilum than in Cypripedium (70% red + 30% blue for P. armeniacum; 90% red + 10% blue for C. flavum). The CO2 response curves of photosynthesis were determined with a range of CO2 concentrations (0–2,000 μmol mol−1) under a controlled light intensity (300 μmol m−2 s−1 for P. armeniacum and 1,000 μmol m−2 s−1 for C. flavum) and temperature (25°C for P. armeniacum and 20°C for C. flavum). Three mature leaves from three individual plants were measured for each species. All measurements were taken at a relative humidity of about 60% and a leaf-to-air vapor pressure deficit of 1.0–1.5 kPa.
Photosynthetic rate (A), stomatal conductance (g s), and transportation rate (E) were recorded automatically during measurements of photosynthesis by Li-6400. WUE was calculated as dividing A by E (Rudmann et al. 2001). Values of g s and WUE measured at the CO2 concentration of 370 μmol mol−1; light intensity of 300 μmol m−2 s−1 for P. armeniacum and 1,000 μmol m−2 s−1 for C. flavum were used for the comparisons of the two species. Mesophyll conductance (g m) was estimated according to the approach of Harley et al. (1992). The photosynthetic response curves to light were made by Photosyn Assistant software (Dundee Scientific, Scotland, UK). Using this function, the light-saturated photosynthetic rate (A max) and dark respiration rate (Rd) were estimated.
The outlines of 15 mature leaves from 15 individual plants for each species (50 leaves from 50 plants were sampled for P. armeniacum and C. flavum) were traced out on graph paper which had a uniform mass distribution with area. The leaf shape was cut out from the graph paper and the copies were weighted. The leaf area (L a) was then gravimetrically evaluated. Leaf fresh weight (FW) was assessed immediately after sampling. Leaf dry mass (DW) was determined after oven-drying at 70°C to constant weight, and LMA was calculated. The water content per leaf area of fresh leaves (LWC) was calculated as LWC = (FW − DW)/L a. The dried leaf samples used for LMA measurement were ground and homogenized for subsequent analyses. Subsequent analyses were made using three different samples obtained from the homogenized leaves for each species.
The stable carbon isotope ratio (δ13C) of leaf tissues was determined using a mass spectrometer (Finnigan MAT 253, Finnigan Inc., FL, USA). δ13C is expressed in delta notation: δ13C (‰) compared with a standard (Pee Dee Belemnite). δ13C can indicate the long-term WUE of a plant, which increases with increasing δ13C value.
Leaf N mass was measured using an elemental analyzer (Leco FP-428, Leco Corporation, MI, USA). P mass was determined using an inductively coupled plasma emission spectroscopy (ICPS-1000 II, Shimadzu Corporation, Kyoto, Japan). Photosynthetic nitrogen use efficiency (PNUE) was calculated by dividing A max by leaf N content (area basis).
Leaf CC (g glucose g−1) was calculated following the formula used by Suárez (2003): CC = [(0.06968H c − 0.065)(1 − ash) + (7.5kN/14.0067)]/E g, where H c is the ash free heat of combustion (kJ g−1), ash is the ash concentration (g g−1), k is the oxidation state of the nitrogen source (=5), N is the organic nitrogen concentration (g g−1), and E g is the growth efficiency (=0.89).
Leaf maintenance cost (mg glucose g−1 day−1) was calculated following the method of Suárez (2003), by averaging the maximum and minimum values calculated from their protein (0.028–0.053), lipids (0.0425) and ash (0.006–0.010) coefficients. Payback time was estimated as the ratio of CC to A max (Navas et al. 2003).
Statistical analysis
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, USA). Differences of leaf traits among the six species were determined by one-way ANOVA and LSD multiple comparisons tests. Comparisons between P. armeniacum and C. flavum were tested by independent sample t test.
Results
Leaf morphology and internal structure
The growing period of Cypripedium in southwest China is mid-May to mid-October, while Paphiopedilum is an evergreen plant. The LL of Cypripedium is approximately 150 days, but Paphiopedilum is usually more than 3 years.
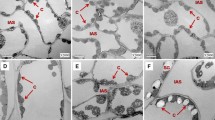
The leaves of Paphiopedilum were thicker and more succulent than those of Cypripedium (Fig. 1). This was mainly caused by the elongated adaxial epidermis cells and the thicker mesophyll layers of Paphiopedilum. The palisade-like adaxial epidermal cells of Paphiopedilum leaf were more or less uniformly elongated over the entire surface and in some species even made up one half of the total leaf thickness (Fig. 1). Adaxial epidermis cells of Paphiopedilum leaves always had a much larger volume than did abaxial cells, but this was not the case in Cypripedium. In some species of Paphiopedilum, the mesophyll cells were distinctly arranged into palisade and spongy mesophyll layers, but there was no differentiation in Cypripedium leaves (Fig. 1). Between one and four layers of palisade cells could be found in most Paphiopedilum species. The thickest mesophyll layer was found in P. dianthum for Paphiopedilum and in C. lichiangense for Cypripedium (Fig. 1). Though the leaf of C. lichiangense was thicker than other species of Cypripedium, it was obviously thinner than that of Paphiopedilum. Both sides of the leaf were heavily cuticularised in Paphiopedilum. Unlike Paphiopedilum leaves, cuticles were not obvious on the surface of Cypripedium leaves (Fig. 1).
Leaf cross sections of Paphiopedilum and Cypripedium under light microscope. a P. bellatulum, b P. armeniacum, c P. dianthum, d C. flavum, e C. lichiangense, f C. yunnanense. Cu cuticle, Ad adaxial epidermis, PT palisade tissue, ST spongy tissue, M mesophyll cells, VB vascular bundle, Ab abaxial epidermis, S stoma. Scale bars 100 μm
Stomatal apparatus
Stomata were only found on the leaf abaxial surface in Paphiopedilum and Cypripedium (Fig. 1). The stomatal density of Cypripedium was similar to that of Paphiopedilum (Fig. 2; Table 2). However, the size of stomatal apparatus and the total stoma area (%) were usually larger in Cypripedium than in Paphiopedilum (Table 2). The largest stomatal apparatus among the six species was found in C. lichiangense. The stoma shape was elliptical for both Paphiopedilum and Cypripedium (Fig. 2). The stomata of Paphiopedilum were slightly sunken into the leaf epidermis, but stomata extruded slightly outside the leaf surface in Cypripedium (Figs. 1, 3, 4). The guard cell walls of Paphiopedilum were heavily cuticularised, but no obvious cuticle was found in Cypripedium. Cuticular lips covering the stomatal pore (i.e., cuticular horns of guard cells) were prominent in Paphiopedilum, resulting in a large antechamber above the stoma (Fig. 4). There were many chloroplasts in the guard cells of Cypripedium, but Paphiopedilum had no guard cell chloroplasts (Fig. 5).
Leaf cross sections of Paphiopedilum and Cypripedium under light microscope, showing stomata. a P. bellatulum, b P. armeniacum, c P. dianthum, d C. flavum, e C. lichiangense, f C. yunnanense. Cu cuticle, GC guard cell, Ab abaxial epidermis, PC prominent cuticularised lips, SP stomatal pore, An antechamber, SC substomatal cavity. Scale bars 10 μm
Leaf traits
The LMA, LWC, and δ13C (‰) values of Paphiopedilum were significantly larger than those of Cypripedium (Table 2). Compared with Cypripedium, Paphiopedilum had lower values of N mass, P mass, A max, A mass, V cmax, J max, Rd mass, g s, g m, and E, and higher CC per area (P < 0.001) and longer payback time (P < 0.001) (Tables 2, 3). The maintenance cost of P. armeniacum was slightly lower than that of C. flavum, but the difference between the two species was not statistically significant (P = 0.104). P. armeniacum had lower PNUE (P = 0.001) and higher WUE (P = 0.017) than C. flavum (Table 3).
Discussion
Compared with Cypripedium, Paphiopedilum had significantly thicker leaves, thicker surface cuticles, huge adaxial epidermis cells, lower total stoma area, sunken stomata, and a lack of guard cell chloroplasts. In addition, leaves of Paphiopedilum had longer LL; higher LMA, WUE, and CC; and lower N mass, P mass, A mass, E, and PNUE than did Cypripedium. The divergence in leaf anatomical structures and physiological functions between Paphiopedilum and Cypripedium reflect adaptations to their habitats.
Leaf anatomical structures of Paphiopedilum and Cypripedium
Unlike Cypripedium, the leaves of Paphiopedilum were thicker, fleshy, and contained more water (Table 2). Moreover, in Paphiopedilum, the leaf water content per area of fresh leaves increased with increasing leaf thickness and vertical thickness of adaxial epidermis cells. Adaxial epidermis cells of Paphiopedilum leaves always had a significantly larger volume than abaxial cells, but this was not the case in Cypripedium. Large epidermal cells in many species of orchids serve as water-storage cells. In some species of orchid, the water stored in epidermal cells can account for up to 80% of the entire leaf volume (Pridgeon and Stern 1982). The mesophyll cells and huge adaxial epidermal cells in Paphiopedilum leaves can store more water than Cypripedium, thereby assisting Paphiopedilum in maintaining its normal physiological metabolism at times of limited water availability.
Cuticles were found on both sides of the Paphiopedilum lamina, but there were no obvious cuticles on leaves of Cypripedium (Fig. 1; Table 2). The cuticle is a thin, hydrophobic, and flexible membrane composed of polymer matrix (cutin) and associated solvent-soluble lipids (cuticular waxes). The major function of cuticle is efficiently preventing water loss from the leaf interior (Bargel et al. 2004; Mill and Stark Schilling 2009). The presence of thick cuticles on the leaf surface is an indicator of aridity (Haworth and McElwain 2008). The cuticles of evergreen plants tend to have a lower permeability than those of deciduous species, possibly reflecting the adaptation of species with long LL foliage can conserve water during dry periods (Gratani and Bombelli 2000). Evolutionary pressures usually favour investment in chemical and structural cuticle defences in plants experiencing environmental stress (Haworth and McElwain 2008). The thick cuticles on Paphiopedilum leaves can reduce water loss and increase water-use efficiency at times when water availability is reduced.
The size and total stoma area (%) of Paphiopedilum were smaller than those of Cypripedium (Table 2). Stomata control the exchange of gases, most importantly water vapour and CO2, between the interior of the leaf and the atmosphere (Buckley 2005). Stomatal distribution, size, density, morphology, and behaviour are closely associated with plant transpiration (Willmer and Fricker 1996). Larger stomata are slower to close and have a greater potential for hydraulic dysfunction under conditions of drought (Aasamaa et al. 2001). Plants with lower stomatal density are usually able to tolerate a more arid environment than plants with higher stomatal density (Kebede et al. 1994). The stomatal density of orchid leaves is low in the entire plant kingdom, especially Paphiopedilum (Karasawa and Saito 1982; Willmer and Fricker 1996). The size and A t value of Paphiopedilum are smaller which indicates that Paphiopedilum can tolerate a more arid environment than Cypripedium.
The stomata of Paphiopedilum were sunken into the leaf epidermis and the structures of stomata were very special compared with common plants (Figs. 1, 3). The guard cell walls were heavily cuticularised and the cuticular lips over the stomatal pore were very prominent in Paphiopedilum, resulting in a very large antechamber above the real stoma (Fig. 4). The antechamber is a structure that prevents liquid water from blocking air exchange in the humid environment (Ziegler 1987) and regulates stomatal opening according to vapour pressure (Maier-Maercker 1983). Rutter and Willmer (1979) noticed the striking thickening of guard cell walls of Paphiopedilum and supposed the extensive wall thickening and sculpturing of the guard cells limited the extent of stomatal opening. In the present study, the g s of Paphiopedilum could only reach 0.046 ± 0.002 mol m−2 s−1 and E reached 0.75 ± 0.12 mmol m−2 s−1 even after 30 min of light induction, which were relatively low values compared with Cypripedium (Table 3). This finding suggests that the most important role of the sunken stoma and the antechamber in Paphiopedilum is to limit stomatal opening and prevent water transpiration. On the other hand, the extruding stomata of Cypripedium could reduce the stomatal resistance by enlarging the stomatal pore and therefore might be an adaptation to lower pressure in high-altitude areas.
Paphiopedilum did not possess guard cell chloroplasts, whereas Cypripedium contained well-developed guard cell chloroplast (Fig. 5). These results confirmed the previous observation that Paphiopedilum lacks guard cell chloroplasts (Zeiger et al. 2002). Guard cell chloroplasts can contribute to stomatal opening via the following mechanisms: photosynthetic production of ATP and reductants, production of osmotically active sugars by photosynthetic carbon assimilation, zeaxanthin functioning as a low-intensity blue-light photoreceptor, and storage of starch (Zeiger et al. 2002). Furthermore, lack of chloroplasts in guard cells decreases gs and photosynthetic rate (Donovan and Arditti 1984). The lower gs and photosynthetic rate of Paphiopedilum compared with Cypripedium were partially caused by the lack of guard cells chloroplasts in Paphiopedilum.
Leaf ecophysiological functions of Paphiopedilum and Cypripedium
Cypripedium had significantly higher photosynthetic capacity (Amax and Amass) than Paphiopedilum. The differences in photosynthetic capacity reflected the differences in leaf physiology, anatomy, and biochemistry. The biochemical changes are often accompanied by changes in the maximum rate of carboxylation (Vcmax) and light-saturated rate of electron transport (Jmax), and there is a strong positive relationship between leaf N and P content and photosynthetic capacity (Zhang et al. 2008). Many studies show that higher photosynthetic rate is often linked to higher gs and gm (Zhang et al. 2008). The lower values of Nmass, Pmass, Vcmax, Jmax, gs, and gm in Paphiopedilum were responsible for the lower photosynthetic capacity compared with Cypripedium.
The CC per unit area of Paphiopedilum leaves was higher than that of Cypripedium, which was mainly due to the larger LMA of Paphiopedilum; while the higher CC in Paphiopedilum leaves was accompanied by its longer payback time. For Paphiopedilum, soil and nutrients are difficult to obtain in its natural habitat. The long LL of Paphiopedilum is beneficial for nutrient conservation in the leaves, indicating a longer duration of the revenue stream from the higher investment of CC. To some extent, the long LL of Paphiopedilum compensates its low photosynthetic capacity. For Cypripedium, having higher photosynthetic capacity, faster growth speed and accumulation rate of assimilation products are adaptations to the shorter growing period at high elevations. Therefore, the leaves of Cypripedium have lower LMA and CC, and shorter payback time and LL.
Leaf traits are often linked to the resource use efficiency of plants (Vendramini et al. 2002). Paphiopedilum had higher WUE and showed more xeromorphic features and conservative water-use strategies than did Cypripedium. Compared with Cypripedium, the lower transpiration rate of Paphiopedilum was mainly caused by the thicker epidermal cuticles, sunken stomata, existence of a stomatal antechamber, lower total stoma area, and lack of guard cell chloroplast. These features, in addition to the thicker leaf and huge adaxial epidermal cells in Paphiopedilum leaves, reflect adaptations to aridity by storing more water or preventing water transpiration. In contrast, no obvious xeromorphic features were observed in Cypripedium leaves. The natural habitat of Paphiopedilum is usually characterized by low soil water content and periodic water deficiency. Compared with Paphiopedilum, the soil layer in areas where Cypripedium grows is deep and can store a lot of water during the rainy season. Moreover, the growing season of Cypripedium coincides with the yearly wet season. Therefore, the growth of Cypripedium is rarely limited by soil water availability. Paphiopedilum had lower PNUE, but the longer LL of Paphiopedilum could enhance nutrient conservation and reduce the speed of nutrition loss. This was benefit to compensate its lower photosynthetic capacity and PNUE, so Paphiopedilum could grow in karst areas. Contrarily, the resource use strategies of Cypripedium were the adaptations to the environment rich in soil nutrients and humidity, but with a short growing season.
Leaf traits such as LL, CTad, CTab, ETad, LMA, LWC, A t, GC, N mass, and P mass were similar in the same genus, but were significantly different between Paphiopedilum and Cypripedium (Table 2). Leaves of plants growing in arid and nutrient-poor habitats usually show xeromorphic features; have lower N mass, P mass, A mass, and Rd mass; higher LMA, WUE, and CC; and longer payback time and LL (Poorter and Bongers 2006; Reich et al. 2007; Vernescu and Ryser 2009). All of these traits were found in Paphiopedilum, which reflected adaptations to low availability of soil water and nutrients in the karst habitat. However, the leaf structures and physiological functions of Cypripedium reflected adaptations to abundant soil nutrients and water availability during a limited growing season.
Overall, the results confirmed our hypothesis that the leaf anatomical structure of Paphiopedilum showed many xeromorphic features linked to reducing water loss and improving water-use efficiency, which contribute to growth and survival in karst habitats. The divergence in leaf anatomical structures and physiological functions between Paphiopedilum and Cypripedium reflects adaptations to their growing environments. Our study provides evidence of the divergent evolution of congeneric orchids under natural selection, thereby contributing to the conservation and cultivation of Paphiopedilum and Cypripedium.
References
Aasamaa K, Sober A, Rahi M (2001) Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust J Plant Physiol 28:765–774. doi:10.1071/PP00157
Atwood JT (1984) The relationships of the slipper orchids (subfamily Cypripedioideae, Orchidaceae). Selbyana 7:129–247
Bargel H, Barthlott W, Koch K, Schreiber L, Neinhuis C (2004) Plant cuticles: multifunctional interfaces between plant and environment. In: Hemsley AR, Poole I (eds) The evolution of plant physiology. Academic Press, London, pp 171–194
Buckley TN (2005) The control of stomata by water balance. New Phytol 168:275–292. doi:10.1111/j.1469-8137.2005.01543.x
Chen XC, Zhu GH, Ji ZH, Lang KY, Luo YB, Cribb P (2005) Orchidaceae. In: Wu ZY, Raven PH (eds) Flora of China, vol 25. Science, Beijing and Missouri Botanical Garden, St. Louis, pp 19–72
Cox AV, Pridgeon AM, Alber VC, Chase MW (1997) Phylogenetics of the slipper orchids (Cypripediodeae, Orchidaceae): nuclear rDNA ITS sequences. Plant Syst Evol 208:197–223. doi:10.1007/BF00985442
Cribb P (1997) The genus Cypripedium. Timber, Portland
Cribb P (1998) The genus Paphiopedilum, 2nd edn. Natural History, Borneo
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol Monogr 69:569–588. doi:10.1890/0012-9615(1999)069[0569:EDILSA]2.0.CO;2
Donovan RD, Arditti J (1984) Carbon fixation by Paphiopedilum insigne and Paphiopedilum parishii (Orchidaceae). Ann Bot 54:583–586
Dunbar-Co S, Sporck MJ, Sack L (2009) Leaf trait diversification and design in seven rare taxa of the Hawaiian Plantago radiation. Int J Plant Sci 170:61–75. doi:10.1086/593111
Gratani L, Bombelli A (2000) Correlation between leaf age and other leaf traits in three Mediterranean maquis shrub species: Quercus ilex, Phillyrea latifolia and Cistus incanus. Environ Exp Bot 43:141–153. doi:10.1016/S0098-8472(99)00052-0
Guan ZJ (2010) Leaf traits of Paphiopedilum and Cypripedium in Orchidaceae. PhD thesis, Kunming Institute of Botany, Chinese Academy of Sciences, China
Harley PC, Loreto F, Marco GD, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98:1429–1436. doi:10.1104/pp.98.4.1429
Haworth M, McElwain J (2008) Hot, dry, wet, cold or toxic? Revisiting the ecological significance of leaf and cuticular micromorphology. Paleogeogr Palaeoclimatol Paleoecol 262:79–90. doi:10.1016/j.palaeo.2008.02.009
Johnson SR (1992) Photosynthetic characters of Paphiopedilum malipoense and P. micranthum (section Parvisepalum). Lindleyana 7:181–184
Karasawa K, Saito K (1982) A revision of the genus Paphiopedilum (Orchidaceae). Bull Hiroshima Bot Gard 5:1–69
Kebede H, Martin B, James N, Gretchen K (1994) Leaf anatomy of two Lycopersicon species with contrasting gas exchange properties. Crop Sci 34:108–113
LeGrand HE (1973) Hydrological ecological problems of karst regions. Science 179:859–864. doi:10.1126/science.179.4076.859
Li ZR, Zhang SB, Hu H, Li DZ (2008) Photosynthetic performance along a light gradient as related to leaf characteristics of a naturally occurring Cypripedium flavum. J Plant Res 121:559–569. doi:10.1007/s10265-008-0186-4
Maier-Maercker U (1983) The role of peristomatal transpiration in the mechanism of stomatal movement. Plant Cell Environ 6:369–380. doi:10.1111/j.1365-3040.1983.tb01269.x
Mill RR, Stark Schilling DM (2009) Cuticle micromorphology of Saxegothaea (Podocarpaceae). Bot J Linn Soc 159:58–67. doi:10.1111/j.1095-8339.2008.00901.x
Navas ML, Ducout B, Roumet C, Richarte J, Garnier J, Garnier E (2003) Leaf life span, dynamics and construction cost of species from Mediterranean old-fields differing in successional status. New Phytol 153:213–228. doi:10.1046/j.1469-8137.2003.00790.x
Pandey SK, Singh H, Singh JS (2009) Species and site effects on leaf traits of woody vegetation in a dry tropical environment. Curr Sci 96:1109–1114
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743. doi:10.1890/0012-9658(2006)87[1733:LTAGPO]2.0.CO;2
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. doi:10.1111/j.1469-8137.2009.02830.x
Pridgeon AM, Stern WL (1982) Vegetative anatomy of Myoxanthus (Orchidaceae). Selbyana 7:55–63
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969. doi:10.1890/0012-9658(1999)080[1955:GOLTRA]2.0.CO;2
Reich PB, Wright IJ, Lusk CH (2007) Predicting leaf physiology from simple plant and climate attributes: a global GLOPNET analysis. Ecol Appl 17:1982–1988. doi:10.1890/06-1803.1
Rudmann SG, Milham PJ, Conroy JP (2001) Influence of high CO2 partial pressure on nitrogen use efficiency of C4 grasses Panicum coloratum and Cenchrus ciliaris. Ann Bot 88:571–577. doi:10.1006/anbo.2001.1503
Rutter JC, Willmer CM (1979) A light and electron microscopy study of the epidermis of Paphiopedilum spp. with emphasis on stomatal ultrastructure. Plant Cell Environ 2:211–219. doi:10.1111/j.1365-3040.1979.tb00072.x
Shelley AJ, David TB (2001) Leaf morphological and anatomical characteristics of heteroblastic Eucalyptus globulus ssp. globulus (Myrtaceae). Aust J Bot 49:259–269
Suárez N (2003) Leaf longevity, construction, and maintenance costs of three mangrove species under field conditions. Photosynthetica 41:373–381. doi:10.1023/B:PHOT.0000015461.36848.c5
Vendramini F, Diaz S, Gurvich DE, Wilson PJ, Thompson K, Hodgson JG (2002) Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol 154:147–157. doi:10.1046/j.1469-8137.2002.00357.x
Vernescu C, Ryser P (2009) Constraints on leaf structural traits in wetland plants. Am J Bot 96:1068–1074. doi:10.3732/ajb.0800312
Willmer C, Fricker M (1996) Stomata, topics in plant functional biology, 2nd edn. Chapman and Hall, London
Wright IJ, Westoby M (2002) Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol 155:403–416. doi:10.1046/j.1469-8137.2002.00479.x
Yuan L, Yang ZL, Li SY, Hu H, Huang JL (2010) Mycorrhizal specificity, preference, and plasticity of six slipper orchids from South Western China. Mycorrhiza. doi:10.1007/s00572-010-0307-5
Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu J (2002) The guard cell chloroplast: a perspective for the twenty-first century. New Phytol 153:415–424. doi:10.1046/j.0028-646X.2001.NPH328.doc.x
Zhang SB, Hu H, Xu K, Li ZH (2006) Photosynthetic performances of five Cypripedium species after transplanting. Photosynthetica 44:425–432. doi:10.1007/s11099-006-0046-1
Zhang SB, Hu H, Li ZH (2008) Variation of photosynthetic capacity with leaf age in an alpine orchid, Cypripedium flavum. Acta Physiol Plant 30:381–388. doi:10.1007/s11738-008-0135-9
Ziegler H (1987) The evolution of stomata. In: Zeiger E, Farquhar GD, Cowan IR (eds) Stomatal function. Stanford University, Stanford, pp 29–57
Acknowledgments
We thank Prof. Cun-Xin Li, Prof. Liu-Sheng Duan, and Dr. Ning Yan for their helpful suggestions regarding the manuscript. Ms. Juliet Lu is acknowledged for improving the English of the manuscript. This work was supported by the National Natural Science Foundation of China (No. 30770225 and No. 30870239) and the Social Development Plan of Yunnan (No. 2007C001Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, ZJ., Zhang, SB., Guan, KY. et al. Leaf anatomical structures of Paphiopedilum and Cypripedium and their adaptive significance. J Plant Res 124, 289–298 (2011). https://doi.org/10.1007/s10265-010-0372-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-010-0372-z