Abstract
Photosynthesis, leaf structure, nitrogen content and nitrogen allocation in photosynthetic functions of Cypripedium flavum were studied in a naturally varying light regime. Light-saturated leaf net photosynthetic rate (A max) was strongly correlated with leaf dry mass per area (LMA), mesophyll conductance (g m) and area-based leaf nitrogen content (Narea), with all variables increasing with increasing irradiance. Such coordinate variation of all these parameters illustrates the plastic response of leaf structure to high light (HL). Leaf Narea was greater under HL than in low light (LL). The fractions of leaf nitrogen partitioning in carboxylation (P R) and bioenergetics (P B) were positively related to LMA. In contrast, P R and P B decreased with increasing mass-based leaf nitrogen content (Nmass). However, no correlation was found between leaf nitrogen investment in light harvesting (P L) and either LMA or Nmass. Like maximum rate of carboxylation (V cmax) and electron transport (J max), the J max/V cmax ratio, which was strongly correlated to LMA, also increased significantly with irradiance. Under HL, leaf maximum photosynthetic nitrogen efficiency (ANUE) and intrinsic water use efficiency (WUE) were greater than in LL conditions, despite a small difference in WUE. This suggests that a functional balance in the photosynthetic machinery favors leaf photosynthetic plasticity of C. flavum in response to different light conditions. Given an ample soil nitrogen supply, C. flavum may offset its susceptibility to HL by efficient nitrogen use and higher stomatal and mesophyll conductance against photoinhibition so as to keep leaf photosynthesis positive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Light is the most important driving force of leaf photosynthesis, which in turn determines a plant’s survival, growth and fitness. However, the quantity of light required by plants under a specific environment, particularly in their natural habitats, may differ by at least two orders of magnitude and vary both spatially and temporarily (Björkman 1981). Upon exposure to a wide range of light regimes, plants show an ability to meet these differing conditions, mainly by alterations in leaf structure and biochemistry (Björkman 1981; Evans 1989; Niinemets and Tenhunen 1997). Generally, leaf morphology—as indicated by leaf dry mass per area (LMA)—predominantly accounts for responses and/or acclimation to a high light (HL) change (Niinemets and Tenhunen 1997; Evans and Poorter 2001; Niinemets and Sack 2006). Higher photosynthetic capacity at HL is closely coupled to a higher LMA and thus anatomical profiles such as greater surface area of leaf chloroplast or mesophyll cells facing intercellular air spaces per area (Evans and Loreto 2000; Terashima et al. 2001; Oguchi et al. 2003), and consequently mesophyll conductance (Piel et al. 2002). Other studies have suggested that changes in leaf photosynthesis under different light conditions are dominated largely by the level and re-allocation of leaf nitrogen (Frak et al. 2001). Efficient nitrogen use in photosynthetic machinery in accordance with the environment may enhance both the fitness of plants and their habitat and range expansion by positively utilizing the resources available (Hikosaka and Terashima 1996).
The light factor, on the other hand, has been justified as the main cause for the decline in distribution of some rare or endangered species, in that habitat loss or fragmentation due to deforestation can lead to significantly varying light environments (Aleric and Kirkman 2005; Liu et al. 2006). Different plants may have different sensitivity and responses to different light conditions, depending on their inherent light requirements. With Heptacodium miconioides, Liu et al. (2006) indicated that leaf photosynthesis would be impaired by light intensities above 716 μmol m−2 s−1, whereas this effect would not occur until approximately 1,000 μmol m−2 s−1 in Cypripedium guttatum (Zhang et al. 2007). Such unfavorable traits, which can be exposed by changes in the environment, may explain distribution differences because they may cause loss of the ability to adjust and compete with other species, thereby resulting in a decline in the distribution range of a species (Gulías et al. 2002). Characteristics such as greater leaf plasticity, including morphology and physiology, are critically indicative of a higher tolerance to changing environmental conditions, even to poor conditions such as more intense light and nutrient limitation (Yamashita et al. 2000; Valladares et al. 2002). Assessment of the trend and magnitude of leaf plasticity in endangered or threatened species in response to different light environments might thus be very important for examining favorable habitat types and the adjustment ability of plants at the leaf or even whole plant level (Muraoka et al. 2002).
Cypripedium flavum, a rare and endangered species, is of great value in horticulture and as an ornamental plant. It often occurs in the understory of mixed deciduous forests, and also survives at relatively open ‘marginal’ forest sites at altitudes of 1,800–3,700 m (Cribb 1997). Currently, the main threats to this species are from habitat loss or destruction through logging, firewood collection and commercial over-collection (Cribb and Sandison 1998), which have caused a severe decline in wild populations. Consequently, these plants must rise to the challenge of more distinct light environments than they experienced before. In this species, however, the relationship between leaf photosynthetic physiology and the corresponding light environment is not well understood. Furthermore, it is important to understand that associated leaf and photosynthetic traits are of potential importance for photosynthesis of plants under different light conditions. Furthermore, lack of such information about the biological and ecological requirements of these plant could disadvantage their conservation and re-establishment (Aleric and Kirkman 2005; Liu et al. 2006).
In this study, we investigated in situ leaf photosynthetic performance in seedlings of C. flavum occurring naturally along three different irradiance levels in order to address plastic responses of leaf photosynthesis. We aimed to answer the following questions: (1) How do photosynthetic and leaf characteristics vary in response to a natural light gradient? (2) How is leaf nitrogen partitioned across light environments, and what significance does this have? (3) Are differing morphological and physiological traits exhibited by plants in different light environments critical in terms of physiological plasticity?
Materials and methods
Study sites and plants
The study was performed in the Shangri-la Alpine Botanical Garden, in Southwest China (27°55′N, 99°33′E, altitude 3,260 m). The annual precipitation varies from 600 to 700 mm, with a yearly mean of ca. 625 mm, ranging mainly from June to September; the mean annual temperature is 5.4°C. The field measurements were conducted in mid-June 2006 at three locations having different light availability due to canopy openness. These sites have similar meteorological conditions since the vertical and horizontal distances between them are less than 3 km.
Cypripedium flavum P. F. Hunt et Summerh, commonly known as slipper orchid, was used for this study. It is a perennial herb native to China, typically a C3 plant with a mean value of −27.8‰ in δ13C [from measurements made on separate samples from five sites with a Delta Plus XL mass spectrometer (Finnigan MAT, Gainesville, FL)]. For details of phenological and life habits, the reader is referred to Zhang et al. (2006). At the study sites, the neighboring plants grown with C. flavum include mainly Pedicularis siphonantha D. Don, Anemone demissa var. villosissima Bruhl, Androsace bulleyana Forrest, Quercus aquifolioides Rehd. et Wils., and Triosteum himalayanum Wall.
Determination of light availability and soil status across sites
The light environment in which the plants are growing was characterized by taking measurements every 15 min with a quantum sensor (LI-190SA) at plant height between 0900 and 1700 hours on a sunny day. All data were recorded automatically with a user-friendly hand-held datalogger LI-1400 (LI-COR, Lincoln, NE). A light gradient was expressed as high light (HL), moderate light (ML) and low light (LL) levels, in terms of the daily mean light intensity. Details of the soil site status are shown in Table 1, where the range of pH (5.60–7.33) matched well with that reported by Cribb (1997).
Field measurements of leaf photosynthesis
Leaf gas exchange was all measured on a clear day using a portable open photosynthesis system (LI-6400, LI-COR). A 6400-02B LED cool light source was mounted on the clamp-on leaf chamber (6 cm2) as a source of different light densities. A source of variable CO2 concentration was provided with a CO2 cylinder attached to the 6400-01 CO2 injector system. All measurements were taken with leaf temperature maintained at 20°C, relative humidity between 50% and 65%, and a leaf to air vapor pressure deficit (VPD) of 0.8–1.2 kPa.
Photosynthetic light and CO2 response curves were measured in situ in plants with recently expanded young leaves occurring naturally across a light gradient. Three leaves from individual plants were chosen within each light regime. For light response curves, CO2 concentration within the chamber was maintained at 370 μmol mol−1, and light intensities were gradually decreased from 2,000 to 0 μmol m−2 s−1. For CO2 response curves, light intensity was maintained at 800 μmol m−2 s−1 and the chamber CO2 concentration was gradually decreased (1,500, 1,200, 1,000, 700, 500, 370, 250, 200, 150, 100, 50, 0 μmol mol−1). Before measurements, induction of photosynthesis at saturating light and ambient CO2 concentration lasted 20–30 min until stable photosynthesis occurred. During these measurements, the minimum waiting time between steps was at least 2 min and data was recorded when steady state was achieved. In addition, in order to characterize maximum photosynthetic nitrogen use efficiency (ANUE, the ratio of net photosynthesis to leaf nitrogen content) and intrinsic water use efficiency (WUE, the ratio of net photosynthesis to stomatal conductance), measurements were also made at optimal conditions of 1,500 μmol m−2 s−1 saturating light intensity, 20°C leaf temperature, ca. 50% relative humidity and 370 μmol mol−1 ambient CO2 concentration for six to eight individuals per site.
Determination of light-curve parameters
Light curve data were fitted by the empirical non-linear equation of Hanson et al. (1987):
where PPFD is photosynthetic photon flux density. Several physiologically important parameters could thus be estimated: maximum rate of photosynthesis at the asymptote of the curves (A max), dark respiration rate (R d), and light compensation point (LCP). Apparent quantum efficiency (AQE), i.e., the initial slope of the curve, was calculated as the slope from a simple linear regression of the first four PPFD values above 0 μmol m−2 s−1 (25, 50, 75, 100 μmol m−2 s−1).
Determination of CO2-curve parameters
A non-rectangular hyperbola type developed by Ethier and Livingston (2004) after the biochemical model of leaf photosynthesis of Farquhar et al. (1980) was used to fit CO2 response curves:
where A c and A j are the Rubisco- and RuBP-limited net photosynthetic rate, respectively. V cmax is the maximum rate of CO2 carboxylation, J is the electron transport rate, g m is internal CO2 transfer conductance or mesophyll conductance, defined as internal conductance to CO2 between the intercellular spaces and chloroplasts, R d is respiratory rate of mitochondria in the light, and Г* is the chloroplastic CO2 photocompensation point. O is the oxygen concentration (200 mmol mol−1), and K c, K o are the Michaelis–Menten constants for CO2 and O2, respectively. In the present case, the values of Г*, K c and K o were derived from Bernacchi et al. (2001) and adjusted to 20°C (Г* = 32.8 μmol mol−1; K c = 231.2 μmol mol−1; K o = 213.9 mmol mol−1). Parameters of V cmax, J, and g m were thus estimated by non-linear least squares fit according to Levenberg–Marquardt method (Bates and Watts 1988). Here, a modified form of photosynthesis model was used because it can validly estimate g m (e.g., Niinemets et al. 2005, 2006), which is obtained by gas exchange analysis without the need for any measurements of leaf chlorophyll fluorescence, if g m alone is considered. If not, V cmax was generally underestimated (Ethier and Livingston 2004). Additionally, the model also fitted our data well and explained variance of the fitting equation to be between 98.6 and 99.6%. The maximum rate of electron transport (J max) was obtained from the empirical equation of Smith (1937) characterized with the PPFD-dependency of potential electron transport rate.
Chlorophyll fluorescence
The F v/F m measurements across light regimes were taken at midday and after sunset to examine the midday depression of PSII quantum yield related to down-regulation of PSII and recovery of photoinhibition (Yamashita et al. 2000). Before measurements, at least 30 min dark adaptation was initiated. Using an FMS-2 pulse modulated fluorometer (Hansatech, King's Lynn, UK), minimal fluorescence (F o) was determined. Maximum fluorescence (F m) was determined after a 0.8 s pulse of 8,000 μmol m−2 s−1 saturating light, and variable fluorescence (F v = F m − F o) was computed.
Analysis of leaf structure and biochemistry
Leaf samples were taken from the same leaves used for gas-exchange measurements. Leaf areas were measured with a leaf area meter (LI-3000A, LI-COR). Leaf dry mass was determined after drying for 72 h at 70°C and LMA (g m−2) was calculated using these data. Total leaf nitrogen (N) content was then measured using an N analyzer (LECO FP-428, St Joseph, MI). Leaf chlorophylls and carotenoids were immediately extracted by direct immersion of six intact discs (0.28 cm2/disc) into the solvent N,N-dimethylformamide (DMF). The absorbance of extracts was measured at 450, 664.5, and 647 nm with a spectrophotometer UV-2550 (Shimadzu, Tokyo, Japan). Leaf chlorophyll a and b content were determined using the formula of Inskeep and Bloom (1985). Total leaf carotenoids were computed according to Jaspars (1965).
Estimation of apparent nitrogen partitioning among components of photosynthetic apparatus
The model proposed by Niinemets and Tenhunen (1997) was used to estimate the proportion of apparent nitrogen investment in Rubisco (P R), bioenergetics (P B) and thylakoid light harvesting (P L) from estimated values of V cmax, J max and measured leaf N and chlorophyll content. In the model, P R (nitrogen in Rubisco) has an overall effect on the capacity for CO2 carboxylation (i.e., V cmax), P B (nitrogen in bioenergetic pools) influences the capacity for photosynthetic electron transport (i.e. J max), and P L (nitrogen in light capture) determines the content of chlorophyll a/b protein complexes associated with photosystems I and II. According to Niinemets and Tenhunen (1997), the values of P R (gram N in Rubisco per gram total leaf N), P B (gram N in cytochrome f, ferredoxin NADP reductase and the coupling factor per gram total leaf N) and P L (gram N in PSI, PSII and LHCII (gram total leaf N)−1) were calculated as follows:
where V cr [μmol CO2 (g Rubisco)−1 s−1] is maximum rate of RuBP carboxylation per Rubisco protein, J mc [mol electron (mol cyt f)−1 s−1] is the capacity for photosynthetic electron transport per cytochrome f, the coefficient 6.25 [g Rubisco (g N in Rubisco)−1] converts nitrogen content to protein content, and 8.06 [μmol cyt f (g N in bioenergetics)−1] is used based on the assumption of a constant 1:1:1·2 molar ratio for cyt f: ferredoxin NADP reductase: coupling factor (Niinemets and Tenhunen 1997). Additionally, the model has considered the V cr and J mc values conserved among C3 species (Niinemets and Tenhunen 1997; Niinemets et al. 2006). From the temperature dependency of V cr and J mc described by Niinemets and Tenhunen (1997), the values of V cr and J mc at 20°C were equal to 12.6 [μmol CO2 (g Rubisco)−1 s−1] and 131.9 [mol electron (mol cyt f)−1 s−1], respectively. Narea, Nmass is leaf nitrogen content per area (g m2) and per leaf dry mass (%), respectively. Chlmass, is leaf chlorophyll concentration (nmol g−1). The factor for adjustment is 1,000. C B is the ratio of chlorophyll binding to N invested in light harvesting and calculated according to Hikosaka and Terashima (1995) and Niinemets and Tenhunen (1997).
Statistical analysis
A non-linear least squares technique was used to examine the photosynthetic responses to irradiance and CO2 concentration. A linear or quadratic relationship was used to test the interrelations between leaf traits and photosynthetic parameters and coefficients of leaf nitrogen partitioning. Comparisons between pairs of means for variables were made by the post hoc Tukey HSD tests. All data analysis was performed using SPSS 13.0 statistical software (SPSS, Chicago, IL).
Results
Effects of light availability on photosynthesis and leaf traits
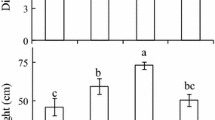
Contrasting light environments significantly affected leaf photosynthetic, biochemical and structural compositions of C. flavum across the study sites (Fig. 1; Table 2). In plants grown under a HL habitat, mean light-saturated photosynthesis per area (A max) was 8.5 μmol m−2 s−1, which was 27.1% or 36.5% greater than that under ML (6.2 μmol m−2 s−1) or LL (5.4 μmol m−2 s−1) conditions, respectively (P < 0.001, Table 2), whereas mass-based light-saturated photosynthesis (A mass) remained fairly constant. Like A max, the maximum rate of carboxylation (V cmax) and electron transport (J max) both increased greatly (P < 0.001, Table 3); furthermore, there was a strong correlation between them (r 2 = 0.96, P < 0.001). The LCP of leaves developed was highest at HL, lowest at ML, and intermediate at LL. Plants grown under HL conditions also had a greater dark respiration rate (R d) than those grown under ML or LL conditions, although the difference was not significant (P > 0.05). The AQE was independent of growth light environment (P > 0.05).
Characteristic responses of leaf net photosynthetic rate (A) to a photosynthetic photon flux density (PPFD) and b intercellular CO2 concentration (Ci) in Cypripedium flavum growing along a natural light gradient. Measurements were made under the following conditions: a 370 μmol mol−1 ambient CO2 concentration, 20°C leaf temperature, and ca. 50% relative humidity; b 800 μmol m−2 s−1 PPFD, 20°C leaf temperature, and ca. 50% relative humidity. The data were fitted to the equation of Hanson et al. (1987) and the modified biochemical model of leaf photosynthesis of Ethier and Livingston (2004), respectively. Each point represents mean ± SE (n = 3). Inlet: linear portion of light response curves used to determine apparent quantum efficiency; the slopes of these regressions were not significantly different (P > 0.05)
A large effect for LMA and mesophyll conductance (g m) was found in this study (P < 0.001, Table 2): both increased strongly with irradiance, and for HL leaves, there was an almost two-fold variation relative to LL leaves. Leaf N content per area (Narea) was highest at HL, and mass-based leaf N content (Nmass) greatest in LL leaves. Leaf chlorophyll content per area (Chlarea) shared the same trend as R d. The ML leaves had a lowest Nmass and Chlarea, but the same Narea as LL leaves (Table 2). The difference in Nmass between ML and LL leaves may thus reflect differing availability of soil N, while the similar Narea indicates some adjustment in leaf morphology due to changes in LMA. In addition, with increasing irradiance, leaf chlorophyll a to b (Chla/Chlb) ratio and carotenoid content per area (Cararea) increased sharply (P < 0.05). In contrast, mass-based leaf chlorophyll content (Chlmass) was lower in HL or ML leaves than in LL leaves. The similarity in measured F v/F m, whether at midday or after sunset for all light levels, being around 0.8 (Table 2), showed that the photosynthetic PSII was not impaired.
Leaf N partitioning within photosynthetic components
The proportions of leaf N partitioning in carboxylation (P R) and bioenergetics (P B) increased with irradiance (P < 0.05), matching well with changes in V cmax and J max (Table 3). Like the strong relationship between V cmax and J max, P R also correlated strongly with P B (r 2 = 0.91, P < 0.001). The proportion of leaf N partitioning in light harvesting (P L) was higher in HL leaves (P < 0.05). In contrast, the LL leaves had a significantly higher amount of chlorophyll binding relative to N invested in light harvesting (C B) than did the other two (P < 0.05), which indicates that the understory-grown leaves have greater efficiency for weak light use.
Relationships between photosynthetic capacity and leaf N, LMA, g m
On a leaf area basis, there was a strong correlation between A max and Narea (Fig. 2a), while there was less significance between values based on leaf mass (r 2 = 0.43, P = 0.059). Likewise, A max correlated highly with both LMA (Fig. 2b) and g m (Fig. 2c). As with Narea and LMA, V cmax followed a similar response pattern (Fig. 3a, b), and so did J max (data not shown). In contrast, the J max/V cmax ratio did not correlate with Narea (Fig. 3d), although it was positively coupled with LMA (Fig. 3c). And LMA and Nmass were negatively correlated (r 2 = 0.67, P = 0.008).
The relationship between light-saturated net photosynthetic rate (A max) and a leaf nitrogen content per area (N area), b leaf dry mass per area (LMA), and c internal mesophyll conductance (g m), across different light regimes. Symbols as in Fig. 1
Effects of LMA (a, c) and Narea (b, d) on maximum rate of carboxylation (V cmax) (a, b), and the ratio of maximum electron transport rate (J max) to V cmax (c, d) across different light regimes. Symbols as in Fig. 1
Effects of LMA and leaf N content on leaf N partitioning
Both P R and P B positively correlated with LMA (r 2 = 0.75, P = 0.003, for P R; r 2 = 0.82, P < 0.001, for P B; Fig. 4a), whereas there was a less significant relationship between P L and LMA (r 2 = 0.44, P = 0.052; Fig. 4b). A quadratic relationship was found between LMA and Chlmass (Fig. 4b).
Effects of LMA and mass-based nitrogen content (Nmass) on leaf nitrogen allocation in photosynthetic functions within a leaf. a Fractions of leaf nitrogen partitioning into Rubisco (P R) and bioenergetic pools (P B) as affected by LMA; b total leaf chlorophyll content (Chl mass) and nitrogen investment in thylakoid light harvesting (P L) as affected by LMA; c P R and P B as affected by Nmass. Symbols as in Fig. 1
Apart from LMA effects, leaf N content per se was also related to leaf N partitioning. Both P R and P B negatively correlated with Nmass (r 2 = 0.71, P = 0.003, for P R; r 2 = 0.62, P = 0.012, for P B; Fig. 4c), but not with Narea (data not shown). P L, however, did not relate to Nmass (r 2 = 0.12, P = 0.37), indicating that leaf N concentration apparently did not change N investment to light capture.
Resource use efficiency
Table 4 shows changes in stomatal conductance (g s), maximum photosynthetic N use efficiency (ANUE) and WUE in C. flavum growing along a natural light gradient. For all variables, the leaves developed at higher light had greater values compared to those at lower light, in spite of no statistically significant change in WUE.
Discussion
The present study suggests that C. flavum shows a considerable amount of leaf photosynthetic plasticity according to light environment, indicating a high correspondence between leaf photosynthetic characteristics and habitat preference. In contrast to leaves under LL, leaves developed under HL had greater A max, LCP and R d, although no statistically significant differences in LCP or R d between light conditions were observed, as also reported by previous studies (Björkman 1981). For a similar range of light regimes, A max increased by a factor of 1.6 with increasing light intensity in C. flavum, as is evident in several other species (Muraoka et al. 2002; Piel et al. 2002; Aleric and Kirkman 2005). The change in A max, however, was smaller than in the case of Alocasia macrorrhiza (Sims and Pearcy 1989), and differed from that of Acer saccharum (Ellsworth and Reich 1992) in that there was no further increase at high light conditions. This difference may result from differences in the intrinsic nature of photosynthetic potential of plant types (e.g., Sims and Pearcy 1989). It has been argued that greater LMA at high light potentially contributes to photosynthetic potential (Terashima et al. 2001), as was the case in this study (Fig. 2b). Typically, this is attributed to the response of leaf mesophyll tissues stimulated with high light (Oguchi et al. 2003; Nomura et al. 2006), which can further increase the content of the enzyme Rubisco and also of other electron carriers in the chloroplast stroma (Björkman 1981; Oguchi et al. 2003). If so, it should be expected that there is a positive correlation between V cmax and LMA (Fig. 3a), similar to the responses of Abies alba (Grassi and Bagnaresi 2001). Another potential result of altered leaf structure lies in changing internal leaf CO2 transfer conductance (g m), which depends greatly on leaf mesophyll organization as affected by light during growth (Piel et al. 2002; Nomura et al. 2006). That there is a marked correlation between g m and both A max and LMA has been well established (Evans and Loreto 2000; Niinemets and Sack 2006); our result are also in agreement with this. Furthermore, in this study, as for the change in A max mentioned earlier, both g m and LMA changed by a factor of about two, which is similar to the case of walnut trees (Piel et al. 2002). Responding to high light, leaves with increased LMA could possess sufficient mesophyll surface (which must also be qualified by smaller cell size, particularly in alpine plants) for efficiently transferring CO2 within leaves in terms of efficient carbon gain (Terashima et al. 2006). Although stomatal conductances (g s) clearly differed among light environments in this case (Table 4), the relative constancy in intercellular CO2 concentration (averaging 283 ± 2 μmol mol−1) due to concurrent changes in A and g s suggests that stomatal limitations to photosynthesis were very similar under these conditions. In C. flavum, therefore, the strong correspondence among LMA, g m, and A max observed in HL-grown plants probably reflects plasticity of the leaf anatomy so as to ease the internal resistance and/or decrease other structural costs within the leaf (Evans and Loreto 2000; Niinemets and Sack 2006), given the high leaf construction cost required at HL for high photosynthetic capacity (Terashima et al. 2001).
On the other hand, leaf physiological plasticity of a species is limited by efficient use of leaf N in the photosynthetic apparatus, which likely affects the plant’s preference for habitat site (Hikosaka and Terashima 1996). Necessitating efficient use of a limiting light resource, a shade-tolerant species often increases the fraction of leaf N in light harvesting (P L) with decreasing irradiance (Evans 1989). In this study, however, the P L value was lower in plants grown under LL (Table 3), contrasting with some studies (Niinemets and Tenhunen 1997; Grassi and Bagnaresi 2001; Katahata et al. 2007), but similar to the case of Quercus rubra and A. sacchrum transferred to high light (Naidu and DeLucia 1998). Possibly, a lower P L at LL partially reflects a slightly lower AQE (Naidu and DeLucia 1998). Conversely, the AQE in C. flavum responded similarly to light environments. One possible reason for this thus may be the low J max under LL (Table 3), given the dual functions of thylakoid chlorophyll–protein complexes, which are involved not only with light capture but also with electron transport (Hikosaka and Terashima 1995; Niinemets and Tenhunen 1997).
Further, a lower ratio of Chla/Chlb at LL (Table 2) means more light harvesting chlorophyll of PSII and fewer PSII reaction centers (Hikosaka and Terashima 1996), which are required for plants adapted to low light in order to balance the light capture capacity in both photosystems (Björkman 1981). Economically, more chlorophyll invested within a leaf-per-leaf mass (Fig. 4b; Murchie and Horton 1997) and a higher ratio of chlorophyll binding to N invested in light harvesting (CB) (Table 3) likewise contribute to the efficient use of low light and reduced associated protein costs to enhance light absorptance per mass (Sims and Pearcy 1989). Moreover, as suggested by Niinemets and Tenhunen (1997), the fact that both P R and P B decreased with increasing Nmass (Fig. 4c) observed supports these conclusions.
Under high irradiance, the realized photosynthetic rate is co-regulated more by investments of leaf N to carboxylation and electron transport (i.e., P R and P B) compared with low irradiance (Hikosaka and Terashima 1995). Our results agree with those of other studies (Niinemets and Tenhunen 1997; Evans and Poorter 2001), but differ from those in Katahata et al. (2007), where the authors found that P R and P B remained relatively stable with different light treatments, and contrasted with Frak et al. (2002). In C. flavum, the estimated proportion of leaf N allocation in all components of the photosynthetic machinery (i.e., the sum of P R + P B + P L) varied from 51% to 70% along a light gradient, which is higher than that of many woody plants (Niinemets and Tenhunen 1997; Grassi and Bagnaresi 2001; Katahata et al. 2007). It is well known that most herbaceous species will invest more than half of total leaf N into proteins associated with photosynthesis, especially the Rubisco enzyme (Evans 1989; Hikosaka and Terashima 1996). With increasing LMA there was a clear pattern of improving allocation of N to Rubisco and electron transport, but less significant increments were found in light capture systems (Fig. 4). This may imply that leaf N re-organization working for high light responses could be an indirect result of changes in leaf structure, because accumulation of leaf dry mass was associated with dilution of leaf N (Niinemets et al. 2006).
The ratio of J max/V cmax is modulated by the extent and nature of coordination of both carboxylation and the electron transport processes that determine photosynthetic potential. Many studies have shown that this ratio remains relatively constant in response to different light conditions (Grassi and Bagnaresi 2001; Piel et al. 2002; Katahata et al. 2007), whether g m is considered or not (von Caemmerer and Farquhar 1981; Piel et al. 2002). In contrast, our results show that the J max/V cmax ratio was significantly greater under HL (Table 3), as in the case of a study by Sims and Pearcy (1989) who argued that high light may increase this ratio, particularly at low growth temperatures. For some plants, leaf photosynthesis at low temperature may be more limited by the capacity for RuBP regeneration (Onoda et al. 2005). Hence, the shifting of the ratio towards electron transport side could lessen the limitation related to RuBP regeneration balancing RuBP carboxylation, and lead to increased net carbon gain (Onoda et al. 2005). The growth and distribution of C. flavum are restricted within alpine regions, where high irradiance together with low temperatures occur frequently during the growing season (Friend and Woodward 1990). Thus, a higher J max/V cmax ratio at HL may benefit plants that adapt to these unfavorable conditions and maintain high photosynthetic activity. Additionally, at HL, higher P B (Table 3) with lower Chlmass and higher Chla/Chlb (Table 2) also favored a higher J max/V cmax ratio. C. flavum may depend on photoprotective mechanisms such as enhanced xanthophylls cycle-dependent energy dissipation as heat under high light (Naramoto et al. 2006). In fact, high soil N availability in high light could contribute to lower susceptibility to photodamage by enhancing the central role of leaf N in fixing CO2 (Kitajima and Hogan 2003).
In response to a wide range of environmental conditions, these favorable traits exhibited by plants might act as important functions in shaping their physiological plasticity and tolerance, and ability to compete with other species (Murchie and Horton 1997; Yamashita et al. 2000; Gulías et al. 2002). For acquisition of available resources, C. flavum needs to be able to cope with co-occurring species such as C. guttatum in its natural distribution ranges. Although the leaf A max of a widely distributed species like C. guttatum was greater than that of C. flavum when grown at 40–50% of full sunlight, g s in both species was comparable, and the latter species had a higher Chlarea (Zhang et al. 2006). Alternatively, when both were exposed to contrasting light conditions, their ability to adjust leaf structure and biochemistry under a given irradiance was very similar (Zhang et al. 2007). Such properties thus may functionally contribute to coexisting species competing in a similar niche, e.g., by a compensatory effect of higher Chlarea (Murchie and Horton 1997). However, the lower photosynthetic capacity in C. flavum may limit its habitat expansion.
In conclusion, adjustments in leaf structure and nitrogen re-allocation in C. flavum leaves in response to the natural growth light environment were realized to maintain a functional balance in the photosynthetic machinery. Furthermore, provided with an adequate supply of soil nitrogen, C. flavum may help protect leaf photosynthesis by efficient nitrogen use, and a higher stomatal and mesophyll conductance against photoinhibition at high light. It is well known that habitat destruction through logging and illegal collection severely threatens the survival and persistence of this species. In the light of the observed photosynthetic plasticity, however, C. flavum could cope with current light regimes. Light factors should therefore be appropriately considered in future management and reintroduction efforts concerning this species.
References
Aleric KM, Kirkman LK (2005) Growth and photosynthetic responses of the federally endanger shrub, Lindera melissifolia (Lauraceae), to varied light environments. Am J Bot 92:682–689
Bates DM, Watts DG (1988) Nonlinear regression and its applications. Wiley, New York
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology I. Encyclopedia of plant physiology, vol 12A. Springer, Berlin, pp 57–107
Cribb P (1997) The genus Cypripedium. Timber Press, Oregon
Cribb P, Sandison MS (1998) A preliminary assessment of the conservation status of Cypripedium species in the wild. Bot J Linn Soc 126:183–190
Ellsworth DS, Reich PB (1992) Leaf mass per area, nitrogen content and photosynthetic carbon gain in Acer saccharum seedlings in contrasting forest light environments. Funct Ecol 6:423–435
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evans JR, Loreto F (2000) Acquisition and diffusion of CO2 in higher plant leaves. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism. Kluwer, Dordrecht, pp 321–351
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthesis CO2 assimilation in leaves of C3 species. Planta 149:78–90
Frak E, Le Roux X, Millard P, Dreyer E, Jaouen G, Saint-Joanis B, Wendler R (2001) Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant Cell Environ 24:1279–1288
Frak E, Le Roux X, Millard P, Adam B, Dreyer E, Escuit C, Sinoquet H, Vandame M, Varlet-Grancher C (2002) Spatial distribution of leaf nitrogen and photosynthetic capacity within the foliage of individual trees: disentangling the effects of local light quality, leaf irradiance, and transpiration. J Exp Bot 53:2207–2216
Friend AD, Woodward FI (1990) Evolutionary and ecophysiological responses of mountain plants to the growing season environment. Adv Ecol Res 20:59–124
Grassi G, Bagnaresi U (2001) Foliar morphological and physiological plasticity in Picea abies and Abies alba saplings along a natural light gradient. Tree Physiol 21:959–967
Gulías J, Flexas J, Abadia A, Medrano H (2002) Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol 22:687–697
Hanson PJ, McRoberts RE, Isebrands JG, Dixon RK (1987) An optimal sampling strategy for determing CO2 exchange rate as a function of photosynthetic photon flux density. Photosynthetica 21:98–101
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Hikosaka K, Terashima I (1996) Nitrogen partitioning among photosynthetic components and its consequences in sun and shade plants. Funct Ecol 10:335–343
Inskeep WR, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol 77:483–485
Jaspars EMJ (1965) Pigmentation of tobacco crown-gall tissues cultured in vitro in dependence of the composition of the medium. Physiol Plant 18:933–940
Katahata S, Naramoto M, Kakubari Y, Mukai Y (2007) Photosynthetic capacity and nitrogen partitioning in foliage of the evergreen shrub Daphniphyllum humile along a natural light gradient. Tree Physiol 27:199–208
Kitajima K, Hogan KP (2003) Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ 26:857–865
Liu P, Yang YS, Xu G, Hao C (2006) Physiological response of rare and endangered seven-son-flower (Heptacodium miconioides) to light stress under habitat fragmentation. Environ Exp Bot 57:32–40
Naidu SL, DeLucia EH (1998) Physiological and morphological acclimation of shade-grown tree seedlings to late-season canopy gap formation. Plant Ecol 138:27–40
Naramoto M, Katahata S, Mukai Y, Kakubari Y (2006) Photosynthetic acclimation and photoinhibition on exposure to high light in shade-developed leaves of Fagus crenata seedlings. Flora 201:12–126
Muraoka H, Tang Y, Koizumi H, Washitani I (2002) Effects of light and soil water availability on leaf photosynthesis and growth of Arisaema heterophyllum, a riparian forest understory plant. J Plant Res 115:419–427
Murchie EH, Horton P (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20:438–448
Niinemets Ü, Sack L (2006) Structural determinants of leaf light-harvesting capacity and photosynthesis potentials. In: Esser K, Lüttge UE, Beyschlag W, Murata J (eds) Progress in botany, vol 67. Springer, Berlin, pp 385–419
Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T (2005) Leaf internal conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ 28:1552–1566
Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T (2006) Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ 29:1159–1178
Nomura N, Setoguchi H, Takaso T (2006) Functional consequence of stenophylly for leaf productivity: comparison of the anatomy and physiology of a rheophyte, Farfugium japonicum var. luchuence, and a related non-rheophyte, F. japonicum (Asteraceae). J Plant Res 119:645–656
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need changes in leaf anatomy? Plant Cell Environ 26:505–512
Onoda Y, Hikosaka K, Hirose T (2005) The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Func Plant Biol 32:903–910
Piel C, Frak E, Le Roux Z, Genty B (2002) Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut. J Exp Bot 53:2423–2430
Sims DA, Pearcy RW (1989) Photosynthetic characteristics of a tropical forest understory herb, Alocasia macrorrhiza, and a related crop species, Colocasia esculenta grown in contrasting light environments. Oecologia 79:53–59
Smith E (1937) The influence of light and carbon dioxide on photosynthesis. Gen Physiol 20:807–830
Terashima I, Miyazawa SI, Hanba YT (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114:93–105
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354
Valladares F, Chico J, Aranda F, Balaguer L, Dizengremel P, Manrique E, Dreyer E (2002) The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 16:395–403
von Caemmerer S, Farquhar GD (1981) Some relationship between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419
Zhang SB, Hu H, Xu K, Li ZR (2006) Gas exchanges of three co-occurring species of Cypripedium in a scrubland in the Hengduan Mountains. Photosynthetica 44:241–247
Zhang SB, Hu H, Xu K, Li ZR, Yang YP (2007) Flexible and reversible responses to different irradiance levels during photosynthetic acclimation of Cypripedium guttantum. J Plant Physiol 164:611–620
Acknowledgments
This work was funded by NSFC (no. 30470182, 30770225, 30770226), Knowledge Innovation Project (no. KSCX2-YW-Z-033) and West Light Foundation of CAS, and National Project for Sci-Tech Construction Platform (no. 2005DRA21006). We thank Dr Ülo Niinemets for clarifying the model of leaf nitrogen partitioning. We also thank Mr. Kun Xu for offering help and Professor Azza Tawfik for improving the English. During the study, we were greatly supported by Mr. Zhengdong Fang and his co-workers from the Shangri-la Alpine Botanic Garden. The anonymous reviewers were acknowledged for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, S., Hu, H. et al. Photosynthetic performance along a light gradient as related to leaf characteristics of a naturally occurring Cypripedium flavum . J Plant Res 121, 559–569 (2008). https://doi.org/10.1007/s10265-008-0186-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-008-0186-4