Abstract
Most strains harboring the feathered (fe) mutation in the Japanese morning glory (Ipomoea nil or Pharbitis nil) show deformed phenotypes such as upcurled leaves and separated or tubular petals. These phenotypes seem to be caused by loss of abaxial identity in lateral organs. The FE gene was isolated using the inserted transposon as a tag. An En/Spm-related transposable element, Tpn102, inserted in the fourth intron of the FE gene, was responsible for the fe mutation. FE encodes a GARP transcription factor closely related to Arabidopsis KANADI1 (KAN1), which promotes an abaxial cell fate. Genetic analyses and molecular studies, which showed that all fe mutant strains have the same fe allele despite their phenotypic differences, revealed that fe strains with strong phenotypes have additional mutations enhancing the fe phenotype. These findings and historical records of fe phenotypes suggest that these enhancer mutations were accumulated in the fe background during selection for strong phenotypes. The mutant phenotypes and molecular analysis of fe strains suggest that FE regulates the abaxial identity of lateral organs redundantly with modifier genes, as KAN1 does in Arabidopsis. FE, however, affects flower phenotype even in the single mutant unlike KAN1, moreover, modifier mutations affect flower phenotype only in the fe mutant background, suggesting that FE may play a more crucial role in promotion of abaxial cell fate in flowers of the Japanese morning glory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Japanese morning glory (Ipomoea nil or Pharbitis nil) is a traditional horticultural plant in Japan. Many spontaneous mutants affecting color and shape of leaves and flowers have been isolated beginning in the 17th century, with most appearing around the early 19th century. Before World War II, extensive genetic studies of the Japanese morning glory were conducted by Japanese geneticists (Imai 1931b, 1938). More than 200 genetic loci were described, and a map consisting of 10 linkage groups was constructed out of 15 groups based on the expected chromosome number (Imai 1929, 1933; Hagiwara 1956). Based on recent molecular studies of these mutations, most are thought to be induced by En/Spm-related transposable elements, Tpn1 (transposable element from Pharbitis nil 1) family (Iida et al. 1999, 2004).
feathered (fe) is one of the classic mutants in the Japanese morning glory; it was first reported in the beginning of the 19th century. The early mutant, called “creased-feathered”, has creased petals and feather-like petals attached to the outside of the flower tube. As a result of continuous selection by Japanese horticulturists, more complicated fe varieties, such as “wind-bell” and “shooting-star” were developed. Although detailed genetic studies of fe were not carried out, it has been thought that these phenotypic differences of the fe strains are caused by allelic differences (Imai 1931b, a). Currently, almost all of the maintained fe strains are either wind-bell or shooting-star types, with a few strains of intermediate phenotype between creased and wind-bell types. Although a creased type was thought to be lost, we have regenerated one (see below). In fe mutants, the abaxial side of the petal has deeper coloration than that of wild-type, and the abaxial side of the leaf also shows accumulation of chlorophyll. Furthermore, strong fe mutants produce filamentous petals and curled leaves. These phenotypes are similar to those of mutants with loss of organ polarity in other species (Waites and Hudson 1995).
During plant development, lateral organs such as the leaf and flower are initiated from the shoot apical meristem (SAM) or flower meristem (FM) and develop along adaxial–abaxial, proximal–distal and medial–lateral axes. Adaxial–abaxial polarity generates morphological differences in leaf tissues, with palisade tissue of the adaxial side adapted for capturing light, and the spongy tissue and stomata of the abaxial side for gas exchange. Similar adaxial–abaxial polarity can be seen in floral organs. In a study that examined the surface structures of 201 species of flower from 60 families, 79% exhibited some form of conical or papillate cells on the adaxial epidermis that orientated towards potential pollinators (Kay et al. 1981). It was suggested that the conical shaped cells of the petal epidermis increase the attractiveness of the flower to its pollinators (Glover and Martin 1998).
In Arabidopsis, three gene families are involved in the establishment of adaxial–abaxial polarity (Bowman et al. 2002). The class III HD-ZIP genes involving PHABULOSA (PHB), PHAVOLUTA (PHV) and REVOLUTA (REV) specify the adaxial identity of lateral organs (McConnell and Barton 1998; McConnell et al. 2001). They are expressed in the adaxial domain of lateral organs, in vascular tissues, and in the apical meristem. Gain-of-function mutations cause adaxialization in lateral organs, while loss-of-function mutations cause abaxialization and defects in meristem formation (Emery et al. 2003; Prigge et al. 2005). On the other hand, abaxial identity is regulated by the KANADI and YABBY genes, both of which encode putative transcription factors (Eshed et al. 1999; Sawa et al. 1999; Siegfried et al. 1999; Eshed et al. 2001; Kerstetter et al. 2001). The expression patterns of KANADI genes are complementary to those of class III HD-ZIP genes, and they negatively regulate each other (Eshed et al. 2001; Emery et al. 2003). It has been suggested that adaxial–abaxial polarity is established primarily by KANADI and class III HD-ZIP genes acting antagonistically, with YABBY genes acting downstream of the pathway, thereby promoting abaxial cell fate and lamina expansion (Eshed et al. 2004).
In other plants, some mutants of class III HD-ZIP and YABBY genes have been characterized. A maize ortholog of REV, rld1 (rolled leaf 1) has been characterized for mutant phenotypes, and its expression pattern is similar to REV of Arabidopsis (Juarez et al. 2004a). On the other hand, maize YABBY genes are expressed on the adaxial side of leaf primordia, and in contrast to observations in Arabidopsis, apparently direct lateral organ outgrowth rather than determine cell fate (Juarez et al. 2004b). In rice, the YABBY gene DROOPING LEAF (DL), an ortholog of Arabidopsis CRABS CLAW (CRC) (Bowman and Smyth 1999; Eshed et al. 1999), regulates carpel specification and midrib formation of leaves, but is not involved in abaxial specification (Yamaguchi et al. 2004). In eudicots, the Antirrhinum YABBY gene GRAMINIFOLIA (GRAM) is expressed along abaxial margins of lateral organ primordia, and promotes abaxial cell fate by repressing adaxial identity. Moreover, GRAM has a contrasting function in promoting adaxial identity redundantly with a paralogous gene, PROLONGATA (PROL) (Golz et al. 2004).
In this study, we isolated the gene responsible for a classic fe mutant phenotype using a transposable element insertion. Analyses of mutant phenotypes and gene structure indicate that FE promotes abaxial identity of lateral organs including flowers and leaves. Genetic and molecular studies revealed that fe mutant strains with strong phenotypes have additional mutations enhancing fe phenotypes.
Materials and methods
Plant materials
Genetic symbols for the Japanese morning glory were described by Imai and Hagiwara (Imai 1931b; Hagiwara 1956). The fe mutant strains (Q422, Q438, Q441, Q449, Q467) and the wild-type strain TKS (Tokyo Kokei Standard, Q1065) used in this study were from the collection at Kyushu University. Because most homozygous fe mutants are sterile, they were maintained using heterozygous stocks except for Q467. Q441 and Q422 are strains of the wind-bell type, and Q449 is a shooting-star type strain. Q467 is a creased type strain derived by crossing Q441 and TKS, and the fertility of fe homozygous plants of this strain is recovered. Q438 is a strain of intermediate type. Q441 is a highly homozygous strain except at the fe locus because it was established at least 50 years ago, and produces phenotypically homogeneous progeny. All plants were grown in a field at Kyushu University from late spring to early winter.
Microscopy
Leaf tissues were fixed in formalin–acetic acid–50% ethanol [1:1:18, v/v] overnight. The samples were dehydrated in a graded ethanol series, embedded in paraffin wax, cut into sections 1.0 μm thick, stained with 0.5% haematoxylin, 1% safranine and 1% fast green, and observed under a light microscope (Eclipse TE-300, Nikon, Tokyo, Japan).
For scanning electron microscopy (SEM), fresh petals were set into molds with epoxy resin and coated with platinum–palladium (Williams et al. 1987). They were observed by SEM (S-3000N microscope, Hitachi, Tokyo, Japan) at 15 kV.
Simplified transposon display (STD)
STD was performed as described previously (Fukada-Tanaka et al. 2001) with slight modifications as follows: Genomic DNA (120 ng) was digested with XspI and ligated with 88 pmol XspI adaptors (5′-GACGATGAGTCCTGAG-3′ and 5′-TACTCAGGACTCAT-3′). The ligated DNA sample was diluted 10-fold with TE (10 mM Tris–HCl, pH 8.0, and 1 mM EDTA). Preamplification was performed in 20 μl reaction mixtures containing 1× ExTaq buffer, 0.2 mM of each dNTP, 0.24 μM XspI primer (5′-GACGATGAGTCCTGAGTAG-3′), 0.24 μM TIR primer (5′-TGTGCATTTTTCTTGTAGTG-3′) complementary to the terminal inverted repeat of Tpn, 2 units TaKaRa ExTaq DNA polymerase (TaKaRa Biomedicals, Kyoto, Japan), and 2 μl of 10-fold diluted DNA sample, under conditions as follows: 20 cycles of 94°C for 30 s, 56°C for 60 s, and 72°C for 60 s. The reaction mixture was again diluted 10-fold with TE. Subsequent selective amplification was performed in 20 μl reaction mixtures containing 1× ExTaq buffer, 0.2 mM of each dNTP, 0.24 μM XspI+N primer (5′-GATGAGTCCTGAGTAGN-3′, where N represents A, C, G or T), 0.12 μM TIR+N primer (5′-TGTGCATTTTTCTTGTAGTGN-3′) labelled with 5-carboxyfluorescein (5-FAM) at the 5′ end, 1 unit TaKaRa ExTaq DNA polymerase (TaKaRa), and 2 μl of 10-fold diluted DNA sample from the preamplification reaction. The temperature profile for PCR was as follows. The first 13 cycles used a touchdown of the annealing temperature with each cycle: 94°C for 30 s, 65°C (initial) for 30 s, and 72°C for 60 s. The annealing temperature was decreased by 0.7°C with each cycle. The final 20 cycles were run at a constant annealing temperature: 94°C for 30 s, 56°C for 60 s, and 72°C for 60 s. Amplified fragments were analyzed using an ABI PRISM 310 genetic analyzer (Applied Biosystems, Foster City, CA, USA). Presumptive fragments were isolated by loading selective amplification products on 6% polyacrylamide/8.3 M urea sequencing gels in an ABI PRISM 377 DNA sequencer (Applied Biosystems). The bands were detected by a fluorescence imaging system (FluorImager, Molecular Dynamics, Sunnyvale, CA, USA). A piece of the gel containing the band was cut out and soaked in 20 μl dH2O for 10 min on ice and heated for 15 min at 95°C. The supernatant was transferred to another tube. Reamplification of the recovered fragment was performed in 20 μl of a reaction mixture containing 1× ExTaq buffer, 0.2 mM of each dNTP, 1 μM XspI primer, 1 μM TIR primer, 2 units TaKaRa ExTaq DNA polymerase (TaKaRa), and 4 μl of the supernatant, under conditions as follows: 30 cycles of 94°C for 30 s, 56°C for 60 s, and 72°C for 60 s. The amplification products were purified from an agarose gel slice using the Wizard SV gel and PCR clean-up system (Promega, Madison, WI, USA). After purification, the sample was ligated with pGEM-T Easy vector (Promega) and transformed into E. coli.
DNA and RNA analysis
Genomic DNA was obtained from leaves. DNA extraction and construction of a genomic DNA library were performed as described previously (Nitasaka 2003). FE genomic clones of wild-type were isolated from a TKS genomic library that was constructed by the following method: 10 μg of genomic DNA from TKS was partially digested with MboI, and DNA fragments larger than 15 kb were gel-purified using the Wizard SV gel and PCR clean-up system (Promega). These fragments were ligated into BamHI-XhoI-digested λDASH II vector (Stratagene, La Jolla, CA, USA). These ligated mixtures were packaged using Max-Plax packaging extract (Epicentre, Madison, WI, USA) and plated with a selective host (XL1-Blue MRA-P2; Stratagene). Three independent clones were obtained out of 600,000 phages from a TKS genomic library using fe-specific Tpn-flanking sequence, obtained using the STD method, as a probe. Genomic clones of a fe mutant were isolated from a Q438 genomic library. Other procedures were identical to the procedure used to construct a TKS genomic library, but a lambda phage vector, λQ1 (constructed by Nitasaka, unpublished results), was used. This library was screened using the FE genomic sequence as a probe. To determine the genomic fe structures of other strains (Q422, Q441, Q449, Q467), we designed primers tT94F (5′-TGCTAATATGAATATATGATTAATT-3′) and tG332R (5′-GGTTCTTTTCACAGTCCTGCAAA-3′) from FE genomic sequence, and Tpn102-2836R (5′-TCCTGTAATCAAACCAACAAGTAAT-3′) and Tpn102-2690F (5′-AGTCTGGGTTTATTATTATTTTTTG-3′) from the inserted transposon (Tpn102) sequence in Q438. The 5′ and 3′ ends of the Tpn102 flanking sequence of each strain were amplified with tT94F/Tpn102-2836R, and tG332R/Tpn102-2690F primer sets, respectively, under conditions as follows: 94°C for 60 s followed by 35 cycles of 94°C for 30 s, 55°C for 120 s, and 72°C for 60 s. Amplified PCR products were subcloned and sequenced.
Total RNA was extracted using a Get PureRNA kit (Dojindo, Kumamoto, Japan), from which cDNA was synthesized with an oligo-dT primer using the SuperScript first-strand synthesis system (Invitrogen, Carlsbad, CA, USA), and used for subsequent PCR. FE cDNA was amplified with primers 1F (5′-ATGCCCTTAGAAGGGGTTTTCATTG-3′) and 1401R (5′-TAATGTGAATTCCAAACTAGGGTT-3′) based on the alignment between the FE genomic sequence and the Arabidopsis KAN1 cDNA sequence, under conditions as follows: 94°C for 60 s followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s. To isolate full-length cDNA products, 5′ and 3′ RACE were performed. 5′ RACE was performed using a 5′ RACE system (Invitrogen) with primers 141R (5′-GCGGCGGCTACCAATTCATTGATACAAGAC-3′) (for first-strand synthesis), 91R (5′-GGTGTTGTTGGGCGGGCTAATGTGGAGAGA-3′) and Abridged Anchor Primer (AAP; 5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG-3′) for the first PCR and 54R (5′-GGGAAGAGGGTTAGAACGAGAGGAG-3′) and Abridged Universal Amplification Primer (AUAP; 5′-GGCCACGCGTCGACTAGTAC-3′) for the second PCR. 3′ RACE was performed using a 3′ RACE system (Invitrogen) with primers 1260F (5′-AAGCAGCAGAGAAGCATGGCTACAAACTAA-3′) and AUAP for the first PCR, and 1315F (5′-ATCAGATCATCATCCTTTCCAACACAACAA-3′) and AUAP for the second PCR. RT-PCR experiments shown in Figs. 4 and 5 were carried out with primers 1F and 1068R (5′-CTTGTCAGTGGTTTTAACAGTTCT-3′), and 1F and 1401R, respectively. Both temperature profiles were as follows: 94°C for 60 s followed by 40 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 60 s.
In situ hybridization
In situ hybridization was performed according to http://www.its.caltech.edu/∼plantlab/protocols/insitu.htm. A 700 bp fragment was amplified from FE cDNA using primers 1F and 770R (5′-GCTCCGATTCCAACTCCGCCGTACT-3′) and cloned into pGEM-T Easy (Promega). Two plasmids, pFEc770A and pFEc770S, with different orientations of the insert, were obtained. These two plasmids were digested with SalI and transcribed with T7 RNA polymerase (Roche, Mannheim, Germany) to generate the antisense and the sense probe for FE, respectively.
Sequence analyses
Restriction fragments from lambda phage clones were subcloned into pBluescript SK–(Stratagene). Most PCR products were subcloned into the pGEM-T Easy vector (Promega). Plasmid sequences were determined using an ABI PRISM 3100 DNA sequencer (Applied Biosystems). To determine the complete FE genomic sequence, DNA sequencing facilitated by the bacterial transposable element γδ was performed as described previously (Kawasaki and Nitasaka 2004). Database searches were carried out using the BLAST programs (Altschul et al. 1990). Amino acid sequences of FE and KANADI genes were aligned using CLUSTAL X software (Macintosh v. 1.64). Based on these alignments, a phylogenetic tree was constructed using the neighbor-joining method (Saitou and Nei 1987).
Genetic analyses
A plant of the Q441 strain (fe/+cma/cma mf1/mf1) was crossed with TKS, and one of the F1 plants heterozygous for fe was self-pollinated, yielding 236 F2 progeny. These F2 progeny were classified according to the strengths of leaf and flower phenotypes, and the patterns of fe segregation in the F3 generation.
Sequence data from this article have been deposited with the GenBank data library under accession numbers AB220968 (FE cDNA) and AB220969 (FE genomic DNA).
Results
The fe mutant phenotype
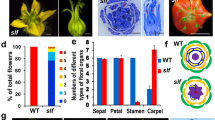
The fe mutation causes a pleiotropic phenotype: the entire body is twisted and distorted, and leaf and flower morphology is especially affected. fe is recessive with respect to flower phenotype, but has a semi-dominant effect on leaf phenotype, i.e., heterozygous fe plants show an intermediate phenotype between the fe mutant and wild-type in cotyledons and leaves but not flowers. Leaves of fe mutants curl upward and the strength of the leaf phenotype is correlated with that of the flowers. fe mutant strains can be classified into several types based on the severity of their mutant phenotype. Wild-type morning glory has a gamopetalous flower with five fused petals and five straight sepals. There are five stamens and one pistil fused with three carpels. All fe mutants have an increased number of petals and five curled sepals. In a weaker “creased” type (Q467) of fe mutant, the corolla is creased and sometimes split between the veins, and supplemental petaloid tissues are attached to the flower tube. The number and shape of reproductive organs (stamens and carpels) are almost normal, but fertility is low.
A stronger wind-bell type (Q441) has tubular petals folded back at their tips like bells. The strongest shooting-star type (Q449) has finer tubular petals than the wind-bell type. In these strong mutants, the petals are completely separated, forming a tube with the adaxial epidermis enclosing the abaxial epidermis. Therefore, especially in the wind-bell type, the side facing the outside of the bell-shaped petals at the tip of tube petals is abaxial epidermis, and very small crimps are observed on the abaxial surface. Strong fe mutants have degenerate stamens and carpels, and filament-like organs without anthers are often observed; thus, these mutants are almost sterile. Moreover, there is an intermediate type (Q438) between creased and wind-bell types (Fig. 1A).
Phenotypes of wild-type and fe mutants. Wild-type (TKS) and creased (Q467), intermediate (Q438), wind-bell (Q441) and shooting-star (Q449) types are shown from the left. (A) Flowers. Top view (left) and bottom view (right) in wild-type and creased types, and two different side views in intermediate, wind-bell and shooting-star types are shown. Extra veins can be seen clearly in the creased type, but other fe mutants also showed an increased number of split or tubular petals. Because these strains, except for wild-type, harbor the flower color mutation magenta (mg), they have reddish flowers. (B) Petal epidermal cells of the adaxial side (left) and abaxial side (right) observed by SEM. Extent of transformation of epidermal cells from abaxial type (flat) to adaxial type (conical) correlates with phenotypic strength. The shooting-star type has smaller cells than other types. (C) Leaf adaxial side (left) and abaxial side (right). The leaf color in shooting-star is pale green because it also harbors a leaf color mutation, yellow (y). (D) Leaf cross-sections. Palisade tissues are stained green. Insets show magnifications of leaf lamina. Scale bar, 1 cm (A,C), 50 μm (B), 500 μm (D) and 100 μm (D, insets)
Flower coloration of wild-type and the creased type differs slightly between the adaxial and abaxial sides; the abaxial side of the petal is lighter than the adaxial side (Fig. 1A). We observed the epidermal surfaces of petals by SEM. Epidermal cells of wild-type and the creased type differed in shape between the adaxial and abaxial sides—adaxial cells were conical and abaxial cells were flatter (Fig. 1B). The different cell shape would change the diffusion of light, resulting in a different color between the adaxial and abaxial sides of the petal. In strong fe mutants, the abaxial side is as dark as the adaxial side (Fig. 1A). We found that abaxial epidermal cells of petals form a conical shape similar to wild-type adaxial cells in strong fe mutants (Fig. 1B). This finding suggests that FE is involved in establishment of abaxial identity in the flowers. In the shooting-star, the strongest type of fe, the cell size of the petals was smaller than other types (Fig. 1B).
Leaves of creased type fe are nearly flat or slightly curl upward, hence, these are difficult to distinguish from wild-type leaves. In strong fe mutants, such as wind-bell and shooting-star types, leaves have thicker laminae, heavily upcurled lobes and elongated petioles compared with the wild-type. Cotyledons also show a similar phenotype to the leaves. The abaxial side of fe leaves appears darker green than wild-type, and ectopic outgrowths of laminae are often observed (Fig. 1C). In wild-type plants, more stomata are distributed on the abaxial than the adaxial side of leaves, while fe mutants show a reduced density of stomata on the abaxial side. In the shooting-star type, the strongest strain of fe, abaxial stomatal density was lower than the adaxial (Fig. 2). Leaf cross-sections revealed that the abaxial subepidermal layer of the spongy mesophyll is transformed into palisade-like mesophyll in fe mutants (Fig. 1D). These observations also suggest that the FE gene is involved in establishment of leaf polarity, which is required for abaxial identity. In strong fe mutants, the internal spongy mesophyll layers between adaxial palisade mesophyll and abaxial palisade-like mesophyll were expanded, making the lamina thicker. Furthermore, the number of abaxial cells was increased in the leaves, resulting in creases on their surface (Fig. 1D). This difference in cell number between adaxial and abaxial sides may be responsible for the upward curling of the leaves.
Comparison of densities of stomata between adaxial and abaxial sides of leaves in wild-type (TKS) and fe strains. All strains except for Q422 are shown in Fig. 1. Both Q441 and Q422 are the wind-bell type. The Q449 strain (shooting-star type) shows a higher stomatal density than other fe strains because of its smaller leaf cell size
Isolation of the FE gene by transposon display
In some fe mutant strains, somatic reversion events from fe/fe homozygotes to FE-r/fe heterozygotes (FE-r; a revertant allele of fe) were rarely observed. Because the fe mutation is recessive for the flower and semi-dominant for the leaf phenotype, these plants produced reversion shoots with fertile wild-type flowers and weakly curled leaves. This reversion event seems to be caused by excision of a Tpn transposon inserted into the FE gene. To isolate the FE gene, we used the STD method (Fukada-Tanaka et al. 2001), which allows the visualization of insertion sites of high copy number transposable elements, such as Tpn, and the isolation of Tpn flanking sequences. We generated siblings homozygous for FE-r and fe after self-pollination of a heterozygous revertant from the Q438 strain, and then compared FE-r with fe with regard to Tpn insertion sites by the STD method. Two fe-specific fragments adjacent to a single Tpn insertion were obtained (Fig. S1 in the supplementary material), and were used as probes to identify the genomic region corresponding to the FE gene. The sequence of the genomic region isolated indicated that it contains a putative coding region highly homologous to the KANADI genes in Arabidopsis (Eshed et al. 2001; Kerstetter et al. 2001). To determine the structure of the FE gene, we obtained FE cDNA by RT-PCR and by 3′ and 5′-RACE. FE consists of six exons; a Tpn transposon was inserted in the fourth intron of the FE gene in the fe mutant of the Q438 strain (Fig. 3A). We tested for the presence of the insert by PCR in 28 FE-r and 21 fe plants from F2 siblings. In all plants tested, the insertion completely coincided with mutant phenotypes, indicating that the fe mutation is caused by this insertion. The inserted Tpn transposon appeared to be Tpn102, the second major type in the Tpn1 family in the Japanese morning glory (Kawasaki and Nitasaka 2004), and this allele was designated fe-1 (Fig. 3A). Insertion of this Tpn102 generates a 3-bp target duplication, and the FE-r allele contained a 3 bp footprint (TTA) generated by Tpn102 excision (Fig. 3B).
Structure of the FE gene. (A) Schematic diagram of the FE gene. Black boxes represent the coding regions. Open boxes represent 5′ and 3′ untranslated regions. The open triangle represents the insertion site of the Tpn transposon in fe-1. Dotted lines indicate the regions covered by different lambda-phage clones. B, BamHI; E, EcoRI. The approximate positions of primers used are indicated by arrowheads. The symbol corresponding to each primer is given as follows: a, 1F; b, 1068R; c, 1401R. The transcriptional direction is from left to right. (B) Sequences at the Tpn102 insertion site in the FE (wild-type), fe-1 and FE-r alleles. The 3-bp TTA indicates the target site and footprint of the Tpn102 insertion. All fe strains tested had the same fe-1 allele
To examine whether the phenotypic differences of fe mutants were caused by differences in alleles, we determined genomic sequences of four additional fe strains showing different phenotypes (Q467, Q441, Q449, Q422; all strains except for Q422 are shown in Fig. 1; Q422 is a wind-bell type fe). Surprisingly, all of the mutants tested had the same fe-1 allele, i.e., Tpn102 was inserted at the same site in the FE gene (Fig. 3B). This finding suggests that fe mutants were derived from a single founder that appeared in the Edo era (∼200 years ago), and that the different phenotypes of fe strains are dependent on different genetic backgrounds. This differs from the previous assumption that creased-feathered is a weak allele of fe (Imai 1931a).
FE encodes a GARP transcripton factor closely related to Arabidopsis KAN1
FE encodes a putative protein of 478 amino acids. A database search revealed that FE is a member of the KANADI genes, which is expressed in the abaxial region of the lateral organ primordia, and promotes abaxial identity (Eshed et al. 2001; Kerstetter et al. 2001). The Arabidopsis genome contains four KANADI genes (KAN1-KAN4), which encode proteins containing a GARP domain (Riechmann et al. 2000), found in plant-specific transcription factors, and six short KANADI-specific motifs (Eshed et al. 2001) (Fig. 4A). FE also contains a highly conserved GARP domain and six KANADI-motifs. In the case of the GARP domain, the amino acid identities between FE and KANADI proteins vary from 93% (KAN3) to 98% (KAN1), and those of KANADI-motifs vary from 46% (KAN4) to 90% (KAN1) on an average of six motifs. Therefore, these conserved domain and motifs are thought to retain functions as KANADI genes. A phylogenetic tree also indicated that FE was most closely related to KAN1 (Fig. 4B).
Comparison of amino acid sequences of FE and KANADI and their relationship. (A) Alignment of FE and KANADI amino acid sequences. Gray boxes represent the KANADI-specific motifs. Boxes with bold letters represent GARP domains. (B) A phylogenetic tree of FE and KANADI genes, indicating that FE is most closely related to KAN1. Numbers at each node indicate bootstrap values of 1000 trials. Accession numbers are as follows: FE (AB220968), KAN1 (AY048688), KAN2 (AY048689), KAN3 (AY048690) and KAN4 (AY048691)
Expression of FE and aberrant transcripts in the fe mutant
We examined the tissue specificity of FE expression by RT-PCR. FE was expressed in all tissues examined (Fig. 5). This result is consistent with the pleiotropic phenotype of fe mutants. Expression of KAN1 is restricted to the lateral organ primordia in Arabidopsis (Eshed et al. 2001; Kerstetter et al. 2001), whereas FE is expressed more ubiquitously, suggesting that FE may act through all developmental stages. Although we could not detect FE signals by in situ hybridization, presumably because its transcriptional level is low, it is supposed that FE is expressed similarly to KAN in fe mutants, on the abaxial side of lateral organs. We next examined transcripts of fe mutants to investigate how the inserted Tpn102 influences expression of the gene. In all fe mutants, expression levels were nearly the same as wild-type, but transcripts were 200 bp longer (Fig. 6A). Sequencing of these transcripts revealed that the captured MYOSIN-related sequence and the 3′ common Tpn exon of Tpn102 (Kawasaki and Nitasaka 2004) was fused to the 3′ region of the FE transcript by alternative splicing within the Tpn102 sequence. A premature termination codon created by the insertion of Tpn102 sequence causes truncation of the C-terminal region of FE containing two conserved KANADI motifs (Fig. 6B). This aberrant FE product might have lost its function. Both the 1.6-kb mutant transcript and the 1.4-kb normal transcript were detected in the mutable Q438 strain (Fig. 6A), perhaps due to excision of Tpn102 in somatic cells.
Transcripts and deduced protein products in fe mutants. (A) RT-PCR analysis of FE transcripts in wild-type (TKS) and fe mutant strains using primers 1F (“a” in Fig. 3A) and 1401R (“c” in Fig. 3A). (B) Genomic structure of the fe-1 allele and its deduced products. Red and blue boxes represent the KANADI-specific motifs and the GARP domain, respectively. Gray boxes represent 5′ and 3′ untranslated regions. Tpn102 (5570 bp), inserted in the fourth intron, contains 5′ and 3′ subterminal regions with tandem repeats (filled triangles), a captured MYOSIN-related coding sequence consisting of two exons (filled boxes) and a part of the 3′ common exon of Tpn1 family (yellow box). In fe mutants, the MYOSIN-related coding sequence and the Tpn common exon has fused to the 3′ region of the FE transcript, and this causes a frame-shift mutation in the fifth exon of FE. The asterisk indicates the site of the termination codon
Stronger fe mutant strains have additional mutations enhancing adaxialization
A heterozygous fe plant of a typical wind-bell type strain (Q441) was crossed with the wild-type strain TKS (Fig. 1), and 236 progeny with various degrees of the fe phenotype segregated in the F2 generation (Tables 1 and 2, Fig. S2 in the supplementary material). Taking together these results with the observation that fe mutant strains with different phenotypes have the same fe-1 allele indicates that fe mutant strains have additional mutation(s) that modify the fe phenotype. Both leaf and flower phenotypes of F2 segregants are correlated, as fe affects both phenotypes.
However, some exceptional progeny were observed, e.g., a weak leaf phenotype with strong flower phenotype or vice versa, suggesting the presence of at least one independent modifier gene for each of the leaf and flower phenotypes.
The crumpled1 (cm1) mutation is recessive, and leaves of cm1 are slightly upcurled and have small concavities on their surfaces. Strong fe mutant strains have the cm1 mutation as an enhancer of leaf phenotype, and the effect of cm1 is semi-dominant to the fe phenotype (Imai 1931a). Thus, heterozygous cm1 also enhances leaf phenotype in a fe homozygous or heterozygous background. Five cm loci (cm1–cm5) were described and their mutant phenotypes are equivalent (Imai 1931a; Hagiwara 1956). The strong fe strains in this study, including Q441, are thought to also harbor the homozygous cm1 mutation according to their phenotypes, the origin of the strain and our preliminary genetic experiments (see Discussion). However, here a genetic symbol cma is used as anonymous cm mutation since Imai’s cm1 stock is not available now. Based on these assumptions and leaf phenotypes, F2 progeny were classified into six genotypic classes of fe and cma combinations, and there was no significant difference between observed and expected segregation ratios (P = 0.097; Table 1).
In contrast to leaf phenotype, flower phenotype appears only in the homozygous fe background. Flower phenotypes were classified into four classes (Fig. S2 B in the supplementary material), indicating that modifier of flower phenotype 1 (mf1) has a semi-dominant effect on flower phenotype, similarly to cma. The observed segregation ratios deviated significantly (P = 0.003) from the expected segregation calculated based on the assumption that fe and mf1 are inherited independently. This result suggests that there is linkage between fe and mf1; the genetic distance calculated from the segregation score was about 34.6 centimorgans (Table 2).
From these genetic and molecular analyses, we concluded that a creased-type fe plant corresponds to the fe single mutant, and the wind-bell type of fe plant has fe and two modifier mutations, cma and mf1. Enhancement of the fe mutant phenotypes by cma or mf1 suggested that cma and mf1 promote abaxial fate redundantly with FE in leaves and flowers, respectively. Single fe mutant show creased flower phenotype (Fig. 1A) unlike kan1 single mutants, which do not show a striking phenotype (Eshed et al. 1999, 2001; Kerstetter et al. 2001). Furthermore, multiple mutants for fe and modifiers show aberrant flower phenotypes only when fe is present in the homozygous state. These observations suggest that some different mechanisms regulate polarity in leaves and flowers, and that FE plays a central role among genes that promote abaxial cell fate, especially in flowers.
Discussion
In this study, we showed that fe mutations cause adaxialization in lateral organs. The mutant phenotypes suggest that FE is required for abaxial identity. Genetic and molecular analyses revealed that all fe mutants are derived from a single founder, and at least two modifier mutations enhance the fe phenotype. A time series of phenotypic changes of fe mutants described in the earlier literature indicates that the simple mutant variety was transformed into complicated varieties over time (Fig. S3 in the supplementary material). In the process of establishment of fe strains with strong phenotypes, such as the wind-bell type, at least two modifier mutations were accumulated in the fe single mutation background (creased type) by phenotypic selection to develop unique and extraordinary plants. Stronger cma and mf1 alleles or additional modifiers probably influence the phenotype of the strongest variety, the shooting-star type (see below).
So far, many genes responsible for flower pigmentation (e.g., anthocyanin biosynthesis) (Inagaki et al. 1994; Fukada-Tanaka et al. 1997; Hoshino et al. 1997; Inagaki et al. 1999; Fukada-Tanaka et al. 2000) and the floral homeotic gene DUPLICATED (DP) (Nitasaka 2003) have been isolated from the Japanese morning glory. Most of these mutant genes are due to insertions of transposable elements belonging to the Tpn1 family. Hence, we thought the fe mutation might also be caused by Tpn insertion, and we succeeded in isolating the FE gene by a new procedure for gene isolation, the STD method, which is a kind of transposon tagging that is effective in high copy number lines (Fukada-Tanaka et al. 2001). This method would be powerful for isolation of other genes responsible for morphological mutations in the Japanese morning glory.
It has been reported that strong fe mutant strains have the cm1 mutation as an enhancer of leaf phenotype; four other cm loci (cm2–cm5) showing almost the same mutant phenotypes have been described (Imai 1931a; Hagiwara 1956). Like cm1, the other cm mutations are thought to enhance the fe phenotype; however, this has not been demonstrated by genetic experiments other than for cm1. In this study, we designated cma as a modifier mutation of fe leaf phenotype. cm1 is located on the same chromosome with variegated-1 (v1) and the genes are linked (Imai 1926). Although we cannot test the allelism of cma since the original genetically analyzed cm mutant strains have been lost, in preliminary experiments, F2 plants from a cross between a wind-bell type fe and a strain carrying v1 showed that fe plants with strongly upcurled leaves had no v1 phenotype, which also suggests linkage between v1 and cma, and allelism between cm1 and cma.
To study the genetic background of modifier mutations of fe strains with strong phenotypes, we intercrossed wind-bell type fe strains. The F1 and F2 generations had no exceptional progeny such as creased type or weakly curled leaves. This indicates that recent wind-bell type fe strains harbor the same modifier mutations mf1 and cma. We also crossed Q449 and another shooting-star type strain, and they also yielded no exceptional progeny. This finding indicates that the background of modifier mutations is equivalent in shooting-star type strains. Intertype crosses between the shooting-star (Q449) and wind-bell type strains yielded F1 fe plants with leaf and flower phenotypes intermediate between the parents. In the F2 generation, wind-bell type, shooting-star type and intermediate type progeny segregated. These results suggest that the shooting-star type also has cma and mf1 mutations, and stronger cma and mf1 alleles or additional modifiers such as another cm mutation influence the phenotype of the shooting-star type.
Almost all members of the Tpn1 family capture host gene sequences, and Tpn102 inserted in the fe mutation captured a MYOSIN-related sequence. Moreover they contain common exons, especially 3′-most one including the transcription termination signal possibly derived from an ancestral autonomous element (Kawasaki and Nitasaka 2004). The transcriptional directions of most Tpn including Tpn102 coincide with those of captured genes. When Tpn is inserted in the same transcriptional direction as the gene, splicing signals of the captured gene and/or the Tpn common exon affect the splicing pattern of transcripts, resulting in fused transcripts. Because the common 3′-most exon of Tpn contains a transcription termination signal, premature termination within Tpn also observed (e.g., the flecked mutation) (Takahashi et al. 1999; Kawasaki and Nitasaka 2004). If the orientation of the captured gene and Tpn is opposite with the inserted gene, the influence on the transcript is less and Tpn is simply spliced out because of the absence of splicing and termination signals (e.g., as for a weak dp allele, dp-pt, unpublished results). In the case of the fe mutation, a new 5′ splicing donor site was created by nucleotide substitution in the common 3′-most exon of Tpn102. fe transcripts are read through the Tpn102 sequence and spliced in FE and Tpn102 sequences, generating fused transcripts of FE, MYOSIN-related sequence and Tpn common sequence. fe mutants show a semi-dominant phenotype, which might be caused by the aberrant products having a dominant-negative function.
In Arabidopsis, appropriate organ formation along the adaxial–abaxial axis is established by three gene families, the class III HD-ZIP, KANADI and YABBY genes, interacting with each other (Hudson 2001; Emery et al. 2003; Eshed et al. 2004; Hawker and Bowman 2004). Although mutants and responsible genes belonging to class III HD-ZIP and YABBY families have been analyzed, no mutants of KANADI-related genes have been isolated from other plants. fe is the first identified mutation of the KANADI gene family in plants other than Arabidopsis, and FE is most closely related to KAN1. KANADI and YABBY genes are functionally redundant in promotion of abaxial identity, and multiple mutations in these genes enhance their phenotype (Eshed et al. 1999, 2004). fe modifier mutations enhance adaxialization in fe mutants, implying that fe modifiers correspond to other members of KANADI or YABBY genes. In the case of YABBY genes, however, the regulatory mechanisms that determine abaxial identity are not shared with Arabidopsis; functions differ depending on the species (Golz et al. 2004; Juarez et al. 2004b; Yamaguchi et al. 2004). The regulation of class III HD-ZIP genes is conserved between Arabidopsis and maize (Juarez et al. 2004a), and we revealed that FE promotes abaxial identity as do KANADI genes, suggesting that the primary role for establishment of adaxial and abaxial polarity by class III HD-ZIP and KANADI genes is conserved among plant species. However, fe mutant phenotypes suggest that FE plays a more crucial role than KANADI to promote abaxial cell fate especially in flower. FE may have been recruited to acquire critical function in flower during plant evolution.
In the Japanese morning glory, the creased flower phenotype occurring with a single fe mutation resulted from an increased number of petals. Furthermore, in plants carrying mutations in fe and modifiers, the number of stamens and carpels increased. Although the relationship between an increase in organ number and adaxialization is unclear, the number of floral organs occasionally increases as a result of floral meristem enlargement (Clark et al. 1993, 1995; Kayes and Clark 1998; Fletcher 2001). Some studies indicate an association between meristem development and the establishment of adaxial-abaxial polarity. Accompanying adaxialization in the Arabidopsis phb-d mutant, SAM size is increased and new axial meristems develop (McConnell and Barton 1998; McConnell et al. 2001). Conversely, the rev mutant of Arabidopsis and the phantastica (phan) mutant of Antirrhinum majus, both of which result in abaxialization, cause meristem arrest (Talbert et al. 1995; Waites and Hudson 1995; Waites et al. 1998). Ectopic expression of FILAMENTOUS FLOWER (FIL) or YABBY3 (YAB3) also results in abaxialization and causes meristem arrest (Sawa et al. 1999; Siegfried et al. 1999). Thus, the increase in the floral organ number in fe mutants could be explained by enlargement of floral meristems as a consequence of adaxialization. fe mutants show various morphological defects in addition to loss of abaxial identity, such as an increase in abaxial cell number and elongated petioles. These phenotypes and ubiquitous expression imply that FE is involved in various aspects of regulating organ formation.
For establishment of organ polarity, various gene families interact in a complicated way (Bowman et al. 2002). To clarify these relationships, many studies including enhancer screening are effective. Further studies on FE and its modifier genes will shed light on detailed mechanisms of plant development.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bowman JL, Eshed Y, Baum SF (2002) Establishment of polarity in angiosperm lateral organs. Trends Genet 18:134–141
Bowman JL, Smyth DR (1999) CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 126:2387–2396
Clark SE, Running MP, Meyerowitz EM (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119:397–418
Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121:2057–2067
Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL (2003) Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol 13:1768–1774
Eshed Y, Baum SF, Bowman JL (1999) Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99:199–209
Eshed Y, Baum SF, Perea JV, Bowman JL (2001) Establishment of polarity in lateral organs of plants. Curr Biol 11:1251–1260
Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL (2004) Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131:2997–3006
Fletcher JC (2001) The ULTRAPETALA gene controls shoot and floral meristem size in Arabidopsis. Development 128:1323–1333
Fukada-Tanaka S, Hoshino A, Hisatomi Y, Habu Y, Hasebe M, Iida S (1997) Identification of new chalcone synthase genes for flower pigmentation in the Japanese and common morning glories. Plant Cell Physiol 38:754–758
Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Iida S (2001) Simplified transposon display (STD): a new procedure for isolation of a gene tagged by a transposable element belonging to the Tpn1 family in the Japanese morning glory. Plant Biotech 18:143–149
Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Saito N, Iida S (2000) Colour-enhancing protein in blue petals. Nature 407:581
Glover B, Martin C (1998) The role of petal cell shape and pigmentation in pollination success in Antirrhinum majus. Heredity 80:778–784
Golz JF, Roccaro M, Kuzoff R, Hudson A (2004) GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development 131:3661–3670
Hagiwara T (1956) Genes and chromosome maps in the Japanese morning glory. Bull Res Coll Agr Vet Sci Nihon Univ 5:34–56
Hawker NP, Bowman JL (2004) Roles for Class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiol 135:2261–2270
Hoshino A, Abe Y, Saito N, Inagaki Y, Iida S (1997) The gene encoding flavanone 3-hydroxylase is expressed normally in the pale yellow flowers of the Japanese morning glory carrying the speckled mutation which produce neither flavonol nor anthocyanin but accumulate chalcone, aurone and flavanone. Plant Cell Physiol 38:970–974
Hudson A (2001) Plant development: two sides to organ asymmetry. Curr Biol 11:R756–R758
Iida S, Hoshino A, Johzuka-Hisatomi Y, Habu Y, Inagaki Y (1999) Floricultural traits and transposable elements in the Japanese and common morning glories. Ann N Y Acad Sci 870:265–274
Iida S, Morita Y, Choi JD, Park KI, Hoshino A (2004) Genetics and epigenetics in flower pigmentation associated with transposable elements in morning glories. Adv Biophys 38:141–159
Imai Y (1926) On the rolled leaves and their linked characters in the Japanese morning glory (Pharbitis Nil). Zeitschr f ind Abst -u Verebgsl 40:205–231
Imai Y (1929) Linkage groups of the Japanese morning glory. Genetics 14:223–255
Imai Y (1931a) Creased flowers of Parbitis Nil. Zeitschr f ind Abst -u Verebgsl 58:248–258
Imai Y (1931b) Description of the genes found in Pharbitis nil. Genetica 12:297–318
Imai Y (1933) Linkage studies in Pharbitis nil. Zeitschr f ind Abst -u Verebgsl 66:219–235
Imai Y (1938) Genetic literature of the Japanese morning glory. Jpn J Genet 14:91–96
Inagaki Y, Hisatomi Y, Suzuki T, Kasahara K, Iida S (1994) Isolation of a Suppressor-mutator/Enhancer-like transposable element, Tpn1, from Japanese morning glory bearing variegated flowers. Plant Cell 6:375–383
Inagaki Y, Johzuka-Hisatomi Y, Mori T, Takahashi S, Hayakawa Y, Peyachoknagul S, Ozeki Y, Iida S (1999) Genomic organization of the genes encoding dihydroflavonol 4-reductase for flower pigmentation in the Japanese and common morning glories. Gene 226:181–188
Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MC (2004a) microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428:84–88
Juarez MT, Twigg RW, Timmermans MC (2004b) Specification of adaxial cell fate during maize leaf development. Development 131:4533–4544
Kawasaki S, Nitasaka E (2004) Characterization of Tpn1 family in the Japanese morning glory: En/Spm-related transposable elements capturing host genes. Plant Cell Physiol 45:933–944
Kay QON, Daoud HS, Stirton CH (1981) Pigment distribution, light reflection and cell structure in petals. Bot J Linn Soc 83:57–84
Kayes JM, Clark SE (1998) CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125:3843–3851
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411:706–709
McConnell JR, Barton MK (1998) Leaf polarity and meristem formation in Arabidopsis. Development 125:2935–2942
McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411:709–713
Nitasaka E (2003) Insertion of an En/Spm-related transposable element into a floral homeotic gene DUPLICATED causes a double flower phenotype in the Japanese morning glory. Plant J 36:522–531
Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17:61–76
Riechmann JL, Heard J, Martin G, Reuber L, Jiang C, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu G (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sawa S, Watanabe K, Goto K, Liu YG, Shibata D, Kanaya E, Morita EH, Okada K (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev 13:1079–1088
Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126:4117–4128
Takahashi S, Inagaki Y, Satoh H, Hoshino A, Iida S (1999) Capture of a genomic HMG domain sequence by the En/Spm-related transposable element Tpn1 in the Japanese morning glory. Mol Gen Genet 261:447–451
Talbert PB, Adler HT, Parks DW, Comai L (1995) The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development 121:2723–2735
Waites R, Hudson A (1995) phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121:2143–2154
Waites R, Selvadurai HR, Oliver IR, Hudson A (1998) The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93:779–789
Williams MH, Vesk M, Mullins MG (1987) Tissue-preparation for scanning electron-microscopy of fruit surfaces—comparison of fresh and cryopreserved specimens and replicas of banana peel. Micron and Microscopica Acta 18:27–31
Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY (2004) The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16:500–509
Acknowledgements
We thank E. Kikuchi and K. Tsuji for providing fe revertant plants, Y. Toh for help with SEM analysis, and S. Iida, T. Tsurimoto, K. Iba and C. Machida for helpful discussions. We also thank National BioResource Project (NBRP; morning glory) for the mutant strains. This study was supported by PRESTO, Japan Science and Technology Corporation, and grants (Nos. 15570007 and Grant-in-Aid for Scientific Research on Priority Areas (C) Genome Science) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Iwasaki, M., Nitasaka, E. The FEATHERED gene is required for polarity establishment in lateral organs especially flowers of the Japanese morning glory (I pomoea nil ). Plant Mol Biol 62, 913–925 (2006). https://doi.org/10.1007/s11103-006-9066-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-006-9066-2