Abstract
Chloroplast photorelocation movement is important for plants to perform efficient photosynthesis. Phototropins were identified as blue-light receptors for chloroplast movement in Arabidopsis thaliana and in the fern Adiantum capillus-veneris, whereas neochrome functions as a dual red/blue light receptor in the latter. However, the signal transduction pathways involved in chloroplast movement remain to be clarified. To investigate the kinetic properties of signalling from these photoreceptors to the chloroplasts, we deduced the speed of signal transfer using Adiantum capillus-veneris gametophytes. When a region of dark-adapted gametophyte cells was subjected to microbeam irradiation, chloroplasts moved towards the irradiated area even in subsequent darkness. We therefore recorded the movement and calculated the speeds of signal transfer by time-lapse imaging. Movement speeds under red or blue light were similar, e.g., about 1.0 μm min−1 in prothallial cells. However, speeds varied according to cell polarity in protonemal cells. The speed of signal transfer from the protonemal apex to the base was approximately 0.7 μm min−1, but roughly 2.3 μm min−1 in the opposite direction. The speed of signal transfer in Arabidopsis thaliana mesophyll cells was approximately 0.8 μm min−1 by comparison. Surprisingly, chloroplasts located farthest away from the microbeam were found to move faster than those in close proximity to the site of irradiation both in Adiantum capillus-veneris and A. thaliana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light plays an essential role in plant development. Consequently, plants exhibit various light-dependent responses such as seed and spore germination (Shinomura et al. 1996; Sugai and Furuya 1967), inhibition of stem elongation (Ahmad and Cashmore 1993), phototropism (reviewed by Iino 2001), flowering (Hayama and Coupland 2003; Ishikawa et al. 2005), and chloroplast photorelocation movement (reviewed by Haupt 1999).
Among these phenomena, chloroplast movement is important for efficient photosynthesis and for plant survival. Chloroplasts move towards weak light to capture light energy efficiently (accumulation response) and move away from strong light to reduce the possibility of photodamage (avoidance response; Kasahara et al. 2002). Without the avoidance response, plants show necrosis and die under strong light (Kasahara et al. 2002). In Arabidopsis thaliana, phototropin 2 (Atphot2) was identified as the photoreceptor for the avoidance response (Jarillo et al. 2001; Kagawa et al. 2001), and both Atphot1 and Atphot2 for the accumulation response (Sakai et al. 2001). Phototropins also mediate other phenomena, such as phototropism and stomata opening (Christie et al. 1998; Kinoshita et al. 2001). In Adiantum capillus-veneris, 11 photoreceptor genes (2 phototropins, 5 cryptochromes, 2 phytochromes, and 1 phytochrome chromophore-binding fragment, and 1 neochrome) have been cloned and some have been characterized extensively (Kanegae and Wada 2006; Wada 2007). Through mutant analyses, Acphot2 was shown to mediate the chloroplast avoidance response induced by strong blue light (Kagawa et al. 2004). Neochrome 1 (Acneo1), which is a chimeric protein between phytochrome and phototropin, was shown to mediate the accumulation response under red light conditions (Kawai et al. 2003). Interestingly, Acneo1 also mediates red light-induced phototropism in protonemal cells (Kadota and Wada 1999; Kawai et al. 2003), negative phototropism in rhizoid cells (Tsuboi et al. 2006), and nuclear migration in prothallial cells (Tsuboi et al. 2007).
Although the photoreceptors involved in chloroplast movement are known in most plant groups (Suetsugu and Wada 2007), the signal transduction pathways, including the signal that is released from photoreceptors and transferred to the chloroplasts, have not been identified. It is clear, however, that the signal for the avoidance response has a short life-time and migrates over a short distance. In contrast, the signal for the accumulation response has a longer life-time and migrates throughout the cell from the irradiated area (Wada et al. 2003). Recent studies using the technique of microbeam irradiation has revealed that chloroplasts do not have a polarity for light-induced accumulation movement and can move freely in any direction both in Adiantum capillus-veneris prothallial cells and in Arabidopsis thaliana mesophyll cells (Tsuboi et al. 2009). These results suggest that a signal released from the photoreceptors could be transferred to the chloroplasts, and that the chloroplasts might show a tactic response towards higher concentrations of this signal, similar to the chemotaxis response of the amoeba cells of Dictyostellium (Vicker 1994). To understand the kinetic properties of the signal for the chloroplast accumulation response, we measured the speed of signal transfer in dark-adapted A. capillus-veneris gametophyte cells and Arabidopsis thaliana mesophyll cells by partial cell irradiation with a red and/or blue microbeam of various light intensities for 1 min or following continuous irradiation.
Materials and methods
Plant materials and culture conditions
Spores of the fern Adiantum capillus-veneris were collected in a greenhouse at Tokyo Metropolitan University in 1998 and stored at about 4°C until use. For protonemal cells, spores were sterilized for 30 s with 0.5% (v/v) Antiformin (Wako Pure Chemical Industries, Osaka), and were sown on the surface of medium containing White’s medium (Kagawa and Wada 1996), solidified with 0.5% INA agar (Funakoshi, Tokyo) in a 3 cm Petri dish and covered with a coverslip and cultured under unilateral red light about 10.7 μmol m−2 s−1 for 7 days. The protonemata obtained were irradiated with white light (20–30 μmol m−2 s−1) for 10 min to induce cell division at the apical region of protonemata and then incubated in the darkness for 24–26 h. The upper 400 μm part of a long basal cell of the two-celled protonemata, i.e., the region close to the septum of the cell division, was used for this experiment. For prothallial cells, sterilized spores of Adiantum capillus-veneris were sown between two layers of cellophane placed on White’s medium, solidified with 0.5% INA agar in a Petri dish (6 cm in diameter), and cultured under continuous white light (20–30 μmol m−2 s−1) for 2–3 weeks. The resulting prothallia were incubated for 24–26 h to allow chloroplasts along the periclinal walls to move towards the anticlinal walls (called “dark positioning”; Tsuboi et al. 2007). Prothallia that contained some chloroplasts still attached to the periclinal walls were used for the following experiments. All cultural and experimental procedures were conducted at 25°C.
For Arabidopsis thaliana, seeds were sown onto soil and cultured under about 60 μmol m−2 s−1 white light (16 h)/dark (8 h) cycle at 23°C for 7 weeks. Chloroplast movement was observed as described elsewhere (Kagawa and Wada 2000).
Light sources
Fluorescent lamps (FL40SD Toshiba Lighting and Technology, Tokyo) were used as the source of white light. Red light was obtained from the same lamp through a red plastic filter (Shinkolite A no. 102, Mitsubishi Rayon, Tokyo).
Microbeam irradiation
Dark-adapted prothallia or protonemal cells were transferred into a custom-made cuvette (25 mm in diameter, 5 mm in height), which was made from two steel rings and two round glasses with a silicon rubber ring spacer in between (Wada et al. 1983). The cuvettes with the prothallia or the protonemal cells were placed on the sample stage of a microbeam irradiator (Yatsuhashi and Wada 1990) under dark conditions. Parts of the cell were irradiated for 1 min or continuously with a microbeam with various light intensities of either red light with a transmission peak at 660 nm obtained through an interference filter (half band-width, 34 nm; IF-BPF-660, Vacuum Optics, Tokyo, Japan) or blue light with a transmission peak at 453 nm obtained through an interference filter (half band-width, 31 nm; IF-BPF-453, Vacuum Optics) and a blue plastic film (#63; Ryuden-sha, Tokyo, Japan). Chloroplast movement was observed using an infrared light obtained by halogen lamp (Focusline 12V–100W HAL, Philips Lighting, The Netherlands) through an infrared filter (IR85, Hoya, Tokyo, Japan) on a monitor screen attached to a camera (C2400-07ER; Hamamatsu Photonics; Hamamatsu, Japan) sensitive to infrared light. The microbeam and its position could be observed under infrared light at a higher fluence rate than the beam used to observe the materials. After setting the irradiation site with the infrared microbeam, the microbeam light was changed from infrared to either red or blue light as needed. All procedures in the dark were performed under a dim green safe light. Images were taken at 1-min intervals and processed and analyzed with “ImageJ” software version 1.33 (shared with http://rsbweb.nih.gov/ij/).
Results
Irradiation-induced movement images of chloroplasts in dark-adapted two-cell protonema of Adiantum capillus-veneris were taken on a time-lapse video imaging apparatus. A red microbeam of 20 μm in width and 1 W m−2 for 1 min was applied to a basal cell region, which was 200 μm away from the cell plate separating the apical cell (Fig. 1a1, shaded area). Chloroplasts located within about 50 μm from the microbeam responded to this light signal within a lag time of 10 min, moving towards the irradiated area. They eventually reached the microbeam-irradiated area within an observation time of 60 min. Chloroplasts located more than ~100 μm away from the microbeam responded to the light signal, but some of them stopped moving and did not reach microbeam-irradiated area (Fig. 1a2–a4). Irradiation-induced chloroplast movement images were also captured in a dark-adapted prothallia cell, using the time-lapse video imaging apparatus. In this case, a red circular microbeam of 10 μm in diameter and 1 W m−2 for 1 min was applied (Fig. 1b1). Chloroplasts located around 10 μm from the irradiation center began to move towards the irradiated area after a lag time of 2–3 min, and these chloroplasts reached the irradiation area within an observation time of 60 min. However, chloroplasts located more than 20 μm away from the microbeam did not respond to the irradiance signal (Fig. 1b2–b4).
a Serial photographs showing chloroplast movement in the upper part of a basal cell of a dark-adapted two-celled protonema of Adiantum capillus-veneris. Chloroplast movement was induced by a 1-min irradiation with a red microbeam (1 W m−2, 20 μm in width), and was continually observed and recorded under infrared light. 1 Photograph showing part of a dark-adapted protonemal cell with some chloroplasts located along the cell wall (arrowheads). An area 200–220 μm from the cell plate (white arrow) between the apical (left side of photographs) and the basal cell of protonema was irradiated with a red microbeam (1 W m−2, shaded area) for 1 min. 2, 3, 4 Photographs taken at 20-min intervals after the start of microbeam irradiation. Note that the chloroplasts located near the microbeam-irradiated area (black arrows) begin to move towards the irradiated area shortly after commencement of microbeam irradiation, but those far from the irradiated area (white arrowheads) start moving after some lag time and stop before reaching the irradiated area. For the analysis of chloroplast behavior, photographs were taken at 1-min intervals under infrared light. Bar 10 μm. b Photographs showing chloroplast movement in a prothallial cell induced by red microbeam irradiation (1 W m−2, 10 μm in diameter) for 1 min. 1 Dark-adapted A. capillus-veneris gametophyte cell with some chloroplasts still attached along the periclinal wall. Chloroplasts at the periphery of the cell (arrowheads) were continually observed and recorded under infrared light. White circle Microbeam-irradiated area. 2, 3, 4 Photographs taken at 15-min intervals after the start of microbeam irradiation. For analytical studies, photographs were taken at 1-min intervals under infrared light. Open circles Microbeam-irradiated area. Bar 10 μm
Chloroplast movement behavior during the accumulation response in protonemal cells was recorded every 1 min, and the relationship between the lag time before the onset of movement and the distance between the center of the microbeam and each chloroplast was plotted (Fig. 2). Chloroplasts started moving from their initial positions towards the microbeam-irradiated area after several minutes lag time. However, the greater the distance between a chloroplast and the microbeam irradiated area, the longer the lag time before the onset of movement was observed. The lag time measured most likely represents the time period required for signal transfer from the microbeam-irradiated area to the chloroplast, and demonstrates that the speed of signal transfer for the chloroplast accumulation response could be calculated. Hence, the relationship between the lag time and distance was studied more thoroughly using many chloroplasts at various positions from the red microbeam (1 W m−2 for 1 min at 200–220 μm from the cell plate). In Fig. 3a, the data presented as black circles were obtained from chloroplasts moving from the apical region of the protenemal cell towards the microbeam area, and shows that the signal was transferred from the cell base to the apex of the cell. Conversely, when chloroplasts moved from the cell base to the apex, the signal was transferred from the opposite direction (white circles). The slope of the approximate line in Fig. 3a indicates the average speed of signal transfer in protonemal cells. The speed of signal transfer towards the protonemal apex under red light (1 W m−2 for 1 min) is about 2.32 μm min−1, whereas the speed towards the protonemal base is about 0.58 μm min−1 (Fig. 3a). These findings therefore suggest that the speed of signal transfer is influenced by cell polarity.
Relationship between the starting times of chloroplast movement towards the microbeam irradiated area and the distance between the center of the microbeam and each chloroplast in protonemal cells. Data were obtained from the same movies used in the time course study shown in Fig. 2. The slopes of the approximate lines indicate the speed of signal transfer in each treatment. Closed circles Data obtained from chloroplasts moving towards the protonemal apex, open circles chloroplasts moving toward the protonemal base. The 200–220 μm region from the cell plate between apical and basal cells was irradiated with a red light microbeam with a fluence rate of 1 W m−2 for 1 min (a) or continuously (b), with a red microbeam of 0.1 W m−2 (c) or 10 W m−2 (d), or with a blue microbeam of 1 W m−2 continuously (e). The 0–20 μm region from the cell plate was irradiated with 1 W m−2 of red light continuously (f)
We next examined whether continuous irradiation with a red microbeam (1 W m−2), in contrast to pulse irradiation (1 min), made any difference to the speed of signal transfer for chloroplast movement (Fig. 3b). However, no dramatic differences were observed in the speeds of signal transfer under continuous irradiation relative to pulse irradiation, i.e., 2.37 μm min−1 from base to apex, and 0.72 μm min−1 from apex to base. Similarly, the effect of fluence rate on the speed of signal transfer for chloroplast movement was examined under continuous irradiation with a red microbeam of 0.1 and 10 W m−2 (Fig. 3c, d) or with a blue microbeam of 1 W m−2 (Fig. 3e). Yet, treatments with different fluence rates and wavelengths of light did not affect the speed of signal transfer for chloroplast movement in either direction. Finally, to compare the speed of signal transfer for chloroplast movement to the opposite direction within the same region (within 200 μm of the cell plate), an area 0–20 μm from the cell plate was irradiated continuously with a red microbeam (1 W m−2) and the speed of signal transfer was calculated from the apical to the basal region (Fig. 3f). The speed of movement towards the protonemal base was about 0.82 μm min−1 and was very different from the speed determined for the opposite direction, i.e., 2.37 μm min−1 (Fig. 3b). However, this velocity was similar to the speed of 0.72 μm min−1 obtained at a region of 220–400 μm from the cell plate (Fig. 3b). These results therefore indicate that the speed of signal transfer for chloroplast movement is influenced by cell polarity, irrespective of their position within the protonemal cell.
The relationship between the initial chloroplast position and the maximum speed of movement (distance moved within 1 min) of each chloroplast, in addition to the region where the maximum speed of movement takes place, is shown in Fig. 4. These data were obtained from the same time-lapse movies used for Fig. 3, thus the light conditions and experimental procedures are the same. In brief, the 200–220 μm region from the cell plate of the dark-adapted protonemal cell was irradiated with a red microbeam pulse (1 min) of 1 W m−2 (Fig. 4a) or continuously (Fig. 4b), or with a red microbeam of 0.1 and 10 W m−2 (Fig. 4c, d, respectively) or with a blue microbeam of 1 W m−2 (Fig. 4e) continuously. The region of 0–20 μm from the cell plate was also irradiated continuously with a red microbeam of 1 W m−2 (Fig. 4f). During photorelocation movement, chloroplasts of protonemal cells moved irregularly but not at a constant speed. They also changed their moving speed frequently, or stopped moving or even moved backward. Nevertheless, they ultimately reached the microbeam-irradiated area. In this regard, calculation of the average speed of chloroplast movement during a long duration is not meaningful for studying the mechanism of chloroplast movement. Hence, the moving speed of chloroplasts was measured every 1 min from the beginning to the end of its movement and the maximum speed during the travel (i.e., the maximum migration distance of each chloroplast in 1 min) was plotted at the position where each chloroplast was localized initially. Interestingly, chloroplasts located farther from the microbeam-irradiated area moved faster irrespective of light conditions or cell polarity. The relationship between the initial positioning of chloroplasts and the region where the highest movement speed takes place was investigated to determine whether a particular area of the protonema cell might contribute to the speed of movement or whether a time lag or distance after the initiation of movement is important for speed (Fig. 4b, e). However, no particular cellular region or timing after the start of movement was found to correlate with fast movement speeds.
Relationship between the highest speed of movement occurring during movement observation, and the initial position of chloroplasts from the microbeam-irradiated area in protonemal cells. The distances travelled every minute in each chloroplast were obtained during the period of chloroplast movement, and the fastest speed during this movement was plotted at the initial position of chloroplasts from the microbeam-irradiated area. Additionally, in b and e, the positions where fastest speed took place were plotted relative to the initial position of the chloroplast. Data were obtained from the same movies used for the analyses in Fig. 3. For other details, see legend to Fig. 3
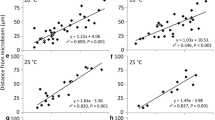
Next, the speed of signal transfer in dark-adapted prothallial cells was measured. A small area (10 μm in diameter as a circular spot) of a dark-adapted prothallial cell was irradiated for 1 min (Fig. 5a) or continuously (Fig. 5b) with a red microbeam of 1 W m−2, or continuously with a red microbeam of 0.1 or 10 W m−2 (Fig. 5c, d, respectively) or a blue microbeam of 1 W m−2 (Fig. 5e). The speed of signal transfer calculated was about 1.0 μm min−1 irrespective of the light conditions.
Relationship between the starting times of chloroplast movement towards the microbeam-irradiated area and the distance between the center of the microbeam and each chloroplast in prothallial cells. Part of the dark-adapted prothallial cell was irradiated with a microbeam (10 μm in diameter) of 1 W m−2 of red light for 1 min (a) or continuously (b), or with a red microbeam of 0.1 W m−2 (c) or 10 W m−2 (d), or with 1 W m−2 of blue light (e) continuously. The slopes of the approximate lines indicate the speed of signal transfer in each treatment
The speed of chloroplast movement (calculated from the distance moved during 1 min) was measured every minute (Fig. 6) in the same prothallial cells used for Fig. 5. The data were obtained from the time-lapse movies recorded under 1 min or with continuous irradiation with a red microbeam of 1 W m−2 (Fig. 6a, b, respectively) or continuous irradiation with a red microbeam of 0.1 or 10 W m−2 (Fig. 6c, d, respectively) or a blue microbeam of 1 W m−2 (Fig. 6e). Interestingly, the same tendency found in protonemal cells was observed; chloroplasts positioned farther from the microbeam moved faster under all light conditions tested.
Relationship between the highest speed of movement during the movement and the initial position of chloroplast from the microbeam-irradiated area in prothallial cells as was shown in Fig. 4 using protonemal cells. Data were obtained from the same movies used for the analyses in Fig. 5. For other details, see legend to Fig. 5
Finally, using Arabidopsis thaliana mesophyll cells we carried out similar experiments to those performed with in Adiantum capillus-veneris. A small area (10 μm in diameter) of a dark-adapted Arabidopsis thaliana mesophyll cell was irradiated continuously with a blue microbeam of 10 W m−2 (Fig. 7). The speed of signal transfer in Arabidopsis thaliana was about 0.70 μm min−1, a little slower compared to that measured for Adiantum capillus-veneris prothallial cells (Fig. 7a). The speed of signal transfer towards all directions of the surrounding cell edge was similar, suggesting that mesophyll cells do not have a cell polarity that could have arisen from the tip and basal axis of the leaf. The behavior and speed of chloroplast movement in Arabidopsis thaliana were also similar to those in Adiantum capillus-veneris. The further away the chloroplasts were located from the microbeam-irradiated area, the faster they moved (Fig. 7b).
Speed of signal transfer and highest speed of chloroplast movement in Arabidopsis thaliana mesophyll cells. Part of a dark-adapted A. thaliana mesophyll cell was irradiated continuously with a microbeam (10 μm in diameter) of 10 W m−2 of blue light. a Relationship between the starting times of chloroplast movement towards the microbeam-irradiated area and the distance between the center of the microbeam and each chloroplast in A. thaliana mesophyll cells. The slope of the approximate line indicates the speed of signal transfer. b Highest speed during chloroplast movement in relation to the initial position of chloroplast from the microbeam-irradiated area in A. thaliana mesophyll cells
Discussion
Chloroplast photorelocation movement is a critical phenomenon for plant survival (Kasahara et al. 2002), but the precise mechanism underlying this mode of organelle movement is not known. Photoreceptors for accumulation and avoidance responses are phot1 and phot2, and phot2, respectively in Arabidopsis thaliana (Kagawa et al. 2001; Jarillo et al. 2001; Sakai et al. 2001). Chloroplast actin filaments that appeared at the front side of moving chloroplasts and mediated chloroplast movement were recently found in Arabidopsis thaliana (Kadota et al. 2009). However, the signal transduction pathways connecting photoreceptors and chloroplast actin filaments remain to be clarified. In this study, the speed of signal transfer from photoreceptors to chloroplasts was measured to gain some insight into the signal that is transmitted from photoreceptor to chloroplast in Adiantum capillus-veneris gametophyte cells and Arabidopsis thaliana mesophyll cells. In protonemal cells, which are tip-growing and linear cells, the average speed of signal transfer was about 2.3 μm min−1 in the basal-to-apical (acropetal) and about 0.7 μm min−1 in the apical-to-basal (basipetal) directions (Fig. 3). These values were almost constant irrespective of light intensity, wavelength, irradiation period, and the region of the cell irradiated. The results suggest that, in protonemal cells, the difference in the speed of signal transfer between the acropetal and basipetal directions may reflect the differential mass flow rates towards the apical and basal directions; the acropetal mass flow rates may be faster than the basipital mass flow rates, due to the higher demand for substances required for tip growth. In gametophyte cells of Adiantum capillus-veneris and mesophyll cells of Arabidopsis thaliana, cellular expansion or growth may be non-polarized compared with that in protonemal cells. Thus, the speed of substance movement, i.e., about 1.0 μm min−1 for gametophyte cells of Adiantum capillus-veneris (Fig. 5) and about 0.7 μm min−1 for mesophyll cells of Arabidopsis thaliana (Fig. 7a), may be equal in every direction and conserved between ferns and seed plants.
Calcium ions have been proposed to act as one of the candidates of the signal (Wada et al. 2003). The necessity of calcium for chloroplast movement was reported in some plants. Chloroplast movement is not induced in the presence of EGTA in protonemal cells of Adiantum capillus-veneris, although chloroplasts show slight movement in random fashion (Kadota and Wada 1992). In Lemna trisulca, chloroplast movement correlates with increased cytoplasmic calcium levels and is inhibited by antagonists of calcium homeostasis (Tlalka and Fricker 1999). The speed of intracellular transfer of calcium ions in plant cells has been measured only in the moss Physcomitrella patens by microinjection of a calcium indicator into protonemal cells (Tucker et al. 2005). The speed of calcium waves found in the cytoplasm was about 3.4 μm s−1. To our knowledge, the transferring speed of substances as signals is not yet known in plant cells except for the above instance. However, in animal cells various experimental data have been accumulated. For example, the transfer speed of calcium waves was about 8 μm s−1 in Xenopus eggs (Kubota et al. 1987) and 15.5 μm s−1 in a SR-free single isolated rabbit cardiac myofibrils (Linke et al. 1993). Comparing these values to the findings presented here, the speed of signal transfer for chloroplast movement in fern gametophytes was 100–200 times slower than those measured for calcium wave transfers, suggesting that the calcium might not be the signal involved in chloroplast movement.
Intracellular transport is dependent on cytoskeleton systems in many cases. Therefore, it is possible that the signal for chloroplast movement is transported by cytoskeleton systems. In Adiantum capillus-veneris protonemal cells, the speed of cytoplasmic streaming depending on the act-myosin system was calculated from the speed of oil drop movement (Wada et al. 1982). The speed was dependent upon the position of long protonema and was about 2 μm min−1 in the apical region and increased gradually to 10 μm min−1 in the basal region where chloroplast movement is more clearly observed. In comparison to the data reported here, the speed of signal transfer involved in chloroplast accumulation was 5–10 times slower than the speed of the act-myosin system.
Surprisingly, chloroplasts situated further from the microbeam-irradiated area move faster under any light intensities or wavelength of microbeam irradiation (Figs. 4, 6). The reason for this, as well as the underlying mechanism, is presently not known. On the other hand, the speed of chloroplast movement in fern gametophytes was reported to be uniform at around 0.3 μm min−1 irrespective of the irradiation wavelength and light fluence (Kagawa and Wada 1996). In this study, the maximum speed refers to the maximum distance migrated within a minute for each chloroplast when measured every minute throughout the whole distance travelled, and not the average speed obtained from whole distance travelled. In a protonemal cell that has a tube-like form and a distinct polarity, the migration speed of each chloroplast is not uniform throughout the direction of travel. By contrast, chloroplasts move rapidly or slowly towards the microbeam-irradiated area, changing their speed frequently. They stop or even move backward occasionally, but gradually and ultimately reach the microbeam-irradiated area. Similar chloroplast behavior has been observed in protonemal cells of the moss Physcomitrella patens (Sato et al. 2001). Hence, the average speed of chloroplast movement measured over the whole distance has no significant meaning for analytical studies of the mechanism underlying chloroplast movement in Adiantum capillus-veneris. Incidentally, the average speed measured for the accumulation response in Adiantum capillus-veneris gametophyte cells was about 0.2 μm min−1 (Tsuboi et al. 2009) and is very similar to that of a previous study (0.3 μm min−1; Kagawa and Wada 1996).
Fern protonemal cells are linear and tube-like in form. When part of the protonemal cell is irradiated with a microbeam, the signal released from the irradiated area is transferred towards only two directions: apical and basal. However, in the case of the prothallial cell, the signal should be transferred two-dimensionally in all directions along the plasma membrane from the microbeam-irradiated area. When the signal is transferred through a tube-like cell like a protonema, the signal concentration may not be reduced as much if there is no dramatic loss during transfer. Alternatively, if the signal is a diffusible material, such as calcium ions, and spreads over the plasma membrane, signal concentration would be reduced during transfer depending on an inverse-square law, i.e., the concentration of the signal is inversely proportional to the square of distance from the signal source, in this case the irradiated area. Considering that the reduction rate of signal concentration during transfer must differ between tube-like protonemal cells and flat gametophyte cells, and that the speed of signal transfer is very similar irrespective of cell shape, the signal must be transferred by an unknown transduction system whereby the signal reduction rate during transfer may not be dependent on an inverse-square law.
References
Ahmad M, Cashmore AR (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162–166
Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282:1698–1701
Haupt W (1999) Chloroplast movement: from phenomenology to molecular biology. Prog Bot 60:3–36
Hayama R, Coupland G (2003) Shedding light on the circadian clock and the photoperiodic control of flowering. Curr Opin Plant Biol 6:13–19
Iino M (2001) Phototropism in higher plants in photomovement. In: Häder D, Lebert M (eds) ESP comprehensive series in photosciences, vol 1. Elsevier, Amsterdam, pp 659–811
Ishikawa R, Tamaki S, Yokoi S, Inagaki N, Shinomura T, Takano M, Shimamoto K (2005) Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 17:3326–3336
Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410:952–954
Kadota A, Wada M (1992) Photoorientation of chloroplasts in protonemal cells of the fern Adiantum as analyzed by use of a video-tracking system. Bot Mag Tokyo 105:265–279
Kadota A, Wada M (1999) Red light-aphototropic (rap) mutants lack red light-induced chloroplast relocation movement in the fern Adiantum capillus-veneris. Plant Cell Physiol 40:238–247
Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoji K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106:13106–13111
Kagawa T, Wada M (1996) Phytochrome and blue light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta 198:488–493
Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41:84–93
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homologue controlling the chloroplast high-light avoidance response. Science 291:2138–2141
Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M (2004) Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol 45:416–426
Kanegae T, Wada M (2006) Photomorphogenesis in ferns. In: Schaefer E, Nagy F (eds) Photomorphogenesis in plants and bacteria, 3rd edn. Springer, Dordrecht, pp 515–536
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290
Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414:656–660
Kubota HY, Yoshimoto Y, Yoneda M, Hiramoto Y (1987) Free calcium wave upon activation in Xenopus eggs. Dev Biol 119:129–136
Linke WA, Bartoo ML, Pollack GH (1993) Spontaneous sarcomeric oscillations at intermediate activation levels in single isolated cardiac myofibrils. Circ Res 73:724–734
Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue-light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98:6969–6974
Sato Y, Wada M, Kadota A (2001) Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J Cell Sci 114:269–279
Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M (1996) Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA 93:8129–8133
Suetsugu N, Wada M (2007) Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol Chem 388:927–935
Sugai M, Furuya M (1967) Photomorphogenesis in Pteris vittata. I. Phytochrome-mediated spore germination and blue light interaction. Plant Cell Physiol 8:737–748
Tlalka M, Fricker M (1999) The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. Plant J 20:461–473
Tsuboi H, Suetsugu N, Wada M (2006) Negative phototropic response of rhizoid cells in the fern Adiantum capillus-veneris. J Plant Res 119:505–512
Tsuboi H, Suetsugu N, Kawai-Toyooka H, Wada M (2007) Phototropins and neochrome1 mediate nuclear movement in the fern Adiantum capillus-veneris. Plant Cell Physiol 48:892–896
Tsuboi H, Yamashita H, Wada M (2009) Chloroplasts do not have a polarity for light-induced accumulation movement. J Plant Res 122:131–140
Tucker EB, Lee M, Alli S, Sookhdeo V, Wada M, Imaizumi T, Kasahara M, Hepler PK (2005) UV-A induces two calcium waves in Physcomitrella patens. Plant Cell Physiol 46:1226–1236
Vicker MG (1994) The regulation of chemotaxis and chemokinesis in Dictyostellium amoebae by temporal signals and spatial gradients of cyclic AMP. J Cell Sci 107:659–667
Wada M (2007) The fern as a model system to study photomorphogenesis. J Plant Res 120:3–16
Wada M, Mineyuki Y, Furuya M (1982) Changes in the rate of organelle movement during progression of the cell cycle in Adiantum protonemata. Protoplasma 113:132–136
Wada M, Kadota A, Furuya M (1983) Intracellular localization and dichroic orientation of phytochrome in plasma membrane and/or ectoplasm of a centrifuged protonema of fern Adiantum capillus-veneris. Plant Cell Physiol 24:1441–1447
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Yatsuhashi H, Wada M (1990) High-fluence rate response in the light-oriented chloroplast movement in Adiantum protonemata. Plant Sci 68:87–94
Acknowledgments
We thank Dr. John Christie, University of Glasgow, for his critical reading and editing of this manuscript. This work was partly supported by the Japanese Ministry of Education, Sports, Science, and Technology (MEXT 13139203, 17084006 to M.W.), the Japan Society of Promotion of Science (JSPS 13304061, 16107002, 20227001 to M.W.), and a Research Fellowship for Young Scientists (to H.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuboi, H., Wada, M. Speed of signal transfer in the chloroplast accumulation response. J Plant Res 123, 381–390 (2010). https://doi.org/10.1007/s10265-009-0284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0284-y