Abstract
The fossil record reveals that seed plant leaves evolved from ancestral lateral branch systems. Over time, the lateral branch systems evolved to become determinate, planar and eventually laminar. Considering their evolutionary histories, it is instructive to compare the developmental genetics of shoot apical meristems (SAMs) and leaves in extant seed plants. Genetic experiments in model angiosperm species have assigned functions of meristem maintenance, specification of stem cell identity, boundary formation, polarity establishment and primordium initiation to specific genes. Investigation of roles of the same or homologous genes during leaf development has revealed strikingly similar functions in leaves compared to SAMs. Specifically, the marginal blastozone that characterizes many angiosperm leaves appears to function in a manner mechanistically similar to the SAM. We argue here that the similarities may be homologous due to descent from ancestral roles in an ancestral shoot system. Molecular aspects of SAM and leaf development in gymnosperms is largely neglected and could provide insight into seed plant leaf evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Evolution of leaves
The sporophyte generation of almost all extant vascular plants has three vegetative organs: roots, stems, and leaves. These organs are distinguished easily from each other on the basis of position, function, external morphology, internal anatomy, and development. The fossil record reveals that the earliest relatives of modern vascular plants had a much simpler sporophyte body that consisted of naked, dichotomously branched axes (e.g., Aglaophyton, Cooksonia; Doyle 1998; Kenrick and Crane 1997; Stewart and Rothwell 1993). Both roots and leaves were later evolutionary modifications of the structure and developmental program of the sporophyte.
Leaves are vascularized, lateral organs, produced at the shoot apex in regular developmental sequence (phyllotaxis), with dorsiventral polarity. Despite common positional and functional aspects of leaves in all vascular plants, comparative morphology and phylogenetic interpretation of the fossil record indicate that leaves evolved independently in the major extant lineages of plants—lycopsids (club mosses), sphenopsids (horsetails), ferns, and seed plants—from leafless ancestors in each clade (Bower 1935; Boyce and Knoll 2002; Doyle 1998; Kenrick and Crane 1997; Stewart and Rothwell 1993; Tomescu 2009, and references therein).
Euphyllophyte leaves evolved from ancestral shoot systems
The fossil record is unclear as to the exact nature of the ancestors of fern and seed plant lineages. There are numerous reconstructed fossil taxa with similarities to ferns, others with similarities to early seed plant relatives (progymnosperms) and still others with similarities to both (Kenrick and Crane 1997; Scheckler et al. 2006; Stewart and Rothwell 1993). However, the ancestral condition for the euphyllophyte clade (including extant sphenopsids, ferns, and seed pants) was resolved by Kenrick and Crane (1997) to be a plant that produced determinate, lateral sterile branches (or appendages) in a helical pattern, which supports earlier conclusions that the precursors of megaphyllous leaves were already present in the ancient stock of plants (the trimerophytes) from which ferns and seed plant lineages later diverged (Gensel 1984; Shou-Gang and Beck 1993). Thus it would seem that determinacy and phyllotaxis in seed plant and fern leaves may be homologous. However, the dorsiventrality and laminar development that define a foliar organ evolved independently within fern and seed plants lineages (Boyce and Knoll 2002; Galtier 1981; Kenrick and Crane 1997; Sanders 2007; Sanders et al. 2009; Tomescu 2009).
Seed plant leaf evolution
Interpretation of the earliest (Upper Devonian) fossil seed plants in fact indicates that spermatophyte “leaves” were highly ramified with equal and unequal dichotomies (Sanders 2007; Sanders et al. 2009; Serbet and Rothwell 1992; Stewart and Rothwell 1993). Laminar development was lacking (e.g., Buteoxylon gordonianum) or occurred after several orders of dichotomous branching in the production of pinnately born pinnules (e.g., Elkinsia polymorpha; Sanders 2007; Sanders et al. 2009; Serbet and Rothwell 1992; Stewart and Rothwell 1993). In Elkinsia, leaf vasculature was dorsiventral and branching of the leaves was in one plane, whereas in Buteoxylon ultimate branching occurred in three dimensions (Sanders 2007; Sanders et al. 2009). Thus, among the earliest seed plants, lateral organs were variable and still rather branch-like (Beerling and Fleming 2007; Galtier 1981). Laminar development, when present, was limited to small outgrowths of terminal branchlets (Sanders 2007; Sanders et al. 2009).
In the slightly later (Lower Carboniferous) lyginopterid seed plants, the leaves were planated but still highly branched, with two to three major dichotomies followed by at least three orders of pinnate dissection (Galtier 1981; Stewart and Rothwell 1993). Lamina were produced in a pinnate pattern on ultimate pinnae but also sometimes on the main rachis (Galtier 1981) suggesting that the program to promote laminar development was not always position-dependent and could be initiated early or late in leaf development. These leaves also produced short branch-like structures from the main rachis or petiole that bore reproductive structures (Stewart and Rothwell 1993). The dichotomous branching of leaves that persisted into the mid-Carboniferous suggests that, unlike leaves of extant seed plants, early seed plant leaves grew by means of a persistent apical meristem (Bower 1884; Dolan and Poethig 1998; Poethig and Sussex 1985).
The fossil record reveals that the leaves of the earliest seed plants were much more branch-like than those of any extant seed plant. Subsequent evolution of seed plants included a trend to earlier expression of laminar growth in leaf development and, at the same time, suppression of stem-like development (apical growth, branching). Given the dramatic differences in the leaves of extant seed plants as compared to the earliest fossils, will there be any homologous components of development in stems and leaves that reflect their common origin (dichotomous branches of the ancestral axis), or will novel pathways have taken over or obscured the axial developmental history of the seed plant leaf? In the following sections we review some of the developmental genetic data for shoot development from the perspective that leaves and the stems that bear them share a common evolutionary and developmental origin and attempt to answer these questions.
Developmental genetics of seed plant shoot apical meristem and leaves
Development of the shoot at the shoot apical meristem
Since seed plant leaves were derived from branch systems, comparisons between the shoot apical meristem (SAM) and leaves of extant seed plants may be revealing. To begin our comparison, let us examine the seed plant SAM. Histological analyses of SAMs allowed its subdivision into three distinct zones, defined by cytoplasmic densities and cell division rates: the peripheral zone, the central zone, and the rib zone (Esau 1977; Foster 1938; Gifford and Corson 1971). The central zone exhibits low rates of cell division and in many species the cells are conspicuously vacuolated. In contrast, cell division rates are higher in the peripheral and rib zones. These three zones are thought to represent functional subdivision of the SAM. Leaves are produced from cells recruited from the peripheral zone, while stem tissue is derived from cells recruited from the rib zone (pith) and the peripheral zone (epidermis, cortex, vasculature). The central zone acts as a reservoir of stem cells that replenish both the peripheral and rib zones as well as maintaining the integrity of the central zone itself. It must be noted that these cells do not act as permanent initials, but rather their behavior is governed in a position-dependent manner. Cyto-histological zonation was originally described in gymnosperms (e.g., Ginkgo) and later in many angiosperm species, suggesting that general mechanistic functioning of the SAM is conserved among seed plants. Studies in angiosperms have shown that the three cyto-histological zones are characterized by distinct gene expression patterns, and genetic studies in model species have provided insight into the molecular bases or the coordinated function of the three zones.
Development of leaves from the SAM
Leaves in flowering plants proceed through distinct stages of morphogenesis (Dengler and Tsukaya 2001; Donnelly et al. 1999; Esau 1977; Hagemann and Gleissberg 1996; Poethig 1997). Angiosperm leaves are initiated from the peripheral zone of the SAM, with the initial anatomical evidence a periclinal cell division in the subepidermal layer. Once established, a leaf primordium proceeds through primary morphogenesis, where marginal elaborations, such as leaflets and serrations, are formed. Subsequently, secondary morphogenesis, characterized by both cell division and cell expansion as well as differentiation, leads to definition of final leaf size and shape. Primary and secondary morphogenesis are not synchronized throughout the developing leaf, with the distal (apical) regions often progressing to secondary morphogenesis earlier than proximal regions, such that a basipetal gradient of leaf differentiation can be observed in each developing leaf. During primary morphogenesis, marginal regions of the leaf appear to play a critical role in patterning events: leaf margins are responsible for maintaining morphogenetic potential for the initiation of lateral elaborations [leaflets and serrations (Fig. 1a, b); Avery 1933; Berger et al. 2009; Hagemann and Gleissberg 1996; Reinhardt et al. 2007]. The marginal region has been termed the marginal blastozone as a reflection of its similarity to other tissues with meristematic potential (Hagemann and Gleissberg 1996). This analogy is particularly striking in features of leaf development in species with dissected leaves, which are reminiscent of events identified in SAM function (Blein et al. 2008; Brand et al. 2007).
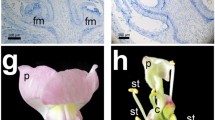
a Cartoon of PIN-mediated auxin flow and CUC and Class I KNOX gene expression in a complex leaf and shoot apical meristem (SAM). Similar auxin flow and gene expression patterns are evident in developing leaves (P1, P2, P3) and developing leaflets (L1, L2, L3). The youngest leaves/leaflets (P3, L3) are at the sites of auxin maxima formed by convergent auxin flow in the epidermis, are bordered by CUC gene expression, and Class I KNOX gene expression is excluded. b Transverse section through an Arabidopsis thaliana shoot apex showing FIL mRNA (brown staining) in developing leaves. Soon after initiation, FIL (YABBY) gene expression becomes restricted to the leaf margins (marginal blastozone), where active growth is occurring. While A. thaliana leaves are simple, they do exhibit marginal serrations (arrows on high magnification of leaf), which in early leaf development are defined by CUC2 expression in the marginal blastozone. Leaves are marked from youngest (1) to oldest (9); leaf 6 is not shown. c Longitudinal section through a developing Ginkgo biloba leaf. Growth occurs via a distal marginal blastozone, with mitotic figures conspicuous in this zone (arrows in inset). A gradient of differentiation is evident, with cells of the distal regions (blastozone) cytoplasmically dense and cells of the proximal region undergoing differentiation. The formation of an open dichotomous venation pattern is evident in the elongating cells of the provasculature and differentiating vasculature
The leaves of gymnosperms are less well studied. They appear to be produced on the SAM in a manner very similar to that of angiosperm leaves. However, gymnosperm leaves have open dichotomous venation in the lamina (like the ancestral leaves) that appears to be produced by a distal marginal meristem (Fig. 1c; Bower 1884; Boyce 2007; Mundry and Stutzel 2004). The compound leaves of cycads develop by an initial apical outgrowth of the rachis with the subsequent initiation of leaflet primordia along “wings” that develop on the margins of the rachis in either an acropetal or basipetal direction (Bower 1884). This suggests the activity of a marginal blastozone to produce leaflets.
Genetic mechanisms for SAM function and leaf development have been the focus for many researchers in plant developmental genetics in the last decade. We describe here, by no means exhaustively, a few key genes that have been proposed to have roles in the establishment and maintenance of the SAM, and describe potentially homologous roles in leaf development (Fig. 1a). Because of the critical and instructive roles that auxin plays in shoot development, control of its synthesis, movement, and catabolism must be tightly regulated. When considering the roles of specific genes in SAM and leaf development, attention must be paid to their interaction, if any, with auxin biology.
Auxin defines sites of growth and differentiation
Auxin maxima define sites of seed plant leaf initiation
The first identified event defining a seed plant leaf primordium is the formation of an auxin maximum in a phyllotactically determined position in the SAM peripheral zone (Benkova et al. 2003; Reinhardt et al. 2000, 2003). PIN-mediated auxin efflux is required for the auxin maximum to form, and for the subsequent channeling of auxin inward from the maximum, which demarcates the position of the primary vascular strand of the leaf. In Arabidopsis thaliana SAMs, auxin maxima are formed in the peripheral zone via the PIN-mediated polar movement of auxin, which converges from sources both peripheral and central to the incipient leaf primordium (Fig. 1a). In addition, local production also contributes to formation of auxin maxima (Cheng et al. 2006). While the central region of the SAM may be a source of auxin that contributes to the formation of peripheral auxin maxima (Heisler et al. 2005), auxin is prevented from flowing basally. Thus, the vascular tissues have a peripheral origin, rather than a central one. Furthermore, if the central zone is a source of auxin, its cells are not competent to respond to auxin in the same manner as cells in the peripheral zone, where the auxin maxima induce leaf formation (Izhaki and Bowman 2007; Reinhardt et al. 2000).
One major difference between seed plant SAMs and those of other extant vascular plants concerns the distribution and movement of auxin. In extant seed plants vasculature does not arise from the central region of the SAM, but rather from auxin maxima associated with leaf development in the peripheral zone of the meristem (Benkova et al. 2003; Esau 1965; Mc Arthur and Steeves 1972; Reinhardt et al. 2003; Schuetz et al. 2008; Young 1954). In contrast, in lycophytes and ferns, it appears that the central region of the SAM acts as a source of auxin, which is transported basally in a polar fashion resulting in vascular tissues originating from the SAM itself (Wardlaw 1946a, b; Wochok and Sussex 1973). It is not known whether auxin maxima are associated with leaf formation in ferns and lycophytes. However, based on patterns of vascular tissue formation, one might predict auxin maxima marking the sites of leaf initiation in ferns where vascular tissues originates from both the SAM and leaves (Wardlaw 1946a, b). In contrast, vascularization of microphyll primordia in lycophytes appears secondarily, perhaps arguing against auxin maxima defining microphyll formation (Floyd and Bowman 2006; Wochok and Sussex 1973).
Auxin maxima define sites of leaflet initiation
In angiosperms, leaflet formation from the leaf margin exhibits a similar series of events as leaf formation from the peripheral zone of the SAM (Barkoulas et al. 2008). The position of the leaflet is marked by an auxin maximum, which is, at least in part, determined by PIN-mediated auxin movement. Subsequently, auxin is channeled inward from the site of leaflet initiation defining the position of leaflet vasculature, an event also marked by PIN expression. In species in which leaflets are initiated in a basipetal manner, such as Solanum lycopersicum and Cardamine hirsuta, the auxin maxima form sequentially in a linear phyllotactic pattern (Barkoulas et al. 2008). In Arabidopsis thaliana, which has entire leaves (i.e., lacking leaflets), auxin maxima form in a similar, phyllotactically linear pattern along the developing leaf margin, with sites marking serrations (or hydathodes) and defining initiation sites for the reticulate pattern of venation (Berleth et al. 2007; Scarpella et al. 2006). Dynamic, PIN-mediated production of auxin maxima induces leaf primordia on the SAM and leaflet primordia or teeth along the leaf margin.
Marking boundaries
CUC activity defines leaf boundaries
In A. thaliana, the delimitation of peripheral zone leaf primordia from the central zone, and leaf primordia from each other within the peripheral zone, is demarcated by the expression of the CUP-SHAPED COTYLEDON (CUC) class of NAC transcription factors (Aida et al. 1999). Loss of CUC activity results in a loss of separation between the cotyledons, such that the entire periphery of the embryo develops as a single cup-shaped cotyledon, a phenotype that can be phenocopied by application of auxin transport inhibitors (Aida et al. 1997; Liu et al. 1993). The activity of CUC genes causes a repression of growth between organs, creating boundaries that are correlated with low auxin levels (Aida et al. 2002; Furutani et al. 2004). CUC genes are initially expressed in a domain extending across the upper region of the globular embryo, dividing the embryo into two halves (Aida et al. 1999). One consequence of CUC expression in the central apical region of the embryo is the activation of STM, a Class I KNOX gene, which is required for establishing the SAM. In cuc1 cuc2 embryos, STM is not activated, resulting in a seedling lacking a SAM (Aida et al. 1997). Subsequent to STM activation, CUC expression is reduced in the SAM center and becomes limited to the boundaries of the SAM (Aida et al. 1999). However, CUC and STM patterns do not appear to be mutually exclusive, with both genes expressed at high levels in the boundary regions (Heisler et al. 2005). Thus, CUC genes play two critical roles in SAM function. First, they are required to activate Class I KNOX gene expression during embryogenesis, and second, they are required to delimit the boundaries of leaves produced in the peripheral zone. Fusion of adjacent floral organs is extensive in cuc1 cuc2 plants, but fusion of leaves is not complete, possibly due to the activity of other closely related genes (e.g. CUC3) (Hibara et al. 2006). Analysis of post-seedling development in which all CUC activity is removed may provide insights into whether CUC activity is required for delimiting the boundary between the central and peripheral zones during leaf initiation. Similar expression patterns and mutant phenotypes suggest that CUC function in the SAM is conserved in angiosperms (Berger et al. 2009; Souer et al. 1996; Weir et al. 2004). The antiquity of the CUC class of NAC genes has not been intensively investigated. However, gymnosperm ESTs representing genes related to the angiosperm CUC clade of genes (S.K.F and J.L.B., unpublished data) warrant further investigation to examine whether the boundary function of CUC genes is likely to be conserved in seed plants.
CUC activity defines leaflet and serration boundaries
CUC gene expression demarcates leaflet, or serration, development along leaf margins (Berger et al. 2009; Blein et al. 2008; Nikovics et al. 2006). In species with defined leaflets, such as S. lycopersicum and C. hirsuta, CUC expression is detected at the distal boundary of leaflets prior to morphological evidence of initiation, in a pattern reminiscent of CUC expression at the boundary of leaf initiation at the SAM (Berger et al. 2009; Blein et al. 2008). At the SAM, CUC expression reflects the phyllotaxy of leaves produced from the peripheral zone, while in the leaf, CUC expression reflects the ‘phyllotaxy’ of leaflet production from the marginal blastozone. CUC gene expression is also detected at positions that will become indentations of serrations of leaflets of compound species as well as the serrations of simple leaves in A. thaliana (Nikovics et al. 2006). Reductions in CUC activity in S. lycopersicum and C. hirsuta result in reduced leaf complexity in terms of both numbers of leaflets and serrations on leaflets (Berger et al. 2009; Blein et al. 2008). Likewise, loss of CUC2 activity in A. thaliana reduces leaf serrations, while increases in CUC2 activity through loss of miR164 regulation increases the number and depth of leaf serrations (Nikovics et al. 2006).
Roles for KNOX
A role for Class I KNOX in preventing differentiation in the SAM
Class I KNOX genes are required for SAM function (Barton and Poethig 1993; Jackson et al. 1994; Long et al. 1996; Vollbrecht et al. 1991). Loss-of-function alleles of STM result in a loss of the SAM (Barton and Poethig 1993). Roles of Class I KNOX gene activity are hypothesized to be suppressing differentiation within the SAM and the formation of organ boundaries. Several lines of evidence indicate that Class I KNOX genes may act to regulate and be regulated by plant hormones to promote maintenance or formation of the SAM (see Veit 2009 for review). Some Class I KNOX genes expressed in the SAM, e.g., STM, promote cytokinin synthesis and down regulate gibberellic acid levels, promoting meristematic activity (Jasinski et al. 2005; Yanai et al. 2005). Class I KNOX gene expression is excluded from the positions of auxin maxima associated with sites of leaf initiation within the SAM (Long and Barton 1998; Long et al. 1996; Vollbrecht et al. 1991). It has been suggested that Class I KNOX genes are downregulated by high levels of auxin and may also act to repress auxin transport (Hay et al. 2006). Thus, both CUC and Class I KNOX gene expression is associated with low levels of auxin. Class I KNOX genes are found in all land plants, and their role in the development of moss sporophytes suggest an ancestral role in preventing premature differentiation in the sporophyte apex of land plants (Champagne and Ashton 2001; Sakakibara et al. 2008; Singer and Ashton 2007).
A role for Class I KNOX in leaf complexity
Concomitantly with the formation of auxin maxima marking the sites of leaf initiation in the peripheral zone, Class I KNOX gene expression (e.g., STM) is down-regulated in incipient leaf primordia (Jackson et al. 1994; Long et al. 1996). In this, and other contexts, there appears to be an antagonistic relationship between Class I KNOX activity and high auxin levels (Hay et al. 2006; Heisler et al. 2005). In species with marginal elaborations, Class I KNOX gene expression is reactivated in leaf primordia post-initiation (Bharathan et al. 2002; Hay and Tsiantis 2006). For example, in C. hirsuta, ChSTM is expressed along leaf margins (i.e., marginal blastozone), with expression disappearing in a basipetal manner as leaves differentiate (Blein et al. 2008; Hay and Tsiantis 2006). In addition, ChSTM is down-regulated at positions of auxin maxima, sites of leaflet initiation, along leaf margins in a manner reminiscent of its expression in the SAM (Hay and Tsiantis 2006). A similar pattern of Class I KNOX expression is observed in other species with dissected leaves (Bharathan et al. 2002). In contrast, in species with ‘simple’ leaves, such as A. thaliana, Class I KNOX genes are not reactivated in leaf primordia post-initiation. However, if Class I KNOX activity is expressed ectopically in these leaves, either through mutation or transgenes, some level of marginal lobing or dissection is produced (e.g., Byrne et al. 2000; Lincoln et al. 1994; Tsukaya and Uchimiya 1997). Likewise, increasing Class I KNOX gene activity in already dissected leaves results in their further dissection, while reductions in Class I KNOX activity can lead to decreases in leaf complexity (Barkoulas et al. 2008; Chen et al. 1997; Hareven et al. 1996).
The relationship between CUC and Class I KNOX activity in leaf margins may be similar to that in the shoot apex. Reduction of CUC activity in C. hirsuta leaves results in a reduction in Class I KNOX gene expression, suggesting CUC activity induces Class I KNOX expression as in the establishment of the SAM in A. thaliana (Blein et al. 2008). However, reducing CUC activity in leaves where Class I KNOX genes are ectopically expressed still results in a reduction in leaf complexity (Blein et al. 2008). Thus, CUC activity is required for leaflet formation, even in the context where Class I KNOX activity is supplied ectopically. In most cases reduction in CUC activity resulted in a reduction in not only leaflet number, but also the overall amount of lamina development, leading to the proposal that CUC activity stimulates adjacent leaflet development in addition to demarcating their boundaries (Berger et al. 2009; Blein et al. 2008). In light of these results, the mechanism of cross regulatory interactions between CUC and Class I KNOX genes warrants further investigation, both in the SAM and in leaves.
Leaf complexity is positively correlated with Class I KNOX leaf expression, leading to the idea that modulation of Class I KNOX activity determines the extent of leaf complexity (Bharathan et al. 2002). This concept can be extended to the evolution of determinate leaves from ancestral shoot systems, and gradual downregulation of Class I KNOX expression likely contributed to ‘overtopping’ and the determinacy of lateral branch systems that would later evolve into seed plant leaves (e.g. Beerling and Fleming 2007; Sanders 2007; Sanders et al. 2009; Tomescu 2008). In this regard, it is of interest to note that down-regulation of Class I KNOX activity does not occur during leaf initiation in ferns, making its down-regulation in seed plants leaf initials a synapomorphy for seed plants (Bharathan et al. 2002; Sano et al. 2005). However, fern leaves undergo extensive apical growth by means of an apical cell (Bower 1884) whereas growth in seed plant leaves is limited to the activity of diffuse marginal, distal and basal meristems. In seed plants with dissected leaves, Class I KNOX expression is reactivated, but expression eventually ceases with its duration correlated with leaf complexity (dissection). In seed plants with simple leaves, Class I KNOX expression in leaves is restricted, or alternatively is not reactivated at all. A. thaliana is a representative of the latter case and is potentially instructive with respect to some aspects of Class I KNOX gene expression. In A. thaliana, Class I KNOX gene expression is reactivated in leaves of asymmetric leaves1 (as1), as2 and YABBY mutants (Byrne et al. 2000; Kumaran et al. 2002; Semiarti et al. 2001). In both these genetic backgrounds Class I KNOX is properly downregulated in incipient leaf primordia, but inappropriately (at least for this species) is reactivated later in leaf development. A possible mechanism for the negative regulation of Class I KNOX by AS1 is the recruitment of HIRA, a chromatin remodeling protein, and the establishment of a repressive chromatin state (Guo et al. 2008; Phelps-Durr et al. 2005). Thus, the variation in leaf complexity observed amongst extant species and the progressive reduction in leaf complexity during seed plant evolution could reflect a progressive restriction of Class I KNOX activity mediated by earlier, or in some cases permanent, establishment of a repression chromatin state at Class I KNOX loci during leaf development. This may correspond to the process of apoxogenesis (gradual shrinking of the meristem) hypothesized to have led to the determinacy of lateral branches in the seed plant ancestors (Scheckler 1976) as well as the further evolutionary simplification of seed plant leaves (Stewart and Rothwell 1993). However, elimination of all apical meristematic activity would result in lack of leaf growth. Thus, evolutionary downregulation of Class I KNOX and other factors favoring apical growth must have occurred together with expression of programs that promoted a different type of meristematic growth—that of laminar outgrowth or the marginal blastozone.
Maintenance of stem cells
A role for WOX in central zone maintenance
Maintenance of the SAM central zone requires the activity of WUSCHEL (WUS), which encodes a WOX-class homeodomain transcription factor (Mayer et al. 1998). In wus mutants, cells that would normally constitute the central domain instead become incorporated into leaf primordia, resulting in a failure to maintain the SAM formed during embryogenesis (Laux et al. 1996). While new SAMs can form in wus plants, they suffer the same fate. The finding that a related WOX gene, WOX5 plays a similar role in the root apical meristem suggests that WOX activity promoting stem cell identity may be a general feature of meristems (Sarkar et al. 2007). In one scenario, an ancestral WOX gene underwent gene duplication, with subfunctionalization of the genes resulting in related WOX genes playing homologous roles in different meristems. WOX activity in the central zone is antagonized by the activity of the CLV genes, which encode a receptor kinase and its secreted ligand, although the molecular mechanism of this antagonism is not known (Fletcher et al. 1999; Reddy and Meyerowitz 2005; Schoof et al. 2000).
A role for WOX in the marginal blastozone?
One feature of the SAM is its requirement for WUS activity to maintain the stem cell pool in the central zone. Does a WOX gene play an analogous role in the marginal blastozone? The WOX genes NARROW SHEATH1 (NS1) and NS2 are required for the recruitment of cells to form marginal leaf domains in maize (Nardmann et al. 2004). In A. thaliana, the orthologous gene, PRESSED FLOWER (PRS) is also implicated in leaf development, with prs mutants lacking stipules, a marginal elaboration of leaves (Matsumoto and Okada 2001; Nardmann et al. 2004). While these results are suggestive of WOX activity having a function in the marginal blastozone, genetic redundancy and/or subfunctionalization among gene family members may be masking a more conspicuous role than revealed by ns and prs mutants. In A. thaliana, the two WOX genes closely related to PRS, WOX1 and WOX6, are candidates for having roles in the marginal blastozone.
From radial stems to asymmetric leaves
Radial patterning of stems
In both stems and leaves of A. thaliana, two gene families, the Class III HD-Zip and KANADI (KAN), influence differentiation in a polar manner. Loss of Class III HD-Zip activity, as in phabulosa phavoluta revoluta (phb phv rev) mutants, results in a loss of central shoot identity (Emery et al. 2003; Prigge et al. 2005). The apical regions of mutant seedlings lack a SAM, and instead comprise a single radial abaxialized cotyledon, with a small, radial vascular bundle. Conversely, gain-of-function PHABULOSA alleles have larger SAMs and adaxialized leaves (McConnell and Barton 1998). Loss- and gain-of-function phenotypes of KANADI activity are largely the converse of those of Class III HD-Zip genes, with loss-of-function alleles having larger meristems and adaxialized leaves, and gain-of-function alleles resulting in abaxialized leaves and loss of meristems (Eshed et al. 2001, 2004; Kerstetter et al. 2001). Stem vasculature is also patterned by these two classes of genes. Gain-of-function REV alleles and KANADI loss-of-function alleles have radialized, rather than colateral, vascular bundles (Emery et al. 2003). Thus, in stems, class III HD-Zip genes promote central (adaxial) identity, while KANADI genes promote peripheral (abaxial) differentiation (Eshed et al. 2001; Kerstetter et al. 2001; McConnell et al. 2001; McConnell and Barton 1998).
In contrast to the CUC and Class I KNOX genes, members of the Class III HD-Zip gene family have expression patterns that correlate with known pathways of auxin flow out of the apex toward incipient leaf primordia and in the provasculature (Emery et al. 2003; McConnell et al. 2001; Otsuga et al. 2001). One phenotypic feature of loss of KANADI activity that is not observed in gain-of-function alleles of Class III HD-Zip genes is the formation of ectopic leaves from the hypocotyl of kan1 kan2 kan4 plants (Izhaki and Bowman 2007). The ectopic leaves are associated with PIN1-mediated ectopic auxin maxima that form during the globular-heart stages of embryogenesis. Thus, it was proposed that KANADI genes act to negatively regulate PIN-mediated auxin movement, and the complementary phenotypes of these two classes of genes may be due to opposite relationships with respect to auxin synthesis and movement (Izhaki and Bowman 2007). The Class III HD-Zip gene family predates the evolution of land plants (Floyd et al. 2006). The expression patterns of Class III HD-Zip genes in the lycophyte Selaginella kraussiana as well as gymnosperms suggest that the relationship of these genes with auxin distribution and their role in the SAM and central shoot differentiation is conserved throughout vascular plants (Floyd and Bowman 2006; Prigge and Clark 2006).
Genes patterning leaf polarity were co-opted from earlier roles in radial patterning of stems
In Arabidopsis PHB, PHV, and REV act to promote differentiation of adaxial cells types of developing leaves (Emery et al. 2003; McConnell et al. 2001; McConnell and Barton 1998; Prigge et al. 2005). These three Class III HD-Zip genes do so, in part, by antagonism with the activity of the KANADI class of transcription factors. In wild-type leaves, Class III HD-Zip genes are expressed adaxially from leaf inception, but in kan1 kan2 kan3 leaves expression is throughout and leaves are adaxialized (Eshed et al. 2004). Gain-of-function alleles of PHB and PHV, due to loss of miR165/166 regulation, have adaxialized leaves (McConnell et al. 2001; McConnell and Barton 1998). Conversely, in phb phv rev triple mutants KANADI genes are ectopically expressed resulting in an abaxialized cotyledon (Emery et al. 2003; Prigge et al. 2005). Since the evolution of vasculature predates the evolution of seed plant leaves, it is hypothesized that the Class III HD-Zip and KANADI genes were co-opted into roles patterning leaf polarity from ancestral functions in radial patterning of stems (Emery et al. 2003). Consistent with this hypothesis is the expression pattern of Class III HD-Zip genes in Selaginella kraussiana, where they are expressed centrally, in the apical meristem and in tissues of developing vascular cylinders (Floyd and Bowman 2006). While mutant phenotypes suggest KANADI function is conserved in angiosperms (Candela et al. 2008; Iwasaki and Nitasaka 2006), the expression patterns of orthologous genes in other vascular plant lineages, e.g. Selaginella kraussiana and the moss Physcomitrella patens, are not known (Floyd and Bowman 2007). Since seed plant leaf primordia are initiated on the flanks of the SAM, they can inherit polar positional information inherent in the SAM, with more central (adaxial) Class III HD-Zip gene expression and more peripheral (abaxial) KANADI gene expression. In this sense, the ancestral spatial and temporal activities of the two gene families may have predisposed them to be co-opted such that lateral shoots acquired polarity at inception, an acquisition that could lead to planation.
Seed-plant-specific YABBY genes are required for lamina development
Unlike the other gene families described above, the origin of the YABBY gene family appears to be coincident with the evolution of seed plants (Floyd and Bowman 2007). YABBY gene expression is confined largely to developing leaves and leaf-derived organs, e.g., cotyledons, floral organs, outer integuments (Sawa et al. 1999; Siegfried et al. 1999; Toriba et al. 2007; Watanabe and Okada 2003). Reduction of YABBY activity results in a progressive reduction in lamina development. In filamentous flower (fil) yabby3 (yab3) double mutants of A. thaliana and the orthologous graminifolia (gram) mutants in Antirrhinum majus lamina growth is restricted (Golz et al. 2004; Siegfried et al. 1999). In these genotypes, polar differentiation of the residual lamina is reduced and many anatomical and morphological marginal characters are lost. Further reductions in YABBY activity as in gram prolongata double mutants of A. majus result in an almost complete loss of lamina development, and, in addition, a failure to maintain the SAM, implying a non-autonomous YABBY-derived activity required for SAM maintenance (Golz et al. 2004). Both loss- and gain-of-function alleles in A. thaliana provide further evidence for non-autonomous YABBY activity, and implicate YABBY activity in maintaining WUS-CLV homeostasis (Goldshmidt et al. 2008). Finally, in A. thaliana, Class I KNOX genes, including STM are ectopically reactivated in fil yab3 leaves (Kumaran et al. 2002). Thus, YABBY gene activity is associated with both lamina growth and repressing KNOX gene expression, suggesting integration of YABBY genes into an ancestral lateral shoot genetic program may have been critical in the evolution of seed plant leaves.
Hypotheses
Evolution of the marginal blastozone from a SAM
Due to their evolutionary history as lateral branch systems, seed plant leaves are likely to retain vestiges of genetic programs active in the ancestral shoot systems. If we assume that the primary and lateral shoots of the ancestral seed plants exhibited similar gene expression and activities of extant seed plant shoots, we can look for homologous roles of gene activity in extant seed plant SAMs and leaves. We hypothesize that the marginal blastozone of seed plant leaves harbors relics of genetic programs originally active in an ancestral shoot system (Fig. 1a, b). Members of each of the gene families discussed in this review play similar roles in the marginal blastozone and SAM.
Consider C. hirsuta leaf development, for which some information of gene expression patterns and gene function are known. Once a leaf primordium is established, convergent PIN-mediated auxin flow in the marginal epidermis leads to formation of auxin maxima in each marginal blastozone at some distance from the apex (Barkoulas et al. 2008). These auxin maxima, and all subsequently formed maxima, define sites of leaflet initiation. Subsequent to the formation of the first auxin maximum, additional auxin maxima form progressively basipetally along the margin, resulting from reiterative reversals of auxin flow below each successively formed leaflet. Thus, with a constant distal auxin flow from the base of the leaf, the reiterative reversals result in successive sites of convergent auxin flow. In each case, the site of auxin reversal away from the distally produced leaflet primordium just produced is marked by CUC expression, similar to the demarcating of leaf primordia from the SAM (Blein et al. 2008). Such reversals of auxin away from primordium production have been observed in real time in the A. thaliana SAM (Heisler et al. 2005). In species with complex leaves, such as C. hirsuta, Class I KNOX gene expression is evident throughout the region of the marginal blastozone that has yet to initiate leaflets, and is punctate in those regions where leaflets have already initiated, being excluded from the leaflet primordia (Blein et al. 2008; Hay and Tsiantis 2006). Expression becomes progressively basal as the leaf differentiates basipetally from the distal tip. Note that the interplay between CUC activity and formation of auxin maxima does not require Class I KNOX activity, since it occurs in simple leaves of A. thaliana.
As in the SAM, Class III HD-Zip activity is required for the marginal blastozone, with loss-of-function alleles lacking lamina growth (Emery et al. 2003; Prigge et al. 2005). Counter intuitively, gain-of-function alleles also lack lamina growth; however, lack of lamina growth is consistent with the idea that leaf polarity per se is required for lamina growth (McConnell and Barton 1998; Sussex 1951; Waites and Hudson 1995). Requirement of polar Class III HD-Zip and KANADI expression for maintenance of meristematic activity in leaves represents a difference from the situation in SAMs, where gain-of-function Class III HD-Zip alleles and loss-of-function KANADI alleles do not result in a loss of meristematic function in the SAM (Eshed et al. 2004; McConnell and Barton 1998). The difference between the meristematic zones of SAMs and leaves suggests the presence of a new regulator. In A. majus, YABBY activity is required to maintain Class III HD-Zip activity in the margins of developing leaves (Golz et al. 2004), suggesting that YABBY genes represent critical key new regulators in the evolution of seed plant leaves (Floyd and Bowman 2006). Loss of marginal leaf tissues and lamina growth, as well as changes in Class I KNOX expression, in YABBY loss-of-function mutants is consistent with the idea that YABBY gene activity coordinates growth and differentiation at the marginal blastozone (Golz et al. 2004; Kumaran et al. 2002; Siegfried et al. 1999). How YABBY gene activity was mechanistically integrated into the evolutionarily older genetic programs is not presently known. It is plausible that YABBY genes may have initially been expressed only at late stages of leaf development (promoting laminar growth of ultimate appendages) but were expressed at progressively earlier stages promoting laminar outgrowth rather than dissection of leaf and leaflet primordia.
The presence or extent of a leaf marginal meristem has been debated for decades. The diffuse distribution of cell division and patterns revealed by clonal analyses in developing leaves have been used to argue against the existence of a marginal meristem (e.g., Bower 1884; Dolan and Poethig 1998; Donnelly et al. 1999; Poethig and Sussex 1985). However, the presence of a marginal meristem or blastozone has been argued based on histological characteristics and initial marginal organogenesis of leaflet/serration development (e.g., Avery 1933; Hagemann and Gleissberg 1996). In addition, based on mutant phenotypes, the leaf margins play a critical role in patterning events in developing angiosperm leaves (e.g. Barkoulas et al. 2008; Berger et al. 2009; Blein et al. 2008; Golz et al. 2004; Reinhardt et al. 2007; Siegfried et al. 1999). If the marginal blastozone is evolutionarily derived from a SAM, the diffuse distribution of cell division observed during angiosperm leaf development might be a consequence of higher cell division rates in tissues derived from the meristem than in the meristem itself, as observed in the SAM and lateral structures derived from the peripheral zone. The transitory nature cells of occupying the marginal blastozone, in conjunction with its diffuse nature and potential to organize patterning events at a distance, may limit large clonal sectors emanating from the blastozone. Alternatively, the more diffuse and basal meristematic activity may represent a derived aspect of angiosperm leaf development associated with the activation of numerous de novo auxin signals (Boyce 2007).
Gymnosperm leaves
Extant gymnosperms, like the earliest seed plants, have laminar outgrowth by means of a distal marginal meristem (Fig. 1c). However, cycad leaflets are produced along the margin of the developing leaf in a manner that suggests the action of a marginal blastozone. Several cycad species also exhibit basipetal initiation of leaflet primordia. If true, this means in cycads, one of the most ancient extant seed plants, a marginal blastozone serves to produce leaflets whereas a distal marginal meristem acts to produce the lamina. This may be inherited from the production of the pinnate ultimate segments of the earliest seed plants. It is then conceivable that the apical marginal growth of simple gymnosperm leaves and the marginal blastozone of angiosperms represent independent evolutionary elaborations of distinct mechanisms for leaf development that both existed in their last common ancestor.
Developmental genetics shows numerous similarities in the developmental genetic mechanisms for patterning and growth in the SAMs and leaves of eudicot angiosperms. We hypothesize here that these largely represent homologies-shared developmental programs in two organs that were once serially homologous branch systems in a euphyllophyte ancestor. One major weakness in this hypothesis is the lack of evidence, both morphological and genetic, for the SAMs and leaves of non-flowering seed plants. With respect to the genes mentioned in this review, Class I KNOX genes are expressed in gymnosperm SAMs in a manner similar to that described for angiosperms, being downregulated in leaf primordia (Bharathan et al. 2002; Pham and Sinha 2003; Sundas-Larsson et al. 1998). Likewise, the pattern of Class III HD-Zip gene expression in Ginkgo biloba and Pseudotsuga menziesii SAMs resembles that in angiosperm SAMs (Floyd and Bowman 2006). While this suggests some conservation of seed plant SAM gene expression, little is known about CUC, WOX, and YABBY genes outside of angiosperms. Extant gymnosperms and angiosperms share a similar organization of the SAM; however, it is not known if the earliest seed plants grew by means of a SAM with cytohistological zonation. The fact that the earliest seed plants were protostelic (had a solid core of xylem surrounded by phloem) rather than eustelic (like all extant seed plants) might suggest a different organization. It certainly indicates that a strong flow of auxin occurred from the center of the meristem. This also indicates that mechanisms for PIN-mediated auxin movement to the periphery of the meristem were either weaker or had not yet evolved. It would be informative to know how PIN genes in gymnosperms are expressed both in the SAM and during leaf development. The models emerging for SAM and leaf development provide the basis for comparison in non-flowering seed plants at least at the level of gene expression patterns. An understanding of the genes expressed during compound cycad leaf development might be particularly informative.
Angiosperm leaves
While the ancestral seed plant leaf was likely to be complex, the ancestral angiosperm leaf is predicted to be simple, implying that complex angiosperm leaves are derived (Doyle 1998). In the hypothesis put forward here, the marginal blastozones of both simple and complex leaves have a common origin in their derivation from an ancestral shoot system. Leaves of many flowering plants differentiate first at the distal tip and then progressing basipetally towards the leaf base. The basipetal wave of differentiation is associated with a concurrent wave of TCP gene expression (Nath et al. 2003). A mild reduction of TCP gene activity results in altered leaf curvature, due to continued growth along the leaf margins, while more severe reductions lead to prolonged proliferation at leaf margins (Efroni et al. 2008; Nath et al. 2003; Palatnik et al. 2003). Consistent with the continued lamina production at the leaf margins, leaves with reduced TCP gene function exhibit gene expression patterns similar to that of young leaves undergoing primary morphogenesis (Efroni et al. 2008). Conversely, loss of miR319 regulation of Lanceolate (La), an S. lycopersicum TCP gene, results in a loss of leaf complexity and significantly reduced leaf size (Ori et al. 2007). One role of TCP function appears to be negative regulation of CUC genes (Koyama et al. 2007). While down-regulation of CUC genes can explain the loss of complexity in La mutants, it does not explain the reduction in leaf size, since loss of CUC function in S. lycopersicum goblet mutants results in leaves lacking complexity but near normal in size (Berger et al. 2009). Thus, evolutionarily derived complex leaves in angiosperms could be primarily a consequence of dissecting the ancestral simple template. However, the extent of growth and complexity would depend upon a competition between maintenance of the marginal blastozone with the wave of TCP-mediated differentiation. Relative heterochronic shifts of either of these activities could lead to more or less complex leaf development.
The phylogenetics of the TCP gene family has not been comprehensively explored. However, the two major classes of TCP gene family members can be found in the Physcomitrella patens and Selaginella moellendorffii genomes, and EST projects have uncovered a gymnosperm (Picea glauca) sequence similar to the clade of genes regulating angiosperm leaf differentiation. More information is required to ascertain whether TCP genes have been recruited from ancestral functions to play a role in leaf differentiation.
While complex leaves are derived within angiosperms, Class I KNOX expression in developing leaves is not, as it represents the ancestral condition in the lateral branch systems from which seed plant leaves are derived. That reactivation of Class I KNOX expression is often sufficient to promote patterned increases in leaf complexity indicates that the remainder of the genetic program required for complex leaf development is already in place. Such genetic programs, e.g. reiterative CUC expression and marginal auxin maximum formation, are evident even in the simple leaves of A. thaliana. The ability to compare gene expression patterns throughout living seed plants will allow more refined hypotheses about shared ancestral pathways and those that may uniquely derived and a better understanding about the evolution of seed plant leaves.
References
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570
Aida M, Vernoux T, Furutani M, Traas J, Tasaka M (2002) Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129:3965–3974
Avery GS (1933) Structure and development of the tobacco leaf. Am J Bot 20:565–592
Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M (2008) A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat Genet 40:1136–1141
Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development 119:823–831
Beerling DJ, Fleming AJ (2007) Zimmermann’s telome theory of megaphyll leaf evolution: a molecular and cellular critique. Curr Opin Plant Biol 10:4–12
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Berger Y, Harpaz-Saad S, Brand A, Melnik H, Sirding N, Alvarez JP, Zinder M, Samach A, Eshed Y, Ori N (2009) The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 136:823–832
Berleth T, Scarpella E, Prusinkiewicz P (2007) Towards the systems biology of auxin-transport-mediated patterning. Trends Plant Sci 12:151–159
Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha N (2002) Homologies in leaf form inferred from KNOX1 gene expression during development. Science 296:1858–1860
Blein T, Pulido A, Vialette-Guiraud A, Nikovics K, Morin H, Hay A, Johansen IE, Tsiantis M, Laufs P (2008) A conserved molecular framework for compound leaf development. Science 322:1835–1839
Bower FO (1884) On the comparative morphology of the leaf in the vascular cryptograms and gymnosperms. Philos Trans R Soc Lond 175:565–615
Bower FO (1935) Primitive land plants. MacMillan, London
Boyce CK (2007) Mechanisms of laminar growth in morphologically convergent leaves and flower petals. Int J Plant Sci 168:1151–1156
Boyce CK, Knoll AH (2002) Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants. Paleobiology 28:70–100
Brand A, Shirding N, Shleizer S, Ori N (2007) Meristem maintenance and compound-leaf patterning utilize common genetic mechanisms in tomato. Planta 226:941–951
Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408:967–971
Candela H, Johnston R, Gerhold A, Foster T, Hake S (2008) The milkweed pod1 gene encodes a KANADI protein that is required for abaxial/adaxial patterning in maize leaves. Plant Cell 20:2073–2087
Champagne C, Ashton N (2001) Ancestry of KNOX genes revealed by bryophyte (Physcomitrella patens) homologs. New Phytol 150:23–36
Chen JJ, Janssen BJ, Williams A, Sinha N (1997) A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. Plant Cell 9:1289–1304
Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799
Dengler NG, Tsukaya H (2001) Leaf morphogenesis in dicotyledons: current issues. Int J Plant Sci 162:459–464
Dolan L, Poethig RS (1998) Clonal analysis of leaf development in cotton. Am J Bot 85:315–321
Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215:407–419
Doyle JA (1998) Phylogeny of vascular plants. Annu Rev Ecol Syst 29:567–599
Efroni I, Blum E, Goldshmidt A, Eshed Y (2008) A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. Plant Cell 20:2293–2306
Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL (2003) Radial patterning of Arabidopsis shoots by class III HD-Zip and KANADI genes. Curr Biol 13:1768–1774
Esau K (1965) Vascular differentiation in plants. Holt, Rinehart and Winston, New York
Esau K (1977) Anatomy of seed plants. Wiley, New York
Eshed Y, Baum SF, Perea JV, Bowman JL (2001) Establishment of polarity in lateral organs of plants. Curr Biol 11:1251–1260
Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL (2004) Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131:2997–3006
Fletcher LC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283:1911–1914
Floyd SK, Bowman JL (2006) Distinct developmental mechanisms reflect the independent origins of leaves in vascular plants. Curr Biol 16:1911–1917
Floyd SK, Bowman JL (2007) The ancestral developmental tool kit of land plants. Int J Plant Sci 168:1–35
Floyd SK, Zalewski CS, Bowman JL (2006) Evolution of Class III homeodomain-leucine zipper genes in streptophytes. Genetics 173:373–388
Foster AS (1938) Structure and growth of the shoot apex in Ginkgo biloba. Bull Torrey Bot Club 65:531–556
Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M (2004) PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131:5021–5030
Galtier J (1981) Structures foliaires de fougeres er pteridospermales du Carbonifere inferieur et leur signification evolutive. Palaeontographica B 180:1–38
Gensel PG (1984) A new Lower Devonian plant and the early evolution of leaves. Nature 309:785–787
Gifford EM, Corson GE (1971) Shoot apex in seed plants. Bot Rev 37:143–229
Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y (2008) Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell 20:1217–1230
Golz JF, Roccaro M, Kuzoff R, Hudson A (2004) GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development 131:3661–3670
Guo MJ, Thomas J, Collins G, Timmermans MCP (2008) Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20:48–58
Hagemann W, Gleissberg S (1996) Organogenetic capacity of leaves: the significance of marginal blastozones in angiosperms. Plant Syst Evol 199:121–152
Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E (1996) The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell 84:735–744
Hay A, Tsiantis M (2006) The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet 38:942–947
Hay A, Barkoulas M, Tsiantis M (2006) ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133:3955–3961
Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15:1899–1911
Hibara K, Karim MR, Takada S, Taoka KI, Furutani M, Aida M, Tasaka M (2006) Arabidopsis CUP-SHAPED COTYLEDON3 regulates postembryonic shoot meristem and organ boundary formation. Plant Cell 18:2946–2957
Iwasaki M, Nitasaka E (2006) The FEATHERED gene is required for polarity establishment in lateral organs especially flowers of the Japanese morning glory (Ipomoea nil). Plant Mol Biol 62:913–925
Izhaki A, Bowman JL (2007) KANADI and class IIIHD-zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell 19:495–508
Jackson D, Veit B, Hake S (1994) Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120:405–413
Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15:1560–1565
Kenrick P, Crane PR (1997) The origin and early evolution of land plants: a cladistic study. Smithsonian Institution Press, Washington
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411:706–709
Koyama T, Furutani M, Tasaka M, Ohme-Takagi M (2007) TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell 19:473–484
Kumaran MK, Bowman JL, Sundaresan V (2002) YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell 14:2761–2770
Laux T, Mayer KFX, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96
Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S (1994) A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 6:1859–1876
Liu CM, Xu ZH, Chua NH (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5:621–630
Long JA, Barton MK (1998) The development of apical embryonic pattern in Arabidopsis. Development 125:3027–3035
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379:66–69
Matsumoto N, Okada K (2001) A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Gene Dev 15:3355–3364
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95:805–815
Mc Arthur ICS, Steeves TA (1972) An experimental study of vascular differentiation in Geum chiloense Balbis. Bot Gaz 133:276–287
McConnell JR, Barton MK (1998) Leaf polarity and meristem formation in Arabidopsis. Development 125:2935–2942
McConnell J, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411:709–713
Mundry M, Stutzel T (2004) Morphogenesis of leaves and cones of male short-shoots of Ginkgo biloba L. Flora 199:437–452
Nardmann J, Ji JB, Werr W, Scanlon MJ (2004) The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 131:2827–2839
Nath U, Crawford BCW, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299:1404–1407
Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, Aida M, Laufs P (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18:2929–2945
Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, Menda N, Amsellem Z, Efroni I, Pekker I, Alvarez JP, Blum E, Zamir D, Eshed Y (2007) Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet 39:787–791
Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE (2001) REVOLUTA regulates meristem initiation at lateral positions. Plant J 25:223–236
Palatnik JF, Allen E, Wu XL, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425:257–263
Pham T, Sinha N (2003) Role of KNOX genes in shoot development of Welwitschia mirabilis. Int J Plant Sci 164:333–343
Phelps-Durr TL, Thomas J, Vahab P, Timmermans MCP (2005) Maize rough sheath2 and its Arabidopsis orthologue ASYMMETRIC LEAVES1 interact with HIRA, a predicted histone chaperone, to maintain knox gene silencing and determinacy during organogenesis. Plant Cell 17:2886–2898
Poethig RS (1997) Leaf morphogenesis in flowering plants. Plant Cell 9:1077–1087
Poethig RS, Sussex IM (1985) The cellular parameters of leaf development in Tobacco—a clonal analysis. Planta 165:170–184
Prigge MJ, Clark SE (2006) Evolution of the class III HD-Zip gene family in land plants. Evol Dev 8:350–361
Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17:61–76
Reddy GV, Meyerowitz EM (2005) Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science 310:663–667
Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12:507–518
Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426:255–260
Reinhardt B, Hanggi E, Muller S, Bauch M, Wyrzykowska J, Kerstetter R, Poethig S, Fleming AJ (2007) Restoration of DWF4 expression to the leaf margin of a dwf4 mutant is sufficient to restore leaf shape but not size: the role of the margin in leaf development. Plant J 52:1094–1104
Sakakibara K, Nishiyama T, Deguchi H, Hasebe M (2008) Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella patens but do function in sporophyte development. Evol Dev 10:555–566
Sanders HL (2007) Developmental changes in the evolution of fundamental plant organography. PhD Thesis, College of Arts and Sciences, Ohio University
Sanders H, Rothwell GW, Wyatt SE (2009) Key morphological alterations in the evolution of leaves. Int J Plant Sci 170:860–868
Sano R, Juarez CM, Hass B, Sakakibara K, Ito M, Banks JA, Hasebe M (2005) KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evol Dev 7:69–78
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446:811–814
Sawa S, Watanabe K, Goto K, Kanaya E, Morita EH, Okada K (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev 13:1079–1088
Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20:1015–1027
Scheckler SE (1976) Ontogeny of progymnosperms I. Shoots of Upper Devonian Aneurophytales. Can J Bot 54:202–219
Scheckler SE, Skog JE, Banks HP (2006) Langoxylon asterochlaenoideum Stockmans: anatomy and relationships of a fern-like plant from the Middle Devonian of Belgium. Rev Palaeobot Palyno 142:193–217
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Schuetz M, Berleth T, Mattsson J (2008) Multiple MONOPTEROS-dependent pathways are involved in leaf initiation. Plant Physiol 148:870–880
Semiarti E, Ueno Y, Tsukaya H, Iwakawa H, Machida C, Machida Y (2001) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128:1771–1783
Serbet R, Rothwell GW (1992) Characterizing the most primitive seed ferns. I. A reconstruction of Elkinsia polymorpha. Int J Plant Sci 153:602–621
Shou-Gang H, Beck CB (1993) Further observations on Eophyllophyton bellum from the Lower Devonian (Siegenian) of Yunnan, China. Paleaeontographica 230:27–41
Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126:4117–4128
Singer SD, Ashton NW (2007) Revelation of ancestral roles of KNOX genes by a functional analysis of Physcomitrella homologues. Plant Cell Rep 26:2039–2054
Souer E, van Houwelingen A, Kloos D, Mol J, Koes R (1996) The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85:159–170
Stewart WN, Rothwell GW (1993) Paleobotany and the evolution of plants. Cambridge University Press, New York
Sundas-Larsson A, Svenson M, Liao H, Engstrom P (1998) A homeobox gene with potential developmental control function in the meristem of the conifer Picea abies. Proc Natl Acad Sci USA 95:15118–15122
Sussex IM (1951) Experiments on the cause of dorsiventrality in leaves. Nature 167:651–652
Tomescu AMF (2009) Megaphylls, microphylls and the evolution of leaf development. Trends Plant Sci 14:5–12
Toriba T, Harada K, Takamura A, Nakamura H, Ichikawa H, Suzaki T, Hirano HY (2007) Molecular characterization the YABBY gene family in Oryza sativa and expression analysis of OsYABBY1. Mol Genet Genomics 277:457–468
Tsukaya H, Uchimiya H (1997) Genetic analyses of the formation of the serrated margin of leaf blades in Arabidopsis: combination of a mutational analysis of leaf morphogenesis with the characterization of a specific marker gene expressed in hydathodes and stipules. Mol Gen Genet 256:231–238
Veit B (2009) Hormone mediated regulation of the shoot apical meristem. Plant Mol Biol 69:397–408
Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350:241–243
Waites R, Hudson A (1995) phantastica—a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121:2143–2154
Wardlaw CW (1946a) Experimental and analytical studies of pteridophytes IX. The effect of removing leaf primordia on the development of Angiopteris evecta Hoffm. Ann Bot Lond 10:223–235
Wardlaw CW (1946b) Experimental and analytical studies of pteridophytes VII. Stelar morphology: the effect of defoliation on the stele of Osmunda and Todea. Ann Bot Lond 9:97–107
Watanabe K, Okada K (2003) Two discrete cis elements control the abaxial side-specific expression of the FILAMENTOUS FLOWER gene in Arabidopsis. Plant Cell 15:2592–2602
Weir I, Lu JP, Cook H, Causier B, Schwarz-Sommer Z, Davies B (2004) CUPULIFORMIS establishes lateral organ boundaries in Antirrhinum. Development 131:915–922
Wochok ZS, Sussex IM (1973) Morphogenesis in Selaginella—auxin transport in stem. Plant Physiol 51:646–650
Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15:1566–1571
Young BS (1954) The effects of leaf primordia on differentiation in the stem. New Phytol 53:445–460
Acknowledgments
We apologize to those researchers whose work we were unable to cite due to space limitations. For those whose work is cited, we assume full responsibility for any errors in interpretation or presentation. We thank Stuart Gardner for rendering Fig. 1a. The authors’ research is funded by the Australian Research Council (DP0771232, FF0561326), the United States National Science Foundation (IOB-0515435) and Monash University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Floyd, S.K., Bowman, J.L. Gene expression patterns in seed plant shoot meristems and leaves: homoplasy or homology?. J Plant Res 123, 43–55 (2010). https://doi.org/10.1007/s10265-009-0256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-009-0256-2