Abstract
Imbalanced Th17/Treg ratio is implicated in the pathogenesis of aplastic anemia. Studies have indicated that bone marrow-derived mesenchymal stem cells-derived exosomes (BMSC-Exo) could correct imbalanced Th17/Treg in aplastic anemia, but the mechanism remains not fully understand. This study was designed to investigate whether BMSC-Exo regulates the Th17/Treg balance in aplastic anemia by transferring miR-23a-3p. Here, miR-23a-3p inhibitor was utilized to knockdown the expression of miR-23a-3p in BMSC-Exo. A co-culture system of CD4+ T cells from aplastic anemia patients and BMSC-Exo was used to explore the effects of BMSC-Exo on the Th17/Treg balance and the underlying mechanism in aplastic anemia. The patients with aplastic anemia exhibited Th17/Treg imbalance favoring the Th17 cells. BMSC-Exo could balance the percentage of Th17 and Treg cells in aplastic anemia, but the effects of BMSC-Exo can be eliminated when miR-23a-3p expression was silenced in BMSCs. IL-6 was a direct target of miR-23a-3p. IL-6 overexpression could abrogate BMSC-Exo-induced balance in Th17/Treg ratio. Overall, BMSC-Exo could balance Th17/Treg ratio in aplastic anemia via suppressing IL-6 expression by transferring miR-23a-3p at least in part. These data indicated miR-23a-3p may be a potential target for the treatment of aplastic anemia. Our study may provide a new idea for the therapy of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aplastic anemia (AA) is a bone marrow hematopoietic failure syndrome characterized by peripheral blood pancytopenia and hypocellular bone marrow. AA associates with a high mortality rate [1, 2]. Immune abnormality is the main pathogenesis of AA [3]. CD4+ T lymphocytes are the major population involved in the cell-mediated immune response. Based on different microenvironments, CD4+T cells can differentiate into T helper type 1 (Th1), Th2, Th17, or regulatory T (Treg) subsets. Among them, more and more attention has been paid to the role of Th17 and Treg cells in hematological diseases associated with bone marrow failure [4]. Th17 cells, a pro-inflammatory cell type, contribute to the occurrence of autoimmune diseases. Oppositely, Treg cells have an immunosuppressive function. Treg cells are essential for the maintenance of immunologic tolerance. The balance between Th17 cells and Treg cells is critically important for the maintenance of immune homeostasis. A large body of evidence have shown that imbalance of Th17/Treg ratio toward the pro-inflammatory Th17 program is associated with many autoimmune diseases, including AA [5, 6].
Mesenchymal stem cells (MSCs) are multi-potent stem cells with self-renewal and multi-lineage differentiation potential. MSCs have immunomodulatory functions, which have been widely used in the treatment of immune diseases [7, 8]. It has been reported that transfusion with bone marrow-derived MSCs (BMSCs) may be a good choice for the treatment of AA [9]. MSCs can exert their immunomodulatory functions through secreting exosomes [10]. Exosomes are nano-sized (30–150 nm) membrane vesicles, which can be released by almost all cell types. Exosomes are crucial mediators for intercellular communication by transmitting bioactive molecules from donor cells to recipient cells [11, 12]. It has been reported that BMSCs could correct the Th17/Treg imbalance in AA through secreting exosomes containing sphingosine 1-phosphate [13].
The dysfunction of microRNAs (miRNAs) has been proved to be associated with various autoimmune diseases, including AA [14,15,16]. The available evidence has suggested that miRNAs are one of the main components of exosomes. A previous study has shown that miR-23a-3p was highly expressed in the exosomes derived from BMSCs [17]. This prompted us to investigate whether miR-23a-3p contributes, at least partially, to the biological function of exosomes. In the present study, we explored whether the exosomes derived from BMSCs regulate Th17/Treg balance in AA by transferring miR-23a-3p. Our data may provide a novel idea for AA therapy.
Materials and methods
Collection of blood sample and isolation of peripheral blood mononuclear cells
Fifteen patients with AA treated in our hospital were enrolled as the AA group. Another 15 healthy volunteers receiving routine physical examination during the same period were selected as the control group. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University. Informed written consents were obtained from all participants. The basic information of patients and healthy volunteers is listed in supplementary table.
Peripheral venous blood samples (30 mL) were collected from healthy volunteers and AA patients and were then kept in anti-coagulated tubes with heparin. Peripheral blood mononuclear cells were isolated from the peripheral venous blood using a Ficoll (Solarbio Life Sciences, Beijing, China) density gradient according to the manufacturer’s instructions.
Isolation and culture of AA CD4 + T cells
CD4+ T cells were isolated from the peripheral blood mononuclear cells of patients with AA using anti-CD4-conjugated magnetic beads and a magnetic-activated cell sorting column (Miltenyi Biotec, Germany). The obtained CD4+ T cells were cultured in human T cell culture medium (Lonza, USA) at 5% CO2 under 37 °C.
BMSC culture and identification
The human BMSCs (ScienCell, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) at 37 °C in an incubator with 5% CO2. The BMSCs at the fourth passage were used in the following experiments. For identification of BMSCs, the immunophenotype of BMSCs was analyzed using antibodies against CD29, CD34, CD45, and CD90 (BD PharMingen, USA) on a flow cytometer (FACSCalibur; Becton Dickinson, USA).
BMSC transfection
The inhibitor of miR-23a-3p and inhibitor negative control were purchased from GenePharma (Shanghai, China). They were transfected into BMSCs using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The BMSCs transfected with miR-23a-3p inhibitor were named as BMSCmiR-23I, and the BMSCs transfected with inhibitor negative control were named as BMSCINC.
Isolation and identification of BMSC-derived exosomes
Exosomes were isolated and purified from the supernatant of BMSCs using the ExoQuick-TC PLUS Exosome Purification Kit (SBI, USA) according to the manufacturer’s instructions. To avert contamination with FBS-derived exosomes, exosome-depleted FBS (Gibco, USA) was used to culture BMSCs. Exosome pellets were resuspended in 200 μL of PBS for identification. The exosomes derived from BMSCmiR-23I and BMSCINC were named as BMSCmiR-23I-Exo and BMSCINC-Exo, respectively.
The morphologic characteristics of exosomes were observed by transmission electron microscopy (TEM). The particle diameter and concentration of exosomes were measured using Nanosizer™ technology (Malvern Instruments, Malvern, UK). Exosomes were quantified using the BCA protein kit (Thermo Scientific, USA) and were subjected to western blot to determine the protein levels of exosomal surface markers including CD9 and CD81.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells or exosomes using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The levels of miR-23a-3p, IL-17 mRNA, IL-6 mRNA, and Foxp3 mRNA were examined by qRT-PCR as described in detail elsewhere [18]. The expression of miR-23a-3p was normalized to U6, and the mRNA levels of other genes were normalized to GAPDH. Data were analyzed using the 2−ΔΔCt method.
The specific primers were as follows:
miR-23a-3p—forward, 5’-ATCACATTGCCAGGGATTTCC-3’;
miR-23a-3p—reverse, 5’-CAGTGCGTGTCGTGGAGT-3’;
U6—forward, 5’-CTCGCTTCGGCAGCACA-3’;
U6—reverse, 5’-AACGATTCACGAATTTGCGT-3’;
IL-17—forward, 5’-CGGACTGTGATGGTCAACCTGA-3’;
IL-17—reverse, 5’-GCACTTTGCCTCCCAGATCACA-3’;
IL-6—forward, 5’-AGACAGCCACTCACCTCTTCAG-3’;
IL-6—reverse, 5’-TTCTGCCAGTGCCTCTTTGCTG-3’;
Foxp3—forward, 5’-GGCACAATGTCTCCTCCAGAGA-3’;
Foxp3—reverse, 5’-CAGATGAAGCCTTGGTCAGTGC-3’;
GAPDH—forward, 5’-GTCTCCTCTGACTTCAACAGCG-3’;
GAPDH—reverse, 5’-ACCACCCTGTTGCTGTAGCCAA-3’.
Detection of Th17 and Treg cells proportion
The proportions of Th17 cells and Treg cells were determined by flow cytometry as previously described [13]. Briefly, the cells were incubated with the APC-conjugated anti-human RORγt (Miltenyi Biotec, USA) and PE-conjugated anti-human Foxp3 (eBioscience, USA) for 40 min at 4 °C in the dark. All stained cells were analyzed utilizing a flow cytometer (FACSCalibur; Becton Dickinson, USA) equipped with the BD CellQuest software.
Enzyme-linked immunosorbent assay (ELISA)
The levels of interleukin (IL)-6, IL-17, IL-10, and transforming growth factor-β1 (TGF-β1) in serum, and cell culture supernatants were measured using their commercial ELISA kits (R&D Systems, USA).
Co-culture of BMSC-Exo and CD4 + T cells
To investigate whether BMSC-Exo regulates the percentage of Th17 and Treg cells in AA by transferring miR-23a-3p, CD4+ T cells were co-cultured with PBS, BMSC-Exo, BMSCINC-Exo, and BMSCmiR-23I-Exo for 24 h. Then, the percentage of Th17 and Treg cells and their related transcriptional factors or cytokines were examined using flow cytometry, ELISA, and qRT-PCR assay.
In order to determine the role of IL-6 in the regulation of BMSC-Exo to Th17/Treg balance in AA, CD4+ T cells were transfected with the IL-6 overexpression vector (GenePharma, Shanghai, China) and then were co-cultured with BMSC-Exo for 24 h. Then, Th17 and Treg cells proportions and their related transcriptional factors or cytokines were examined utilizing flow cytometry, ELISA, and qRT-PCR assay.
Luciferase activity assay
The 3’-UTR of IL-6, which putatively harbors the binding sites of miR-23a-3p, was amplified by PCR and subsequently cloned into the pGL3 vector (Promega, USA), generating pGL3-IL-6-wild-type vector. The pGL3-IL-6-mutant vector was generated by mutagenesis at the miR-23a-3p binding sites of the 3’-UTR region. For luciferase activity assay, HEK293T cells were co-transfected with the pGL3-IL-6-wild-type vector or the pGL3-IL-6-mutant vector, and miR-23a-3p mimic or mimic negative control (NC). MiR-23a-3p mimic and mimic NC were purchased from GenePharma (Shanghai, China). The luciferase activity was examined using the dual luciferase assay kit (Promega, USA) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 7. The Student’s t-test was used to analyze the differences between two independent groups, and one-way analysis of variance (ANOVA) was used to analyze the differences among groups. P values < 0.05 were considered statistically significant.
Results
The patients with AA exhibited Th17/Treg imbalance favoring the Th17 cells
The percentage of Th17 cells was significantly higher, whereas the percentage of Treg cells was notably lower in the peripheral blood of most AA patients when compared to control subjects (Fig. 1a). We then performed ELISA analysis to determine the levels of Th17-related cytokines (IL-17 and IL-6) and Treg-related cytokines (TGF-β1 and IL-10) in peripheral blood of AA patients and control volunteers. Our results showed that the levels of IL-17 and IL-6 were noticeably higher, whereas the levels of TGF-β1 and IL-10 were significantly lower in most patients with AA than those in control subjects (Fig. 1b).
The patients with AA exhibited Th17/Treg imbalance favoring Th17 cells. The peripheral blood samples were obtained from AA patients (n = 15) and healthy volunteers (n = 15). a. The proportions of Th17 and Treg cells were determined by flow cytometry. b. The levels of IL-17, IL-6, TGF-β1, and IL-10 were determined by ELISA analysis. **P < 0.01, vs. Control
Characterization of BMSCs and BMSC-Exo
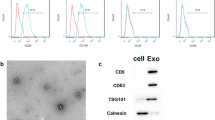
Flow cytometry analysis revealed that the MSC cell markers (CD29 and CD90) were positively expressed in the BMSCs, and the hematopoietic stem cell markers (CD34 and CD45) were negatively expressed in BMSCs (Fig. 2a). Exosomes were isolated from the supernatant of BMSCs and then were identified by TEM, NTA, and western blot. The results showed that the BMSCs-derived nanoparticles exhibited a sphere-shaped morphology with a size distribution between 0 and 150 nm (Fig. 2b, c). Moreover, our results proved that exosome markers, including CD9 and CD81, were enriched in these nanoparticles (Fig. 2d). All these data confirmed that these nanoparticles were exosomes.
Characterization of BMSCs and BMSC-Exo. a. Detection of the MSC cell markers (CD29 and CD90), and hematopoietic stem cell markers (CD34 and CD45) utilizing flow cytometry. b. Representative transmission electron microscopy images of BMSC-Exo. c. The particle size and concentration of BMSC-Exo were measured using Nanosizer™ technology. d. The protein levels of exosomal surface markers, CD9 and CD81, in BMSC-Exo were examined by western blot. e. The levels of miR-23a-3p in both BMSCs and BMSC-Exo were examined by qRT-PCR analysis. **P < 0.01, vs. BMSC. The data are presented as mean ± standard deviation from three independent experiments
BMSC-Exo balanced Th17/Treg ratio in AA by transferring miR-23a-3p
As shown in Fig. 2e, the level of miR-23a-3p was significantly higher in BMSC-Exo than in BMSCs. To elucidate the contribution of miR-23a-3p to the effect of BMSC-Exo on Th17/Treg balance, we developed miR-23a-3p-deficient BMSC-Exo. BMSCs were transfected with miR-23a-3p inhibitor (miR-23I) or inhibitor NC (INC). Then, exosomes were isolated from the supernatants of INC-transfected BMSCs (named as BMSCINC-Exo) and miR-23I-transfected BMSCs (named as BMSCmiR-23I-Exo). The qRT-PCR results confirmed that miR-23a-3p level was successfully downregulated in BMSCmiR-23I-Exo (Fig. 3a). Then, we co-cultured CD4+ T cells with PBS, BMSC-Exo, BMSCINC-Exo, or BMSCmiR-23I-Exo for 24 h. BMSC-Exo treatment significantly increased the level of miR-23a-3p in CD4+ T cells. However, the upregulation of miR-23a-3p induced by BMSC-Exo can be partly reversed when the expression of miR-23a-3p was silenced in BMSCs (Fig. 3b). Importantly, BMSC-Exo treatment notably downregulated Th17 cell proportion and the expression of Th17-related cytokines (IL-17 and IL-6), along with a significantly increased in Treg cell proportion and the expression of Treg-related transcriptional factor (Foxp3) and cytokines (TGF-β1 and IL-10). However, these effects of BMSC-Exo were eliminated when miR-23a-3p expression was inhibited in BMSC-Exo (Fig. 3c-e). These results suggested that BMSC-Exo corrected imbalance in Th17/Treg in AA by transferring miR-23a-3p.
BMSC-Exo balanced Th17/Treg ratio in AA by transferring miR-23a-3p. a. The miR-23a-3p expression was detected by qRT-PCR analysis in BMSCINC-Exo and BMSCmiR-23I-Exo. CD4+T cells were incubated with PBS, BMSC-Exo, BMSCINC-Exo, and BMSCmiR-23I-Exo for 24 h. Then, b. The miR-23a-3p expression was determined by qRT-PCR analysis. c. The proportions of Th17 and Treg cells were determined by flow cytometry. d. The mRNA levels of IL-17 and Foxp3 were determined by qRT-PCR analysis. e. The levels of IL-17, IL-6, TGF-β1, and IL-10 were measured by ELISA analysis. *P < 0.05, **P < 0.01, vs. PBS; #P < 0.05, ##P < 0.01, vs. BMSCINC-Exo. The data are presented as mean ± standard deviation from three independent experiments
BMSC-Exo corrected Th17/Treg imbalance in AA through miR-23a-3p-mediated targeting of IL-6
Using target prediction software (TargetScan), we found the presence of binding sites for miR-23a-3p in IL-6 3’-UTR. Evidences have indicated that IL-6 contributes Th17 differentiation [19]. These findings prompted us to investigate whether exosomal miR-23a-3p inhibits Th17 cell differentiation and promotes Treg cell differentiation by targeting IL-6. To this end, we performed luciferase activity assay and found that miR-23a-3p mimic transfection significantly decreased the luciferase activity in IL-6 WT group, but not in IL-6 Mut group, suggesting IL-6 as a direct target of miR-23a-3p (Fig. 4a). Furthermore, BMSC-Exo treatment decreased the IL-6 mRNA expression in CD4+ T cells, while the effect of BMSC-Exo was impaired when miR-23a-3p expression in BMSCs was knocked down (Fig. 4b). Moreover, transfection with miR-23a-3p mimics in CD4+ T cells significantly suppressed IL-6 mRNA expression (Fig. 4c). These results together indicated that BMSC-Exo might downregulate IL-6 expression level in CD4+ T cells by transferring miR-23a-3p. We also found that IL-6 overexpression significantly increased Th17 cell proportion and the expression of Th17-related cytokines (IL-17 and IL-6), but decreased Treg cell proportion and the expression of Treg-related transcriptional factor (Foxp3) and cytokines (TGF-β1 and IL-10). Importantly, IL-6 overexpression could abrogate the regulation of BMSC-Exo to Th17/Treg cell balance in AA (Fig. 4d-f).
BMSC-Exo balanced Th17/Treg cells in AA by miR-23a-3p-mediated suppressing of IL-6. (a) The interaction between miR-23a-3p and IL-6 3’-UTR was analyzed by luciferase activity assay. (b). CD4+T cells were maintained with PBS, BMSC-Exo, BMSCINC-Exo, and BMSCmiR-23I-Exo for 24 h, and then, the level of IL-6 mRNA was determined by qRT-PCR analysis. (c) The mRNA level of IL-16 was determined by qRT-PCR analysis in the AA CD4+T cells transfected with mimic NC or miR-23a-3p mimics. The proportions of Th17 cells and Treg cells were determined by flow cytometry (d), mRNA levels of IL-17 and Foxp3 were determined by qRT-PCR analysis (e), and levels of IL-17, IL-6, TGF-β1, and IL-10 were determined by ELISA analysis (f) in the groups of BMSC-Exo, BMSC-Exo + NC, and BMSC-Exo + IL-6. *P < 0.05, **P < 0.01, vs. mimic NC or PBS or BMSC-Exo + NC; ##P < 0.01, vs. BMSCINC-Exo. The data are presented as mean ± standard deviation from three independent experiments
Discussion
T lymphocytes are an important class of immune cells, implied in the establishment and maintenance of memory, homeostasis, and immune response [20]. In recent years, the research for exploring the functions of Th17 and Treg cells has been paid more attention. Th17 cells secrete IL-17A, IL-17F, and IL-22, and express master transcription factor RORγt. Treg cells secrete IL-10 and TGF-β, and express Foxp3 [21, 22]. Increasing evidences have indicated that imbalanced Th17/Treg cells ratio associates with the pathogenesis of many autoimmune diseases, including AA. Lu et al. have reported that the percentages of Th22 and Th17 cells were notably higher in the serum of AA patients than that in the serum of control individual [23]. Cheng et al. have indicated that the percentage of Th17 cells was significantly higher in the serum of AA patients with poor curative effect when compared to the AA patients with good curative effect [24]. Furthermore, the percentage of Treg cells has been proved to be notably decreased in mouse AA model [25]. Here, we also found that the percentages of Th17 and Treg cells were imbalanced in the serum of AA patients. The proportion of Th17 cells was increased, while Treg cells proportion was decreased in AA.
Currently, the standard treatment of AA for patients who lack a transplant option is immunosuppressive treatment; some drug-like horse antithymocyte globulin and eltrombopag were used for the treatment [2, 26]. However, the treatment of AA is challenging. Dysfunction of MSCs contributes to immune imbalance [27]. MSCs display genetic, functional, and morphologic alterations in AA. Recently, Zhao et al. have demonstrated that the MSCs derived from human gingiva could effectively improve the bone marrow failure through reducing the infiltration of CD8+T cells, Th1, and Th17 cells and increasing the proportion of Treg cells [4]. More and more studies have suggested that AA could be relieved as the imbalance of Th17/Treg to be corrected. The balance of Th17 versus Treg cells can be regulated by various factors. Among them, MSCs have attracted increasing attention [28]. MSCs could balance Th17/Treg cell ratio, and this effect has been showed to be mediated, at least in part, by their secreted exosomes containing many bioactive molecules [29]. Since exosomes derived from BMSCs also contained a substantial quantity of miR-23a-3p, we speculated that miR-23a-3p might similarly account for a part of the effects of BMSC-Exo on the Th17/Treg balance in AA [17]. Our results showed that decreasing the level of miR-23a-3p in BMSCs-Exo could partly subvert BMSC-Exo-induced declining in Th17 cell percentage and increasing in Treg cell percentage.
Furthermore, miRNAs mostly play their functions by targeting the 3’-UTR of their target genes. As an important member of the miRNAs family, miR-23a-3p acts as an oncogene or a tumor suppressor [30, 31]. It has been shown that IL-6 contributed Th17 differentiation [19]. In this present study, we proved that IL-6 was a novel target of miR-23a-3p. Exosomal miR-23a-3p could inhibit Th17 cell differentiation and promote Treg cell differentiation by suppressing IL-6. However, many questions need to be explored. Many different mRNAs can be regulated by the same miRNAs. It is no clear that whether miR-23a-3p could regulate the Th17 and Treg cells through targeting other molecules.
Conclusions
In summary, this study demonstrated that BMSC-Exo-transferred miR-23a-3p mediated, at least in part, the correction of imbalanced Th17/Treg in AA through suppressing IL-6. This study added a novel mechanism for how BMSC-Exo corrected Treg/Th17 imbalance in AA, and our data might encourage the use of MSCs infusions in patients with AA.
Availability of data and materials
The data could be obtained upon request to the corresponding author.
Abbreviations
- AA:
-
Aplastic anemia
- BMSCs:
-
Bone marrow-derived MSCs
- IL:
-
Interleukin
- miRNAs:
-
MicroRNAs
- MSCs:
-
Mesenchymal stem cells
- TGF-β1:
-
Transforming growth factor-β1; Th1: T helper type 1
- Treg:
-
Regulatory T
References
Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. https://doi.org/10.1111/bjh.13853.
Fattizzo B, Kulasekararaj AG, Hill A, et al. Clinical and morphological predictors of outcome in older aplastic anemia patients treated with eltrombopag. Haematologica. 2019;104:e494–6. https://doi.org/10.3324/haematol.2019.216374.
Boddu PC, Kadia TM. Molecular pathogenesis of acquired aplastic anemia. Eur J Haematol. 2019;102:103–10. https://doi.org/10.1111/ejh.13182.
Zhao J, Chen J, Huang F, et al. Human gingiva tissue-derived MSC ameliorates immune-mediated bone marrow failure of aplastic anemia via suppression of Th1 and Th17 cells and enhancement of CD4+Foxp3+ regulatory T cells differentiation. Am J Transl Res. 2019;11:7627–43.
Li H, Wang L, Pang Y, et al. In patients with chronic aplastic anemia, bone marrow-derived MSCs regulate the Treg/Th17 balance by influencing the Notch/RBP-J/FOXP3/RORγt pathway. Sci Rep. 2017;7:42488. https://doi.org/10.1038/srep42488.
Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017. https://doi.org/10.3390/molecules22010134.
Zhao L, Chen S, Yang P, Cao H, Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10:182. https://doi.org/10.1186/s13287-019-1287-9.
Wang S, Zhu R, Li H, Li J, Han Q, Zhao RC. Mesenchymal stem cells and immune disorders: from basic science to clinical transition. Front Med. 2019;13:138–51. https://doi.org/10.1007/s11684-018-0627-y.
Wang H, Wang Z, Xue M, Liu J, Yan H, Guo Z. Co-transfusion of haplo-identical hematopoietic and mesenchymal stromal cells to treat a patient with severe aplastic. Cytotherapy. 2010;12:563–5. https://doi.org/10.3109/14653241003695059.
Toh WS, Zhang B, Lai RC, Lim SK. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. 2018;20:1419–26. https://doi.org/10.1016/j.jcyt.2018.09.008.
Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–5. https://doi.org/10.1016/j.biocel.2011.10.005.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. https://doi.org/10.1038/s41556-018-0250-9.
Li Y, Wang F, Guo R, et al. Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life. 2019;71:1284–92. https://doi.org/10.1002/iub.2035.
Hosokawa K, Muranski P, Feng X, et al. Identification of novel microRNA signatures linked to acquired aplastic anemia. Haematologica. 2015;100:1534–45. https://doi.org/10.3324/haematol.2015.126128.
Hosokawa K, Kajigaya S, Feng X, et al. A plasma microRNA signature as a biomarker for acquired aplastic anemia. Haematologica. 2017;102:69–78. https://doi.org/10.3324/haematol.2016.151076.
Giudice V, Banaszak LG, Gutierrez-Rodrigues F, et al. Circulating exosomal microRNAs in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2018;103:1150–9. https://doi.org/10.3324/haematol.2017.182824.
Ferguson SW, Wang J, Lee CJ, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8:1419.
Wu D-M, Wen X, Han X-R, et al. Bone marrow mesenchymal stem cell-derived Exosomal MicroRNA-126-3p inhibits pancreatic cancer development by targeting ADAM9. Mol Ther Nucleic Acids. 2019;16:229–45. https://doi.org/10.1016/j.omtn.2019.02.022.
Guo D, Chen Y, Wang S, et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology. 2018;154:132–43. https://doi.org/10.1111/imm.12874.
Kumar BV, Connors TJ, Farber DL. Human T Cell Development, localization, and Function throughout Life. Immunity. 2018;48:202–13. https://doi.org/10.1016/j.immuni.2018.01.007.
Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15:458–69. https://doi.org/10.1038/s41423-018-0004-4.
Naufel AO, Aguiar MCF, Madeira FM, Abreu LG. Treg and Th17 cells in inflammatory periapical disease: a systematic review. Braz Oral Res. 2017;31:e103. https://doi.org/10.1590/1807-3107bor-2017.vol31.0103.
Lu T, Liu Y, Li P, et al. Decreased circulating Th22 and Th17 cells in patients with aplastic anemia. Clin Chim Acta. 2015;450:90–6. https://doi.org/10.1016/j.cca.2015.07.031.
Cheng H, Cao J, Chen W, Qi KM, Li ZY, Xu KL. Th17 Cell Subset levels in peripheral blood of patients with aplastic anemia and their clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:218–24. https://doi.org/10.19746/j.cnki.issn.1009-2137.2020.01.037.
Liu HY, Lin ZH, Liu H, Lu W, Zhang YP. The changes of regulatory T cells and Th17 cells in a novel mouse severe aplastic anemia model. Zhonghua Xue Ye Xue Za Zhi. 2012;33:653–6.
Fattizzo B, Levati G, Cassin R, Barcellini W. Eltrombopag in Immune Thrombocytopenia, Aplastic Anemia, and Myelodysplastic Syndrome: From Megakaryopoiesis to Immunomodulation. Drugs. 2019;79:1305–19. https://doi.org/10.1007/s40265-019-01159-0.
Fattizzo B, Giannotta JA, Barcellini W. Mesenchymal stem cells in aplastic anemia and myelodysplastic syndromes: The “Seed and Soil” crosstalk. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155438.
Chen QH, Wu F, Liu L, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther. 2020;11:91. https://doi.org/10.1186/s13287-020-01612-y.
Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020–30. https://doi.org/10.1002/iub.2147.
Quan J, Pan X, Li Y, et al. MiR-23a-3p acts as an oncogene and potential prognostic biomarker by targeting PNRC2 in RCC. Biomed Pharmacother. 2019;110:656–66. https://doi.org/10.1016/j.biopha.2018.11.065.
Ding F, Lai J, Gao Y, et al. NEAT1/miR-23a-3p/KLF3: a novel regulatory axis in melanoma cancer progression. Cancer Cell Int. 2019;19:217. https://doi.org/10.1186/s12935-019-0927-6.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Q-Z Shi designed the study; Q-Z Shi, H-M Yu, H-M Chen, M Liu, and X Cheng conducted the experiments and analyzed the data; Q-Z Shi drafted the paper; and all authors reviewed and approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University.
Informed consent
Informed written consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, Qz., Yu, Hm., Chen, Hm. et al. Exosomes derived from mesenchymal stem cells regulate Treg/Th17 balance in aplastic anemia by transferring miR-23a-3p. Clin Exp Med 21, 429–437 (2021). https://doi.org/10.1007/s10238-021-00701-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-021-00701-3