Abstract
Bone marrow mesenchymal stem cells (BMSCs) are associated with immune thrombocytopenia (ITP), the underlying mechanism has not been fully elucidated. Here, we attempted to investigate whether BMSCs can regulate Th17/Treg imbalance in ITP through the exosome pathway. We first assessed the proportions of Th17 cells and Tregs in ITP patients, showing that ITP patients exhibited an evident imbalance of Th17/Treg. BMSCs-exosomes’ treatment significantly reduced Th17/Treg ratio in the CD4+ T cells of ITP patients. Moreover, miR-146a-5p was highly expressed in BMSCs-exosomes. The expression of miR-146a-5p was obviously increased in CD4+ T cells following the treatment of BMSCs-exosomes. BMSCs-exosomal miR-146a-5p silencing promoted the proportions of Th17 cells and repressed the proportions of Tregs in CD4+ T cells. In addition, miR-146a-5p directly interacted with IL-1R-associated kinase-1 (IRAK), and repressed IRAK1 expression. IRAK1 overexpression promoted Th17/Treg ratio in CD4+ T cells, which was abolished by BMSCs-exosomal miR-146a-5p. In conclusion, these findings demonstrate that BMSC-derived exosomal miR-146a-5p regulates Th17/Treg imbalance in ITP by repressing IRAK1 expression. Thus, this work suggests that BMSCs-exosomal miR-146a-5p may be a potential therapeutic target for ITP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune thrombocytopenia (ITP) is a clinically common acquired autoimmune bleeding disease. The typical clinical manifestations of ITP are decrease of peripheral blood platelet count, bone marrow megakaryocyte maturation disorder, and clinical bleeding symptoms [1]. The pathogenesis of ITP is very complicated and has not yet been clearly explained. The increased destruction and insufficient production of platelets caused by abnormal humoral and cellular immunity are the main causes of ITP. [2]. Autoimmune abnormalities caused by autoimmune tolerance defects are considered as the central link in the occurrence of ITP [3]. Therefore, it is worth to better understand the pathogenesis of ITP, and develop more efficient therapies for ITP.
The pathogenic role of T-cell imbalance in ITP has become a consensus [4]. Helper T (Th) cells are the most important regulatory cells in T-cell immune system, and play a crucial role in autoimmune diseases, infectious diseases, and tumors. According to the type of cytokine secreted, Th cells can be divided into Th1 cells, Th2 cells, Th17 cells, and regulatory T cells (Tregs). The imbalance of Th cell subgroups is an important pathogenesis of ITP [5]. Th17 and Tregs are two important subgroups of Th cells, and Th17 and Treg cells can achieve mutual transformation in different immune environments. The increase of Th17 cells is accompanied by the decrease of Tregs, indicating that there is continuous inflammation. Elevated Tregs inhibit the progression of inflammation. The balance between Th17 cells and Tregs is closely associated with various infectious diseases and autoimmune diseases [6, 7]. Previous studies have shown that the plasma of ITP patients exhibits an increase of Th17/Tregs ratio [8, 9]. Liu et al. have studied immune-related miRNAs in peripheral blood mononuclear cells (PBMC) of ITP patients, founding that down-regulation of miR-146a, miR-326, and miR-142-5p may be associated with ITP [10]. MiR-142-5p and miR-146a are negatively correlated with the levels of Th17 cells, and miR-146a is positively correlated with the levels of Tregs and platelet counts. Therefore, immune-related miRNAs may be inherently related to Th17/Treg balance in ITP.
Bone marrow mesenchymal stem cells (BMSCs) exert an immunomodulatory function in autoimmune tolerance. Previous studies have confirmed that the biological signs and immunological characteristics of BMSCs are abnormal in various autoimmune diseases including ITP [11,12,13]. Thus, the dysfunction of stem cells may participate in the pathogenesis of ITP, and stem cells may be the potential therapeutic target for ITP. Ma et al. have found that transplantation of human umbilical cord mesenchymal stem cells significantly ameliorates the dysfunction of megakaryocytes and promotes platelet counts in ITP patients [14]. After transplantation of BMSCs into ITP mice, the platelet production, the number of Tregs, and the levels of IL-10 and TGF-β are significantly increased [15]. Additionally, mesenchymal stem cells (MSCs) play a role in paracrine manner, such as exosomes [16]. Exosomes are membranous vesicles that secreted by cells through exocytosis. Exosomes contain proteins, lipids, transcription factors, DNA, mRNA, and microRNA (miRNA), which act on target cells and perform their functions by binding to target-tissue receptors or fusing with plasma membranes [17]. In addition, MSC-exosomes exhibit an up-regulation of miR-146a, suggesting that MSC-exosomal miR-146a may regulate Th17/Treg imbalance in ITP [18]. Predictive analysis of the target genes of miR-146a revealed that miR-146a interacted with IL-1R-associated kinase-1 (IRAK1). IRAK1 has been confirmed to be involved in the regulation of Th17/Treg differentiation and balance [19, 20]. Therefore, we speculated that exogenous BMSCs may secrete exosomal miR-146a to CD4+ T cells of ITP patients, and regulate the imbalance of Th17/Treg by targeting IRAK1 in ITP.
Materials and methods
Participant
A total of 20 ITP patients were recruited at The First Affiliated Hospital of Nanchang University in this study. Twenty healthy controls (HC) were recruited and served as control. There was no significant difference in age and sex between ITP patients and healthy volunteers. One patient undergoing a total hip replacement was recruited for bone marrow extraction. The clinical characteristics of all participants are shown in Table 1. All patients signed informed consents. All protocols were authorized by the Ethics Committee of The First Affiliated Hospital of Nanchang University.
Isolation and identification of human BMSCs
BMSCs were separated from bone marrow of patient who undergoing a total hip replacement by density-gradient centrifugation. BMSCs were cultured in DMEM/F12 (Gibco, Camarillo, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (Solarbio, Beijing, China) at 37 °C and 5% CO2.
For identification of BMSCs, BMSCs were cultured to the third generation. BMSCs were resuspended in the 0.1 M PBS at a concentration of 1 × 106 cell/mL. BMSCs were blocked with 10% bovine serum albumin (BSA) at 4 °C for 10 min. Then, BMSCs were incubated with 10 μL CD29-FITC, CD106-APC (Abcam, Cambridge, MA, USA), CD34-PE, or CD45-PE (Abcam) at darkness, 4 °C for 30 min. FITC, APC or PE-conjugated IgG1 used as an isotype control. After that, BMSCs were fixed with 4% paraformaldehyde. The expression of surface molecule of BMSCs was detected using an FACSCalibur (BD Biosciences, San Jose, CA, USA).
Isolation and identification of exosomes
BMSCs were cultured in exosome-free DMEM/F12 medium (Gibco) to the third generation. Exosomes were separated from culture supernatant of BMSCs by ultracentrifugation. The culture supernatant of BMSCs was collected by centrifugation at 3000 g for 45 min, and then filtrated by a 0.22 µm filter under sterile condition to remove debris. Subsequently, the supernatants were centrifuged at 200,000 g, 4 °C for 2 h to collect the pellets. The pellets were washed with PBS for several times, and centrifuged again at 200,000 g, 4 °C for 2 h. The ultrastructure of BMSCs-exosomes was analyzed using a transmission electron microscopy (Thermo Fisher Scientific, Waltham, MA, USA). Western blot (WB) was performed to assess the expression of exosome positive markers, CD9, CD63, and TSG101, and exosome negative marker Calnexin.
Isolation and culture of CD4+ T cells
CD4+ T cells were separated form peripheral blood of ITP patients and healthy controls, respectively. Peripheral blood was extracted from 20 ITP patients and 20 healthy controls. The peripheral blood from 20 ITP patients or 20 healthy controls was fully mixed. Then, PBMC were isolated from the mixed peripheral blood by density-gradient centrifugation with Ficoll. Then, CD4+ T cells were separated from PBMC using Dynabeads™ CD4 Positive Isolation Kit (Thermo Fisher Scientific) as the manufacturers' protocol described. CD4+ T cells were cultured in DMEM/F12 medium contained 10% FBS and 1% penicillin/streptomycin at 37 °C and 5% CO2. CD4+ T cells were incubated with BMSCs-exosomes (BMSCs-Exo) or phosphate buffer saline (PBS).
Flow cytometry analysis of the proportions of Th17 cells and Tregs
Flow cytometry was performed to examine the proportions of Th17 cells and Tregs in peripheral blood and CD4+ T cells. Peripheral blood from ITP patients and healthy individuals were mixed with 100 μL heparin to and resuspended in DMEM/F12 medium. Peripheral blood was incubated with 5 μL bredeldin, 4 μL ionomycin, and 5 μL PMA at 37 °C for 6 h. CD4+ T cells were resuspended in DMEM/F12 medium. Cell suspension (100 μL) was incubated with 10 μL CD25-PE or CD4-APC (Abcam) at 4 °C for 30 min in darkness. After fixation and permeabilization, cell suspension was stained with 10 μL Foxp3-APC or IL-17-PE (Abcam) at 4 °C for 30 min in darkness. The proportions of Th17 cells and Tregs in peripheral blood or CD4+ T cells were detected using a FACSCalibur (BD Biosciences). Furthermore, CD25 and Foxp3 were used to mark Tregs. IL-17 and CD4 were used to mark Th17 cells.
Cell transfection
Full length of IRAK1 was subcloned into the vector pcDNA3.1 (pcDNA3.1-IRAK1) (GeneChem, Shanghai, China). The empty pcDNA3.1 (pcDNA3.1-NC) served as control. The miR-146a-5p mimic and miR-146a-5p inhibitor and the corresponding NC (mimic NC or inhibitor NC) were synthesized by GeneChem. BMSCs and CD4+ T cells were transfected with these plasmids using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) as the protocol described.
Quantitative real-time PCR (qRT-PCR)
Exosome DNA/RNA Extraction Kit (Baiaolaibo, Beijing, China) and TRIzol reagent (Invitrogen) was used to extract total RNA from exosomes or cells as the protocol of manufacturer. RNA integrity was examined by 1.5% agarose gel electrophoresis. The extracted RNA was reversed-transcribed into complementary DNA using PrimeScript™ RT Reagent Kit (Takara, Tokyo, Japan). The relative expression of genes was assessed by performing qRT-PCR using SYBR Green PCR Mix Kit (Takara) as the protocol of manufacturer. QRT-PCR was performed on a Real-Time PCR Instrument (Applied Biosystems, Carlsbad, CA, USA). The results were analyzed using \(2^{{ - \Delta \Delta C_{{\text{T}}} }}\) method for quantification. GAPDH was used as reference gene for normalization. The primer sequences were as follows: miR-146a-5p: forward: 5ʹ-GCG AGG TCA AGT CAC TAG TGG T-3ʹ, reverse: 5ʹ-CGA GAA GCT TGC ATC ACC AG AGA ACG5-3ʹ; IRAK1: forward: 5ʹ-TCA GCT TTG GGG TGG TAG TG-3ʹ, reverse: 5ʹ-TAG ATC TGC ATG GCG ATG GG-3ʹ; GAPDH: forward: 5ʹ-CTT TGG TAT CGT GGA AGG ACT C-3ʹ, reverse: 5ʹ-GTA GAG GCA GGG ATG ATG TTC T-3ʹ.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-17, IL-10, and TGF-β in peripheral blood and CD4+ T cells were assessed by performing using Human ELISA Kit (SenBeiJia, Nanjing, China) according to the manufacturer’s instructions. The optical density values of samples were detected using a microplate reader (Thermo Fisher Scientific).
WB
RIPA lysis buffer (Auragene, Changsha, China) was used to extract total protein from CD4+ T cells following the protocol described. The concentration of proteins was estimated using Bicinchoninic acid protein assay kit (Beyotime Biotechnology, Shanghai, China). Protein samples (25 µg) were separated by 10% SDS-PAGE protein electrophoresis, and then transferred onto PVDF membranes (Merck Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk at room temperature for 2 h to block the non-specific sites. Subsequently, the membranes were incubated with the primary antibody IRAK1 (1:1000, Proteintech, Wuhan, China) at 4 °C for 12 h. The horseradish peroxidase-linked secondary antibodies (1:5000, Proteintech) were incubated with the membranes at room temperature for 2 h. For normalization, β-actin antibody (1:5000, Proteintech) was used as a reference protein. The data were analyzed by Image J software.
Luciferase reporter assay
To explore whether miR-146a-5p interacted with 3′ untranslated region (UTR) of IRAK1, pmir-Glo-IRAK1-WT and pmir-Glo-IRAK1-Mut vectors containing the wild-type (WT) or mutant type (Mut) of 3′UTR of IRAK1 were constructed (GeneChem). The sense and anti-sense strands of the oligonucleotides of the IRAK1-3'-UTR contained predicted binding sites of miR-146a-5p. WT/Mut 3′UTR of IRAK1 vector was transfected into 293 cells together with miR-146a-5p mimic or mimic NC. After 48 h of transfection, dual-luciferase assay kit (Promega, Madison, USA) was used to measure the activities of firefly and renilla luciferase on luciferase assay system (Ambion, Austin, TX, USA). The relative Rluc/Luc ratio was calculated.
RNA-binding protein immunoprecipitation (RIP)
The interaction between miR-146a-5p and IRAK1 in CD4+ T cells was verified through RIP assay applying Millipore Magna RIP Kit (Merck Millipore). CD4+ T-cell lysate were stained with anti-IRAK1 (1:1000, Proteintech) at 4 °C for 12 h. Subsequently, the intracellular protein–RNA complex was collected and digested with proteinase K to elute RNAs. Finally, qRT-PCR was performed to detect the expression of miR-146a-5p in the immunoprecipitated RNAs. Cell lysate were served as input.
RNA pull-down
RNA pull-down was performed to examine the relationship between miR-146a-5p and IRAK1 in CD4+ T cells using Pierce™ Magnetic RNA–Protein Pull-Down Kit (Thermo Fisher Scientific). Streptavidin magnetic beads were used to capture the labeled RNA form the CD4+ T-cell lysate. Then, total protein of CD4+ T cells was incubated with magnetic beads-RNA to obtain RNA-binding protein complexes. The expression of IRAK1 in the eluted proteins from the RNA-binding protein complexes were analyzed by WB.
Statistical analysis
Each assay was performed for 3 times. Data were reported as mean ± standard deviation. SPSS 22.0 statistical software (IBM, Armonk, NY, USA) was used for statistical analysis. Two-tailed Student’s t, one-way or two-way ANOVA was used to analyze the statistical difference. P < 0.05 was considered as a significant difference.
Results
BMSCs-exosomes repressed the proportions of Th17 cells and enhanced the proportions of Tregs in ITP
To investigate the mechanism of action of BMSCs-derived exosomes in ITP, we separated and identified BMSCs from healthy individual. Flow cytometry data showed that BMSCs were positive for CD29 (92.9%) and CD106 (93.5%), whereas negative for CD34 (3.6%) and CD45 (3.7%) (Fig. 1a). Exosomes were extracted from BMSCs and identified by transmission electron microscopy and WB analysis. The diameter of exosomes was approximately 100 nm (Fig. 1b). Compared with BMSCs, exosomes exhibited an up-regulation of exosome positive markers, CD9, CD63, and TSG101. The negative marker Calnexin was hardly expressed in the exosomes (Fig. 1c). Thus, BMSCs and BMSCs-exosomes were isolated and identified successfully, and can be used for further analysis.
Identification of BMSCs and BMSCs-exosomes. a Flow cytometry was performed to identify the surface molecule of BMSCs. b, c Exosomes were extracted from BMSCs, and the ultrastructure and positive markers (CD9, CD63, and TSG101) and negative marker (Calnexin) of exosomes were identified by transmission electron microscopy and WB. N = 3
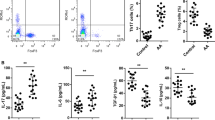
We first compared the proportions of Th17 cells and Tregs between peripheral blood of ITP patients and healthy individuals by flow cytometry. Compared with healthy individuals, ITP patients exhibited a significant increase in the proportions of Th17 cells, whereas the proportions of Tregs were decreased in ITP patients (Fig. 2a–c). The ratio of Th17/Treg was notably enhanced in ITP patients as compared with healthy individuals (Fig. 2e). We also found that ITP patients displayed a boost in the levels of IL-17 with respect to healthy individuals (Fig. 2e). However, there was no significant difference in the levels of IL-10 between ITP patients and healthy individuals (Fig. 2f). The levels of TGF-β were lower in ITP patients than that in healthy individuals (Fig. 2g). Next, we separated CD4+ T cells from the mixed peripheral blood of 20 ITP patients. CD4+ T cells were then incubated with BMSCs-exosomes to determine the influence of BMSCs-exosomes on Th17/Treg imbalance. Flow cytometry data revealed that the treatment of BMSCs-exosomes significantly repressed the proportions of Th17 cells in CD4+ T cells, and enhanced the proportions of Tregs in CD4+ T cells (Fig. 3a–c). Thus, Th17/Treg ratio was severely decreased in CD4+ T cells in the presence of BMSCs-exosomes (Fig. 3d). ELISA results showed that BMSCs-exosomes significantly suppressed the levels of IL-17 in CD4+ T cells, while BMSCs-exosomes notably enhanced the levels of IL-10 and TGF-β in CD4+ T cells (Fig. 3e–g). Additionally, we examined whether BMSCs-exosomes affected Th17/Treg imbalance in CD4+ T cells of healthy individuals. The data obtained from flow cytometry and ELISA all revealed that BMSCs-exosomes had no impact on Th17/Treg imbalance in CD4+ T cells of healthy controls (Supplementary figure 1A-G). Thus, CD4+ T cells from ITP patients were used for further analysis.
The ratio of Th17/Treg was enhanced in ITP patients. a–d Flow cytometry was performed to assess the proportions of Th17 cells and Tregs in peripheral blood of ITP patients and healthy individuals. e–g ELISA was performed to examine the concentrations of IL-17, IL-10, and TGF-β in peripheral blood of ITP patients and healthy individuals. N = 3. **P < 0.01 vs. HC
BMSCs-exosomes repressed Th17/Treg ratio in CD4+ T cells of ITP patients. CD4+ T cells were isolated from peripheral blood of ITP patients. CD4+ T cells were incubated with BMSCs-exosomes or PBS. Normal CD4+ T cells served as control. a–d Flow cytometry was performed to assess the proportions of Th17 cells and Tregs in the CD4+ T cells. e–g ELISA was performed to examine the concentrations of IL-17, IL-10, and TGF-β in the CD4+ T cells. N = 3. **P < 0.01 vs. PBS
Taken together, these data demonstrated that BMSCs-exosomes repressed the proportions of Th17 cells and enhanced the proportions of Tregs in ITP, suggesting that BMSCs-exosomes may be participated in regulating the imbalance between Th17 cells and Tregs in ITP.
BMSCs-derived exosomal miR-146a-5p regulated the imbalance of Th17 cells and Tregs in the CD4+ T cells
To investigate whether BMSCs-exosomes exerted function by delivering miR-146a-5p, we compared the expression of miR-146a-5p between BMSCs and BMSCs-exosomes. The results of qRT-PCR revealed that miR-146a-5p was highly expressed in BMSCs-exosomes as compared with BMSCs (Fig. 4a). Then, we treated CD4+ T cells with BMSCs-exosomes. Figure 4b shows that miR-146a-5p was up-regulated in CD4+ T cells in the presence of BMSCs-exosomes. Moreover, we silenced miR-146a-5p in BMSCs, and miR-146a-5p expression was severely decreased in the BMSCs-exosomes following the transfection of miR-146a-5p inhibitor (Fig. 4c). Then, CD4+ T cells were incubated with the exosomes from miR-146a-5p-silenced BMSCs (miR-146a-5pI-Exo). Compared with NCI-Exo group, miR-146a-5p was down-regulated in CD4+ T cells in the presence of miR-146a-5pI-Exo (Fig. 4d). In addition, we estimated the influence of miR-146a-5pI-Exo treatment on the proportions of Th17 cells and Tregs in CD4+ T cells by flow cytometry. MiR-146a-5pI-Exo enhanced the proportions of Th17 cells, and repressed the proportions of Tregs in CD4+ T cells. Th17/Treg ratio was significantly enhanced in CD4+ T cells following miR-146a-5pI-Exo treatment (Fig. 5a–d). Moreover, the levels of IL-17, IL-10, and TGF-β in CD4+ T cells were assessed by ELISA. Compared with NCI-Exo group, the levels of IL-17 were significantly enhanced in miR-146a-5pI-Exo group. The levels of IL-10 and TGF-β in CD4+ T cells were decreased in the presence of miR-146a-5pI-Exo (Fig. 5e–g). Thus, these data suggested that BMSCs-exosomes regulated the imbalance of Th17 cells and Tregs through delivery of miR-146a-5p.
BMSCs-exosomes promoted miR-146a-5p expression in CD4+ T cells through delivery of miR-146a-5p. Exosomes were extracted from BMSCs. The expression of miR-146a-5p in BMSCs and BMSCs-exosomes was examined by qRT-PCR. b CD4+ T cells were incubated with BMSCs-exosomes or PBS. Normal CD4+ T cells served as control. The expression of miR-146a-5p in the CD4+ T cells was estimated by qRT-PCR. c BMSCs were transfected with miR-146a-5p inhibitor or inhibitor NC, and the exosomes were extracted from the transfected BMSCs (miR-146a-5pI-Exo, NCI-Exo). QRT-PCR was performed to assess miR-146a-5p expression of miR-146a-5pI-Exo, NCI-Exo. d CD4+ T cells were incubated with miR-146a-5pI-Exo or NCI-Exo. CD4+ T cells incubated with normal BMSCs-exosomes served as control. QRT-PCR was performed to estimate the expression of miR-146a-5p in the CD4+ T cell. N = 3. **P < 0.01 vs. BMSC. ##P < 0.01 vs. PBS; $$P < 0.01 vs. NCI-Exo
BMSCs-exosomal miR-146a-5p regulated the proportions of Th17 cells and Tregs in the CD4+ T cells. BMSCs were transfected with miR-146a-5p inhibitor or inhibitor NC, and the exosomes were extracted from the transfected BMSCs (miR-146a-5pI-Exo, NCI-Exo). CD4+ T cells were incubated with miR-146a-5pI-Exo or NCI-Exo. CD4+ T cells incubated with normal BMSCs-exosomes served as control. a–d Flow cytometry was performed to assess the proportions of Th17 cells and Tregs in the CD4+ T cells. e–g ELISA was performed to examine the concentrations of IL-17, IL-10, and TGF-β in the CD4+ T cells. N = 3. $P < 0.05, $$P < 0.01 vs. NCI-Exo
BMSCs-exosomal miR-146a-5p repressed IRAK1 expression in CD4+ T cells by interacting with IRAK1
We further explored the molecular mechanism of exosomal miR-146a-5p in regulating the imbalance of Th17 cells and Tregs. Bioinformatics analysis revealed that IRAK1 may be the target gene of miR-146a-5p. We performed luciferase reporter assay to verify the relationship between miR-146a-5p and IRAK1, showing that miR-146a-5p interacted with IRAK1 (Fig. 6a). RIP and RNA pull-down assays also demonstrated that miR-146a-5p directly targeted IRAK1 in CD4+ T cells (Fig. 6b, c). Subsequently, we examined the influence of miR-146a-5p overexpression or knockdown on IRAK1 expression in CD4+ T cells by qRT-PCR and WB. MiR-146a-5p overexpression caused a down-regulation of IRAK1 gene and protein in CD4+ T cells. The gene and protein expression of IRAK1 was enhanced in CD4+ T cells in the presence of miR-146a-5p inhibitor (Fig. 6d, e). Furthermore, we determined the effect of BMSCs-exosomal miR-146a-5p on IRAK1 expression in CD4+ T cells. After treated with BMSCs-exosomes, the gene and protein expression of IRAK1 was significantly decreased in CD4+ T cells. However, the gene and protein expression of IRAK1 was obviously enhanced in the CD4+ T cells in the presence of miR-146a-5pI-Exo (Fig. 6f, g). Thus, these data demonstrated that BMSCs-exosomes repressed IRAK1 expression in CD4+ T cells through delivery of miR-146a-5p.
Exosomal miR-146a-5p repressed IRAK1 expression in CD4+ T cells. a Luciferase reporter assay was performed to verify the interaction between miR-146a-5p and IRAK1. RIP (b) and RNA pull-down (c) assays were performed to determine the relationship between miR-146a-5p and IRAK1 in CD4+ T cells. d, e CD4+ T cells were transfected with miR-146a-5p mimic, mimic NC, miR-146a-5p inhibitor, or inhibitor NC. QRT-PCR and WB were performed to estimate the gene and protein expression of IRAK1 in the CD4+ T cells. f, g BMSCs were transfected with miR-146a-5p inhibitor or inhibitor NC, and the exosomes were extracted from the transfected BMSCs (miR-146a-5pI-Exo, NCI-Exo). CD4+ T cells were incubated with miR-146a-5pI-Exo, NCI-Exo, normal BMSCs-exosomes, or PBS. Normal CD4+ T cells served as control. QRT-PCR and WB were performed to estimate the gene and protein expression of IRAK1 in the CD4+ T cells. N = 3. **P < 0.01 vs. mimic NC; ##P < 0.01 vs. inhibitor NC; $$P < 0.01 vs. PBS; &&P < 0.01 vs. NCI-Exo
BMSCs-exosomal miR-146a-5p repressed Th17/Treg ratio in CD4+ T cells by repressing IRAK1 expression
Finally, we investigated the biological role of miR-146a-5p/IRAK1 in the regulation of Th17/Treg ratio. CD4+ T cells were transfected with miR-146a-5p mimic and pcDNA3.1-IRAK1. Flow cytometry data showed that miR-146a-5p overexpression reduced the proportions Th17 cells, and enhanced Treg proportions in CD4+ T cells. However, up-regulation of IRAK1 caused an increase in the proportions of Th17 cells, and led to a decrease in the proportions of Tregs in CD4+ T cells. MiR-146a-5p overexpression abolished the influence of IRAK1 up-regulation on the proportions of Th17 cells and Tregs in CD4+ T cells (Fig. 7a–c). Th17/Treg ratio was decreased in CD4+ T cells in the presence of miR-146a-5p mimic. IRAK1 up-regulation promoted the Th17/Treg ratio in CD4+ T cells, which was rescued by miR-146a-5p overexpression (Fig. 7d). We also found that the levels of IL-17 were repressed by miR-146a-5p overexpression in CD4+ T cells, whereas miR-146a-5p overexpression enhanced the levels of IL-10 and TGF-β in CD4+ T cells. However, RAK1 up-regulation enhanced the levels of IL-17, and reduced the levels of IL-10 and TGF-β in CD4+ T cells. The influence conferred by RAK1 up-regulation was abolished by miR-146a-5p overexpression (Fig. 7e–g). In addition, CD4+ T cells were transfected with pcDNA3.1-IRAK1, IRAK1 protein expression was significantly increased in CD4+ T cells in the presence of pcDNA3.1-IRAK1 (Supplementary figure 2). IRAK1-overexpressed CD4+ T cells were incubated with BMSCs-exosomes. BMSCs-exosomes reduced the proportions of Th17 cells and Th17/Treg ratio, and enhanced the proportions of Tregs in CD4+ T cells. IRAK1 overexpression caused an increase in the proportions of Th17 cells and Th17/Treg ratio, and a decrease of Treg proportions of in CD4+ T cells. The influence conferred by IRAK1 up-regulation was rescued by BMSCs-exosomes (Fig. 8a–d). ELISA data revealed that BMSCs-exosomes reduced the levels of IL-17 and enhanced the levels of IL-10 and TGF-β in CD4+ T cells. IRAK1 up-regulation promoted IL-17 levels and inhibited the levels of IL-10 and TGF-β in CD4+ T cells, which was partly abolished by BMSCs-exosomes (Fig. 8e–g). Taken together, these findings confirmed that BMSCs-exosomal miR-146a-5p regulated the imbalance of Th17/Treg in CD4+ T cells by repressing IRAK1 expression.
MiR-146a-5p overexpression repressed Th17/Treg ratio in CD4+ T cells by repressing IRAK1 expression. CD4+ T cells were transfected with miR-146a-5p mimic or mimic NC and pcDNA3.1-IRAK1 or pcDNA3.1-NC. a–d Flow cytometry was performed to assess the proportions of Th17 cells and Tregs in the CD4+ T cells. e–g ELISA was performed to examine the concentrations of IL-17, IL-10, and TGF-β in the CD4+ T cells. N = 3. **P < 0.01 vs. mimic NC + Vector; ##P < 0.01 vs. mimic NC + IRAK1
BMSCs-exosomal miR-146a-5p repressed Th17/Treg ratio in CD4+ T cells by repressing IRAK1 expression. CD4+ T cells were transfected with pcDNA3.1-IRAK1 or pcDNA3.1-NC, and then incubated with BMSCs-exosomes or PBS. a–d Flow cytometry was performed to assess the proportions of Th17 cells and Tregs in the CD4+ T cells. e–g ELISA was performed to examine the concentrations of IL-17, IL-10, and TGF-β in the CD4+ T cells. N = 3. **P < 0.01 vs. PBS + Vector; ##P < 0.01 vs. PBS + IRAK1
Discussion
BMSCs have been reported to be associated with the pathogenesis of ITP. ITP patients exhibit an significant increase in apoptosis of MSCs. MiR-98-5p inhibits PI3K/Akt signaling pathway by interacting with IGF2BP1, and promotes p53 expression by regulating ubiquitylation of p53, thereby inducing MSCs deficient in ITP [21]. The numeric and functional abnormities of suppressor T cells participate in the progression of ITP. Umbilical cord-derived MSCs efficiently ameliorate the numbers and dysfunction of suppressor T cells and platelet recovery in ITP [22, 23]. BMSCs also have been confirmed to be beneficial to the increase of platelet counts in ITP [15]. Moreover, accumulating evidences have demonstrated that MSCs play a role by secreting exosomes. Miao et al. have found that human umbilical cord-MSCs-derived miR-1246-contiaining exosomes effectively attenuate hepatic ischemia reperfusion injury. Exosomal miR-1246 mediates the balance between Th17 cells and Tregs through regulating IL-6-gp130-STAT3 axis in hepatic ischemia reperfusion injury [24]. In aplastic anemia, MSCs-derived exosomal S1P regulates Th17/Treg balance [25]. Whether BMSCs-derived exosomes can regulate Th17/Treg balance in ITP has not been reported. We compared the proportions Th17 cells and Tregs between ITP patients and healthy individuals, founding that ITP patients displayed an imbalance of Th17/Treg. The proportions Th17 cells were enhanced and the proportions of Tregs were decreased in ITP patients, which were consistent with the previous report [26]. However, BMSCs-exosomes had no impact on Th17/Treg imbalance in CD4+ T cells of healthy controls. Subsequently, we found that BMSCs-exosomes effectively repressed Th17/Treg ratio in CD4+ T cells. The levels of Th17 cytokine IL-17 were decreased, and the levels of Treg cytokines IL-10 and TGF-β were enhanced in CD4+ T cells in the presence of BMSCs-exosomes. Thus, these data suggested that BMSCs-exosomes regulated the imbalance of Th17/Treg in ITP.
MiR-146a-5p participates in the progression of various diseases. In the serum and tissue of non-small cell lung cancer patients, the expression of miR-146a-5p is significantly increased. MiR-146a-5p has a promoting effect on proliferation, migration and apoptosis resistance of non-small cell lung cancer cells by targeting TRAF6/NF-κB-p65 axis [27]. MiR-146a-5p acts as a key factor in KCNQ1OT1/miR-146a-5p/ACER3 axis, and it represses radiosensitivity and promotes the tumorigenesis of hepatocellular carcinoma [28]. Moreover, human MSCs prevent group 2 innate lymphoid cell-dominant allergic airway inflammation in mice by delivering miR-146a-5p [29]. In early syphilis patients, miR-146a-5p is highly expressed, and treponema pallidum-stimulated macrophage-derived miR-146a-5p-containing exosomes reduce endothelial cells permeability and monocyte transendothelial migration through interacting with JAM-C [30]. In our work, we further explored the underlying mechanism of BMSCs-exosomes in regulating the imbalance of Th17/Treg in ITP. We found that miR-146a-5p was abundant in BMSCs-exosomes. Moreover, BMSCs-exosomes enhanced the expression of miR-146a-5p in CD4+ T cells, and promoted Th17/Treg ratio in CD4+ T cells. Thus, these results demonstrated that BMSCs-exosomes regulated Th17/Treg imbalance in CD4+ T cells through the delivery of miR-146a-5p.
IRAK1 is a target gene of miR-146a-5p. Previous study has found that miR-146a-5p regulates autophagy to repress particulate matter caused inflammation in THP-1 cells by targeting IRAK1 [31]. IRAK1 is highly expressed in breast cancer, and miR-146a-5p represses IRAK1 expression by directly targeting IRAK1, thereby inhibiting migration, and invasion of breast cancer cells [32]. Consistently, we also confirmed that miR-146a-5p interacted with IRAK1, and repressed IRAK1 expression in CD4+ T cells. BMSCs-exosomes inhibited IRAK1 expression in CD4+ T cells through the delivery of miR-146a-5p. IRAK1 overexpression enhanced the proportions of Th17 cells, and repressed the proportions of Tregs in CD4+ T cells. Overexpression of miR-146a-5p or exosomal miR-146a-5p effectively abrogated the influence conferred by IRAK1 overexpression on Th17/Treg ratio in CD4+ T cells. Taken together, these data confirmed that exosomal miR-146a-5p regulated the imbalance of Th17/Treg in ITP by interacting with IRAK1.
In conclusion, this work demonstrates that BMSCs-derived exosomes regulate the imbalance of Th17/Treg in ITP through the delivery of miR-146a-5p. MiR-146a-5p repressed IRAK1 expression by targeting IRAK1 in ITP. Thus, this work suggests that BMSCs-exosomal miR-146a-5p may be a potential therapeutic target for ITP.
References
Cooper N, Kruse A, Kruse C, Watson S, Morgan M, Provan D, et al. Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol. 2020. https://doi.org/10.1002/ajh.26045.
Stasi R. Immune thrombocytopenia: pathophysiologic and clinical update. Semin Thromb Hemost. 2012;38(5):454–62. https://doi.org/10.1055/s-0032-1305780.
Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–32. https://doi.org/10.1016/j.autrev.2017.04.012.
Kostic M, Zivkovic N, Cvetanovic A, Marjanović G. CD4 T cell phenotypes in the pathogenesis of immune thrombocytopenia. Cell Immunol. 2020;351:104096. https://doi.org/10.1016/j.cellimm.2020.104096.
Wang Q, Li J, Yu TS, Liu Y, Li K, Liu S, et al. Disrupted balance of CD4 T-cell subsets in bone marrow of patients with primary immune thrombocytopenia. Int J Biol Sci. 2019;15(13):2798–814. https://doi.org/10.7150/ijbs.33779.
Sehrawat S, Rouse BT. Interplay of regulatory T cell and Th17 cells during infectious diseases in humans and animals. Front Immunol. 2017;8:341. https://doi.org/10.3389/fimmu.2017.00341.
Jadidi-Niaragh F, Mirshafiey A. The deviated balance between regulatory T cell and Th17 in autoimmunity. Immunopharmacol Immunotoxicol. 2012;34(5):727–39. https://doi.org/10.3109/08923973.2011.619987.
Guo NH, Fu X, Zi FM, Song Y, Wang S, Cheng J. The potential therapeutic benefit of resveratrol on Th17/Treg imbalance in immune thrombocytopenic purpura. Int Immunopharmacol. 2019;73:181–92. https://doi.org/10.1016/j.intimp.2019.04.061.
Wu D, Liu Y, Pang N, Sun M, Wang X, Haridia Y, et al. PD-1/PD-L1 pathway activation restores the imbalance of Th1/Th2 and treg/Th17 cells subtypes in immune thrombocytopenic purpura patients. Medicine. 2019;98(43):e17608. https://doi.org/10.1097/md.0000000000017608.
Liu L, Hua M, Liu C, He N, Li Z, Ma D. The aberrant expression of microRNAs and correlations with T cell subsets in patients with immune thrombocytopenia. Oncotarget. 2016;7(47):76453–63. https://doi.org/10.18632/oncotarget.12949.
Zhang JM, Feng FE, Wang QM, Zhu XL, Fu HX, Xu LP, et al. Platelet-derived growth factor-BB protects mesenchymal stem cells (MSCs) derived from immune thrombocytopenia patients against apoptosis and senescence and maintains MSC-mediated immunosuppression. Stem Cells Transl Med. 2016;5(12):1631–43. https://doi.org/10.5966/sctm.2015-0360.
He Y, Xu LL, Feng FE, Wang QM, Zhu XL, Wang CC, et al. Mesenchymal stem cell deficiency influences megakaryocytopoiesis through the TNFAIP3/NF-κB/SMAD pathway in patients with immune thrombocytopenia. Br J Haematol. 2018;180(3):395–411. https://doi.org/10.1111/bjh.15034.
Rossi F, Tortora C, Palumbo G, Punzo F, Argenziano M, Casale M, et al. CB2 receptor stimulation and dexamethasone restore the anti-inflammatory and immune-regulatory properties of mesenchymal stromal cells of children with immune thrombocytopenia. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20051049.
Ma L, Zhou Z, Zhang D, Yang S, Wang J, Xue F, et al. Immunosuppressive function of mesenchymal stem cells from human umbilical cord matrix in immune thrombocytopenia patients. Thromb Haemost. 2012;107(5):937–50. https://doi.org/10.1160/th11-08-0596.
Zhang P, Zhang G, Liu X, Liu H, Yang P, Ma L. Mesenchymal stem cells improve platelet counts in mice with immune thrombocytopenia. J Cell Biochem. 2019. https://doi.org/10.1002/jcb.28405.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142–57. https://doi.org/10.3390/ijms15034142.
Seo Y, Kim HS, Hong IS. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019;2019:5126156. https://doi.org/10.1155/2019/5126156.
Wang Y, Ma WQ, Zhu Y, Han XQ, Liu N. Exosomes derived from mesenchymal stromal cells pretreated with advanced glycation end product-bovine serum albumin inhibit calcification of vascular smooth muscle cells. Front Endocrinol. 2018;9:524. https://doi.org/10.3389/fendo.2018.00524.
Maitra U, Davis S, Reilly CM, Li L. Differential regulation of Foxp3 and IL-17 expression in CD4 T helper cells by IRAK-1. J Immunol. 2009;182(9):5763–9. https://doi.org/10.4049/jimmunol.0900124.
Stürner KH, Verse N, Yousef S, Martin R, Sospedra M. Boswellic acids reduce Th17 differentiation via blockade of IL-1β-mediated IRAK1 signaling. Eur J Immunol. 2014;44(4):1200–12. https://doi.org/10.1002/eji.201343629.
Wang Y, Zhang J, Su Y, Wang C, Zhang G, Liu X, et al. miRNA-98-5p targeting IGF2BP1 induces mesenchymal stem cell apoptosis by modulating PI3K/Akt and p53 in immune thrombocytopenia. Mol Ther Nucleic Acids. 2020;20:764–76. https://doi.org/10.1016/j.omtn.2020.04.013.
Li H, Guan Y, Sun B, Dou X, Liu X, Xue F, et al. Role of bone marrow-derived mesenchymal stem cell defects in CD8 CD28 suppressor T-lymphocyte induction in patients with immune thrombocytopenia and associated mechanisms. Br J Haematol. 2020. https://doi.org/10.1111/bjh.16953.
Gong X, Sun D, Li Z, Shi Q, Li D, Ju X. Three-dimensional culture of umbilical cord mesenchymal stem cells effectively promotes platelet recovery in immune thrombocytopenia. Biol Pharm Bull. 2020;43(7):1052–60. https://doi.org/10.1248/bpb.b19-01069.
Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71(12):2020–30. https://doi.org/10.1002/iub.2147.
Li Y, Wang F, Guo R, Zhang Y, Chen D, Li X, et al. Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life. 2019;71(9):1284–92. https://doi.org/10.1002/iub.2035.
Tang M, Cheng L, Li F, Wu B, Chen P, Zhan Y, et al. Transcription factor IRF4 dysfunction affects the immunosuppressive function of treg cells in patients with primary immune thrombocytopenia. Biomed Res Int. 2019;2019:1050285. https://doi.org/10.1155/2019/1050285.
Liu X, Liu B, Li R, Wang F, Wang N, Zhang M, et al. miR-146a-5p plays an oncogenic role in NSCLC via suppression of TRAF6. Front Cell Dev Biol. 2020;8:847. https://doi.org/10.3389/fcell.2020.00847.
Yang G, Zhou L, Xu Q, Meng F, Wan Y, Meng X, et al. LncRNA KCNQ1OT1 inhibits the radiosensitivity and promotes the tumorigenesis of hepatocellular carcinoma via the miR-146a-5p/ACER3 axis. Cell Cycle. 2020;19(19):2519–29. https://doi.org/10.1080/15384101.2020.1809259.
Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles. 2020;9(1):1723260. https://doi.org/10.1080/20013078.2020.1723260.
Hu W, Xu B, Zhang J, Kou C, Liu J, Wang Q, et al. Exosomal miR-146a-5p from Treponema pallidum-stimulated macrophages reduces endothelial cells permeability and monocyte transendothelial migration by targeting JAM-C. Exp Cell Res. 2020;388(1):111823. https://doi.org/10.1016/j.yexcr.2020.111823.
Shang Y, Liu Q, Wang L, Qiu X, Chen Y, An J. MicroRNA-146a-5p negatively modulates PM caused inflammation in THP-1 cells via autophagy process. Environ pollut. 2020. https://doi.org/10.1016/j.envpol.2020.115961.
Long JP, Dong LF, Chen FF, Fan YF. miR-146a-5p targets interleukin-1 receptor-associated kinase 1 to inhibit the growth, migration, and invasion of breast cancer cells. Oncol Lett. 2019;17(2):1573–80. https://doi.org/10.3892/ol.2018.9769.
Funding
This work was supported by the National Natural Science Foundation of China (NFSC 81460037) and Postgraduate Innovation Special Fund of Jiangxi Province (YC2020-B031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2021_547_MOESM1_ESM.tif
Supplementary file1 (TIF 338 KB) Supplementary figure 1 BMSCs-exosomes had no effect on Th17/Treg ratio in CD4+ T cells of healthy individuals. CD4+ T cells were isolated from peripheral blood of healthy individuals. CD4+ T cells were incubated with BMSCs-exosomes or PBS. Normal CD4+ T cells served as control. (A-D) Flow cytometry was performed to assess the proportions of Th17 cells and Tregs in the CD4+ T cells. (E-G) ELISA was performed to examine the concentrations of IL-17, IL-10 and TGF-β in the CD4+ T cells. N = 3.
13577_2021_547_MOESM2_ESM.tif
Supplementary file2 (TIF 131 KB) Supplementary figure 2 The expression of IRAK1 in CD4+ T cells. CD4+ T cells were transfected with pcDNA3.1-IRAK1 or pcDNA3.1-NC. The expression of IRAK1 in CD4+ T cells was assessed by WB. N = 3. **P < 0.01 vs. Vector.
Rights and permissions
About this article
Cite this article
He, Y., Ji, D., Lu, W. et al. Bone marrow mesenchymal stem cell-derived exosomes induce the Th17/Treg imbalance in immune thrombocytopenia through miR-146a-5p/IRAK1 axis. Human Cell 34, 1360–1374 (2021). https://doi.org/10.1007/s13577-021-00547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00547-7