Abstract
Expressed on the cell surface of most of NK cells and some T cells, CD161 has been shown to deliver inhibitory signal in human NK cells. To determine whether the CD161-expressing cell quantities and the cell surface expression levels of CD161 in NK and T cells were altered in systemic lupus erythematosus (SLE) patients, we analyzed the CD3, CD56 and CD161 expression patterns of peripheral blood lymphocytes by flow cytometric analysis to identify different NK and T cell subpopulations. The cell surface expression levels of CD161 were estimated by the mean florescence intensities (MFIs) of CD161. It was found that SLE patients had lower frequencies of CD161+CD56+CD3− and CD161+CD56+CD3+ cells among the lymphocyte population than normal controls, whereas the frequencies of CD161−CD56+CD3− and CD161+CD56−CD3+ cells were not statistically different between two groups. In addition, SLE patients also had decreased absolute counts of all CD161-expressing NK cells and T cells and had reduced frequencies of CD161+ cells in CD56+CD3−, CD56+CD3+ and CD56−CD3+ cell populations. Moreover, SLE patients had reduced MFIs of CD161 in CD161+CD56+CD3+ and CD161+CD56−CD3+, but not CD161+CD56+CD3−, cell populations. Our results indicated that CD161-expressing cell frequency and the CD161 expression levels were reduced in some NK and T cell subpopulations of SLE patients, suggesting possible important role of CD161 and CD161-expressing immune cells in the SLE pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a human autoimmune disease characterized by lymphocyte hyperactivity, autoantibody production and immune-mediated tissue damages. Although the pathogenesis of SLE still remains elusive, the possible contribution of natural killer (NK) cell defect to the SLE pathogenesis has been evidenced in several studies, showing that SLE patients had decreased NK cell numbers in the peripheral blood, decreased NK cell cytotoxicity function, impaired NK cell differentiation and altered cytokine production from NK cells [1–10]. Since NK cells can modulate innate and adaptive immune responses and are able to inhibit autoreactive Th17 cells, it is possible that the NK cell defect may be related to regulating autoreactive lymphocyte activation and the development of autoimmunity in SLE patients [11–13].
NK cells are traditionally defined as immune cells expressing CD56, but not CD3, on the cell surface (CD56+CD3−), although some CD3+ T cells also express CD56 and are therefore referred to as (CD56+CD3+) NK T cells in this study. In addition to CD56, several NK cell surface receptors have been identified to deliver activating and inhibitory signals for regulating NK cell functions. CD161 is a type II transmembrane C-type lectin glycoprotein that appears to function as an inhibitory receptor in human NK cells. CD161 is also referred to as killer cell lectin-like receptor subfamily B member 1 (KLRB1), NK cell receptor protein 1A (NKR-P1A) or C-type lectin domain family 5 member B (CLEC5B) [14]. Although CD161 is expressed in the majority of NK cells, it also has been shown to be expressed on peripheral blood NK T cells, subsets of CD4+ and CD8+ T cells predominantly with the effector/memory phenotype and tissue-infiltrating T cells [15–17]. Recently, CD161 was also found to be expressed on human Th17 cells and IL-17-producing T cells at a high level and on FoxP3+ T cells [18–20]. The ligand for human CD161 is lectin-like transcript-1 molecule (LLT1) (also referred to as osteoclast inhibitory lectin or CLEC2D) that can inhibit NK cell cytotoxicity after interacting with CD161 [21, 22]. Therefore, the interaction between LLT1 and CD161 may play an important role in modulating the functions of NK cells and T cells, especially Th17 cells.
To explore the possible important role of CD161 in the SLE pathogenesis, in this study, we performed flow cytometric analysis to evaluate the absolute counts and frequencies of CD161-expressing cells in the peripheral blood NK cell and T cell populations for SLE patients and normal controls. The CD161 expression levels were also determined by the mean florescence intensities in the flow cytometric analysis. The data from SLE patients were statistically compared with those from normal controls to determine whether SLE patients had any abnormality in the quantities of CD161-expressing cells and/or the CD161 expression levels.
Materials and methods
Patients and normal controls
SLE patients (n = 20) and normal control individuals (n = 16) were recruited into this study from Cathay General Hospital, Taipei, Taiwan. The classification criteria of American College of Rheumatology were used for the diagnosis of SLE [23]. The disease activities of SLE patients were determined using the systemic lupus erythematosus disease activity index (SLEDAI) scoring method [24]. Only SLE patients with positive antinuclear and anti-ds DNA antibodies and with the active disease activity (SLEDAI score more than 3) were recruited into this study. This study was approved by the institutional review board for research ethics at Cathay General Hospital, Taiwan. Informed consent was provided from all blood donors.
Preparation of peripheral blood mononuclear cells (PBMCs)
Heparinized whole blood was obtained from each study individual, and PBMC preparation was conducted within 4 h after blood samples were collected. PBMCs were isolated by Ficoll–Hypaque density gradient centrifugation.
Flow cytometric analysis
To analyze different NK cell and T cell subpopulations by flow cytometric analysis, PBMCs isolated from each of normal individuals and SLE patients were incubated with FITC-conjugated anti-CD3 antibody (BD Biosciences, San Jose, CA, USA), PE-conjugated anti-CD56 antibody (BD Biosciences, San Jose, CA, USA) and PE-Cy5-conjugated anti-CD161 antibody (Beckman Coulter, Fullerton, CA, USA). Then, cells were washed with phosphate-buffered saline twice and were then immediately analyzed by a Becton Dickinson FACScan analyzer. In the flow cytometric analysis, the lymphocyte population was gated first to identify CD3-positive and CD3-negative lymphocyte populations, and then, the CD3-positive or CD3-negative lymphocyte population was gated for the subsequent analysis of the expression patterns of CD56 and CD161. The mean fluorescence intensities (MFIs) in the flow cytometric analysis for different cell subpopulations were used to estimate the expression levels of CD56 and CD161.
Statistical analysis
The nonparametric Mann–Whitney rank sum test was used to test the significance of the difference in the cell population frequencies and mean florescence intensities between SLE patient and normal control groups. P-values were calculated and considered significant if less than 0.05.
Results
Profiling the flow cytometric patterns of CD161 expression in the peripheral blood NK cells and T cell populations
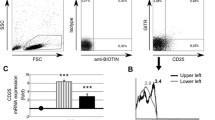
The patterns of CD161 expression in CD56+CD3− NK cell, CD56+CD3+ NK T cell and CD56−CD3+ T cell populations were analyzed by flow cytometric analysis, using florescence-labeled anti-CD3, anti-CD56 and anti-CD161 antibodies to stain peripheral blood mononuclear cells. As shown in Fig. 1, the CD161 expression was detected in NK cells, NK T cells as well as in T cells. The majority of NK cells and NK T cells expressed CD161, whereas only small portion of T cells expressed CD161. More importantly, SLE patient and normal control seemed to have the distinct flow cytometric patterns of CD161 and CD56 expression in CD3-positive and CD3-negative lymphocyte populations (Fig. 1).
Flow cytometric analysis to identify various CD161-expressing NK cell, NK T cell and T cell subpopulations in the peripheral blood. Shown is the representative flow cytometric analysis for the expression patterns of CD56 and CD161 in CD3-positive and CD3-negative lymphocyte populations from a normal control and an SLE patient
Quantifying the frequencies and absolute counts of peripheral blood NK and T cell subpopulations
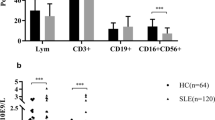
By using CD161, CD56 and CD3 as cell lineage markers in flow cytometric analysis of lymphocyte population, we were able to identify four NK and NK T cell subpopulations, CD161+CD56+CD3−, CD161−CD56+CD3−, CD161+CD56+CD3+ and CD161−CD56+CD3+ cells, and two T cell subpopulations, CD161+CD56−CD3+ and CD161−CD56−CD3+ cells. To evaluate whether SLE patients have altered frequency of any one of these NK and T cell subpopulations among lymphocytes, the frequency of each cell subpopulation among lymphocytes was determined by the flow cytometric analysis for each of SLE patient and normal control groups. Then, the frequencies of each cell subpopulation in SLE patient group were statistically compared with those in normal control group. It was found that the frequencies of CD161+CD56+CD3− and CD161+CD56+CD3+ cells among the lymphocyte population in SLE patients were lower than those in normal controls, as shown in Fig. 2, whereas SLE patients and normal controls did not have statistically significant difference in the frequencies of CD161−CD56+CD3−, CD161−CD56+CD3+ and CD161+CD56−CD3+ cell subpopulations, although SLE patients seemed to have slightly lower frequencies of CD161−CD56+CD3+ and CD161+CD56−CD3+ cell subpopulations (Fig. 2). When we calculated the absolute cell counts (per microliter) of each cell subpopulation for SLE patients and normal controls, we found that SLE patients had decreased absolute cell counts of all NK and T cell subpopulations (Fig. 3). We then calculated the percentage of CD161+ cells in CD56+CD3−, CD56+CD3+ and CD56−CD3+ cell subpopulations for each individual of SLE patients and normal controls for the subsequent statistical comparison of these two study groups. We found that SLE patients had lower percentages of CD161+ cells in CD56+CD3−, CD56+CD3+ and CD56−CD3+ cell subpopulations than normal controls (Fig. 4). Thus, our data indicated that SLE patients had defect in NK cells and T cells, but the defect was more prominent for CD161+ cells.
Frequencies of different NK cell, NK T cell and T cell subpopulations among the lymphocyte population. The frequencies of CD161+CD56+CD3− and CD161−CD56+CD3− NK cell subpopulations, CD161+CD56+CD3+ and CD161−CD56+CD3+ NK T cell subpopulations, and CD161+CD56−CD3+ T cells were obtained from flow cytometric analysis for each individual of SLE and normal control groups. The data of SLE group (n = 20) and normal control group (n = 16) were shown as box plots with the 25th to 75th percentiles for each cell subpopulation. The p values of the statistical comparisons between SLE and control groups for different cell subpopulations were indicated
Absolute counts of different NK cell, NK T cell and T cell subpopulations in the peripheral blood. The absolute counts of CD161+CD56+CD3−, CD161−CD56+CD3−, CD161+CD56+CD3+, CD161−CD56+CD3+, CD161+CD56−CD3+ and CD161−CD56−CD3+ cell subpopulations were calculated from cell frequencies in the flow cytometric analysis for each individual of SLE and normal control groups. The data of SLE and normal control groups were shown as box plots with the 25th to 75th percentiles for each cell subpopulation. The p values of the statistical comparisons between SLE and control groups for different cell subpopulations were indicated
Frequencies (percentages) of CD161-expressing cells among peripheral blood NK cell, NK T cell and T cell populations. The frequencies of CD161-expressing cells among CD56+CD3− NK cells, CD56+CD3+ NK T cells and CD56−CD3+ T cells were determined by flow cytometric analysis for each individual of SLE and normal control groups. The data of SLE and normal control groups were shown as box plots with the 25th to 75th percentiles for each cell population. The p values of the statistical comparisons between SLE and control groups for different cell populations were indicated
Evaluating the expression levels of CD161 in NK and T cell subpopulations
We then evaluated whether SLE patients had altered expression levels of CD161 in different NK and T cell subpopulations, CD161+CD56+CD3−, CD161+CD56+CD3+ and CD161+CD56−CD3+ cells, by using the mean fluorescence intensities (MFIs) of CD161 in flow cytometric analysis to represent the CD161 expression levels. The MFIs of CD161 in the CD161−CD56−CD3− cell subpopulation of SLE patients and normal controls were used to normalize the MFIs of CD161 in different NK and T cell subpopulations, and calculated MFIs of CD161 in SLE group were statistically compared with those in normal control group. As shown in Fig. 5, SLE group exhibited lower MFIs of CD161 in CD161+CD56+CD3+ and CD161+ CD56−CD3+ cell subpopulations than normal controls with the statistical significance, whereas both study groups had comparable MFIs of CD161 in CD161+CD56+CD3− cell subpopulation. The data indicated that SLE patients had decreased CD161 expression in NK T cells and in T cells. To determine whether this alteration in SLE patients is specific to CD161, we also analyzed the CD56 expression levels by using flow cytometric MFIs of CD56 in the CD161−CD56−CD3− cell population to normalize and calculate MFIs of CD56 in CD161+CD56+CD3−, CD161−CD56+CD3−, CD161+CD56+CD3+ and CD161−CD56+CD3+ cell subpopulations of SLE patients and normal controls for the subsequent statistical comparison. It was found that SLE group exhibited higher MFIs of CD56 in CD161+CD56+CD3− and CD161−CD56+CD3− cell subpopulations than normal control group, whereas both groups had comparable MFIs of CD56 in CD161+CD56+CD3+ and CD161−CD56+CD3+ cell populations, indicating that CD56 expression was increased in NK cells, but not NK T cells, of SLE patients (Fig. 6). Our results demonstrated the differential alteration in the CD161 and CD56 expression on NK and T cell subpopulations of SLE patients.
CD161 protein expression levels in peripheral blood NK cells, NK T cells and T cells. The mean florescence intensities (MFIs) of CD161 in flow cytometric analysis were normalized and used to represent the CD161 protein expression levels in CD161+CD56+CD3− NK cells, CD161+CD56+CD3+ NK T cells and CD161+CD56−CD3+ T cells of SLE patients and normal controls. The CD161 MFIs of SLE and normal control groups were shown as box plots with the 25th to 75th percentiles for each cell population. The p values of the statistical comparisons between SLE and control groups for different cell populations were indicated
CD56 protein expression levels in peripheral blood NK cell, NK T cell and T cells. The mean florescence intensities (MFIs) of CD56 in flow cytometric analysis were normalized and used to represent the CD56 protein expression levels in CD161+CD56+CD3− and CD161−CD56+CD3− NK cells and in CD161+CD56+CD3+ and CD161−CD56+CD3+ NK T cells of SLE patients and normal controls. The CD56 MFIs of SLE and normal control groups were shown as box plots with the 25th to 75th percentiles for each cell population. The p values of the statistical comparisons between SLE and control groups for different cell populations were indicated
Discussion
SLE patients have been shown to have decreased amounts of peripheral blood CD56+CD3− NK cells. In this study, we used CD161 as a surface marker to determine whether SLE patients particularly had the defect in CD161-expressing NK and NK T cells, and it was found that SLE patients have decreased cell frequencies of CD161+CD56+CD3− and CD161+CD56+CD3+, but not CD161−CD56+CD3− or CD161−CD56+CD3+, NK or NK T cell subpopulations among peripheral blood lymphocytes. These data suggest that the SLE patients have relatively selective defect in quantities of CD161-expressing cells in NK cells and NK T cells, although the absolute counts of all NK cell and NK T cell subpopulations were found decreased in SLE patients.
CD161 is expressed in subsets of human CD4+ and CD8+ T cells. In this study, we found that SLE patients had decreased absolute counts of CD161+CD56−CD3+ T cells and decreased cell frequencies of CD161-expressing cells among CD56−CD3+ T cells, suggesting that SLE patients may have selective decrease in CD161-expressing T cells in the peripheral blood, although lymphopenia is a general phenomenon in SLE patients. Our results appear to be in agreement with the finding that SLE patients had decreased CD161+CD8+ T cells [25]. Indeed, CD161+CD4+ T cell deficiency also has been found in patients with rheumatoid arthritis, another human autoimmune disease [26]. Therefore, it remains to be determined whether SLE patients also have defect in CD161+CD4+ T cells.
SLE patients had been reported to have the aberrant expression of certain NK cell receptors, including NKG2A, NKG2D, DNAM-1, CD16, CD69 and CD158 [8, 9, 27, 28]. CD161 is an NK cell receptor that can deliver inhibitory signal for the cytotoxicity function of human NK cells. In this study, we demonstrated that the CD161 expression on CD56+CD3− NK cells was not affected in SLE patients, but NK cells of SLE patients exhibited higher expression levels of CD56, another NK cell surface protein which is also known as neural cell adhesion molecule (NCAM) and may be involved in cell adhesion function. This finding seems to be concordant with the previous report that SLE patients have increased CD56bright NK cell proportion [29]. In contrast, the CD161 expression was found to be down-regulated in CD56+CD3+ NK T cells and T cells of SLE patients. It is possible that the aberrant expression of CD161 and CD56 may contribute to the functional abnormality in NK, NK T and T cells of SLE patients. In addition, it will be interesting to determine whether SLE patients have aberrant CD161 expression in Th17 cells, since CD161 is also expressed on Th17 cells, a T cell lineage linked to some human inflammatory and autoimmune disorders [30].
Taken together, our results showed that CD161-expressing cell frequency and the CD161 expression levels were reduced in some NK and T cell subpopulations of SLE patients. This evidence supports the possibility that CD161 and CD161-expressing immune cells may contribute to the SLE pathogenesis.
References
Sibbitt WL Jr, Mathews PM, Bankhurst AD. Natural killer cell in systemic lupus erythematosus. Defects in effector lytic activity and response to interferon and interferon inducers. J Clin Invest. 1983;71:1230–9.
Yabuhara A, Yang FC, Nakazawa T, et al. A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. J Rheumatol. 1996;23:171–7.
Stohl W, Elliott JE, Hamilton AS, Deapen DM, Mack TM, Horwitz DA. Impaired recovery and cytolytic function of CD56+ T and non-T cells in systemic lupus erythematosus following in vitro polyclonal T cell stimulation. Studies in unselected patients and monozygotic disease-discordant twins. Arthritis Rheum. 1996;39:1840–51.
Erkeller-Yuksel FM, Lydyard PM, Isenberg DA. Lack of NK cells in lupus patients with renal involvement. Lupus. 1997;6:708–12.
Riccieri V, Spadaro A, Parisi G, et al. Down-regulation of natural killer cells and of γ/δ T cells in systemic lupus erythematosus. Does it correlate to autoimmunity and to laboratory indices of disease activity? Lupus. 2000;9:333–7.
Park Y-W, Kee S-J, Cho Y-N, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–63.
Cho Y-N, Kee S-J, Lee S-J, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology. 2011;50:1054–63.
Hervier B, Beziat V, Haroche J, et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011;63:1698–706.
Puxeddu I, Bongiorni F, Chimenti D, et al. Cell surface expression of activating receptors and co-receptors on peripheral blood NK cells in systemic autoimmune diseases. Scand J Rheumatol. 2012;41:298–304.
Henriques A, Teixeira L, Inês L, et al. NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol. 2013;32:805–13.
Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6:751–60.
Wu W, Shi S, Ljunggren H-G, et al. NK cells inhibit T-bet-deficient, autoreactive Th17 cells. Scand J Immunol. 2012;76:559–66.
Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther. 2013;15:216.
Mesci A, Ljutic B, Makrigiannis AP, Carlyle JR. NKR-P1 biology: from prototype to missing self. Immunol Res. 2006;35:13–26.
Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–28.
Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211–6.
Billerbeck E, Kang Y-H, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010;107:3006–11.
Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–16.
Maggi L, Santarlasci V, Capone M, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–81.
Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood. 2013;121:2647–58.
Aldemir H, Prod’homme V, Dumaurier M-J, et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–5.
Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–9.
Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7.
Bombardier C, Gladman D, Urowitz M, Caron D, Chang C. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40.
Mitsuo A, Morimoto S, Nakiri Y, et al. Decreased CD161+CD8+ T cells in the peripheral blood of patients suffering from rheumatic diseases. Rheumatology. 2006;45:1477–84.
Chalan P, Kroesen B-J, van der Geest KSM, et al. Circulating CD4+CD161+ T lymphocytes are increased in seropositive arthralgia patients but decreased in patients with newly diagnosed rheumatoid arthritis. PLoS One. 2013;8:e79370.
Li W-X, Pan H-F, Hu J-L, et al. Assay of T- and NK-cell subsets and the expression of NKG2A and NKG2D in patients with new-onset systemic lupus erythematosus. Clin Rheumatol. 2010;29:315–23.
Bai Y, Zhang Y, Yang Q, et al. The aberrant expression of stimulatory and inhibitory killer immunoglobulin-like receptors in NK- and NKT-cells contributes to lupus. Clin Lab. 2014;60:717–27.
Schepis D, Gunnarsson I, Eloranta M-L, et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–6.
Bedoya SK, Lam B, Lau K, Larkin J III. Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789.
Acknowledgments
I thank Yanfeng Lu for review of the manuscript. This study was supported by a grant from the joined research found of Cathay General Hospital and National Taiwan University and by a grant from the National Science Council of Taiwan (Grant#101-2314-B-281-006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Lin, YL., Lin, SC. Analysis of the CD161-expressing cell quantities and CD161 expression levels in peripheral blood natural killer and T cells of systemic lupus erythematosus patients. Clin Exp Med 17, 101–109 (2017). https://doi.org/10.1007/s10238-015-0402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0402-1